Abstract
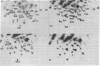
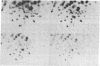
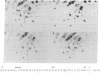
Avian retroviruses of subgroups B and D efficiently infect chicken (C/E) but not turkey (T/BD) cells. We describe here three variants of subgroup B and D viruses that infect both cell types equally well. One of these viruses, NTRE-4, was a recombinant between transformation-defective Prague (Pr) strain Rous sarcoma virus (RSV) subgroup B and the endogenous virus RAV-0; the second, SR-DE-1, was a recombinant between Schmidt-Ruppin RSV subgroup D and defective endogenous virus information. T1 oligonucleotide fingerprint analysis of the genomes of these two viruses showed only a small alteration in the portion of the env gene responsible for subgroup specificity, as indicated by the presence of a single subgroup E oligonucleotide in an otherwise purely subgroup B or D gene. The third virus, hrBO1Pr-B, was a variant of Pr-RSV-B that did not appear to be a recombinant and whose altered host range we attribute to mutation. Analysis of the host range of all three viruses by infection of selectively resistant cells and by interference testing indicates that all use the subgroup B receptor on chicken cells and the subgroup E receptor on turkey cells. These viruses may be analogous to the polytropic recombinant viruses recently found to be associated with leukemia in some strains of mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B., Motta J. V. The role of the tvb locus in genetic resistance to RSV(RAV-O). Virology. 1975 Oct;67(2):327–334. doi: 10.1016/0042-6822(75)90434-1. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Rommelaere J., Hopkins N. Large T1 oligonucleotides of Moloney leukemia virus missing in an env gene recombinant, HIX, are present on an intracellular 21S Moloney viral RNA species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2964–2968. doi: 10.1073/pnas.75.6.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Neiman P. E. Infection of chick cells by subgroup E viruses. Virology. 1976 Sep;73(2):508–520. doi: 10.1016/0042-6822(76)90412-8. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L. Intracellular restriction on the growth of induced subgroup E avian type C viruses in chicken cells. J Virol. 1976 Jun;18(3):856–866. doi: 10.1128/jvi.18.3.856-866.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Coffin J., Eisenman R. Recombinant avian oncoviruses. I. Alterations in the precursor to the internal structural proteins. Virology. 1978 Jun 15;87(2):326–338. doi: 10.1016/0042-6822(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. DEAE-dextran: enhancement of cellular transformation induced by avian sarcoma viruses. Virology. 1967 Sep;33(1):175–177. doi: 10.1016/0042-6822(67)90109-2. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Weiss R. A., Hanafusa H. Proposal for numbering mutants of avian leukosis and sarcoma viruses. J Virol. 1974 Feb;13(2):551–554. doi: 10.1128/jvi.13.2.551-554.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]