Abstract
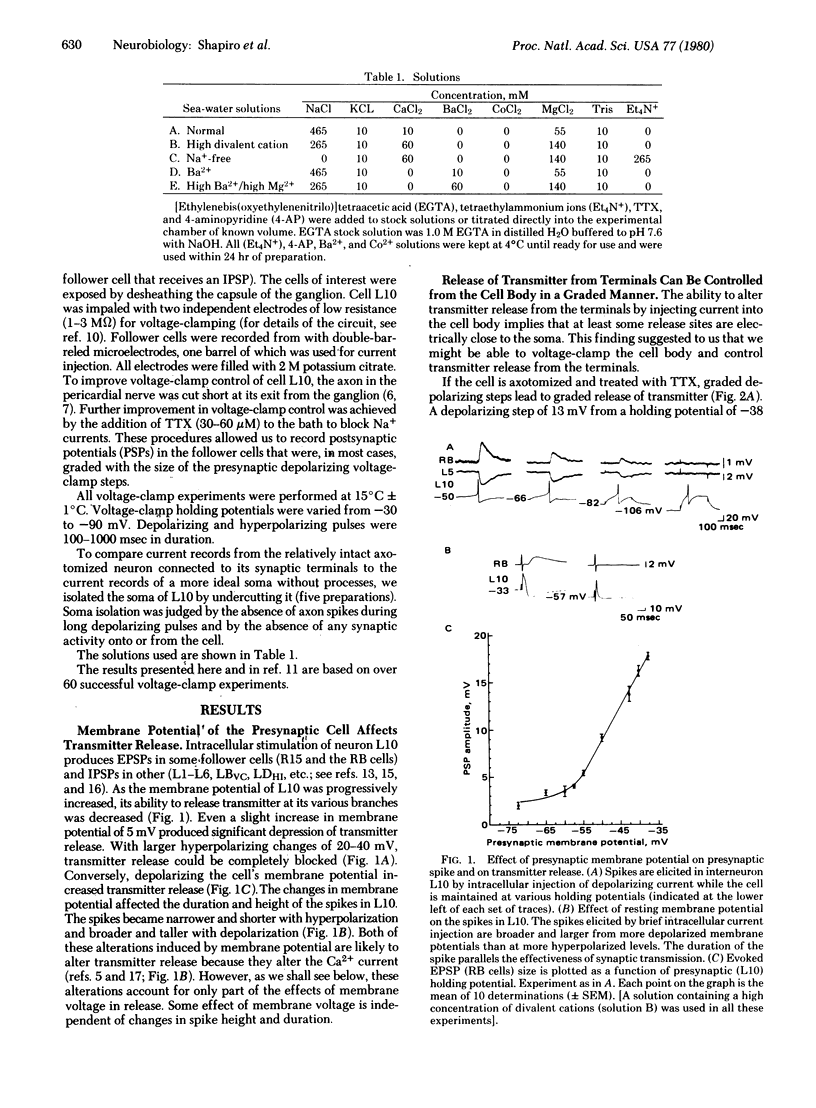
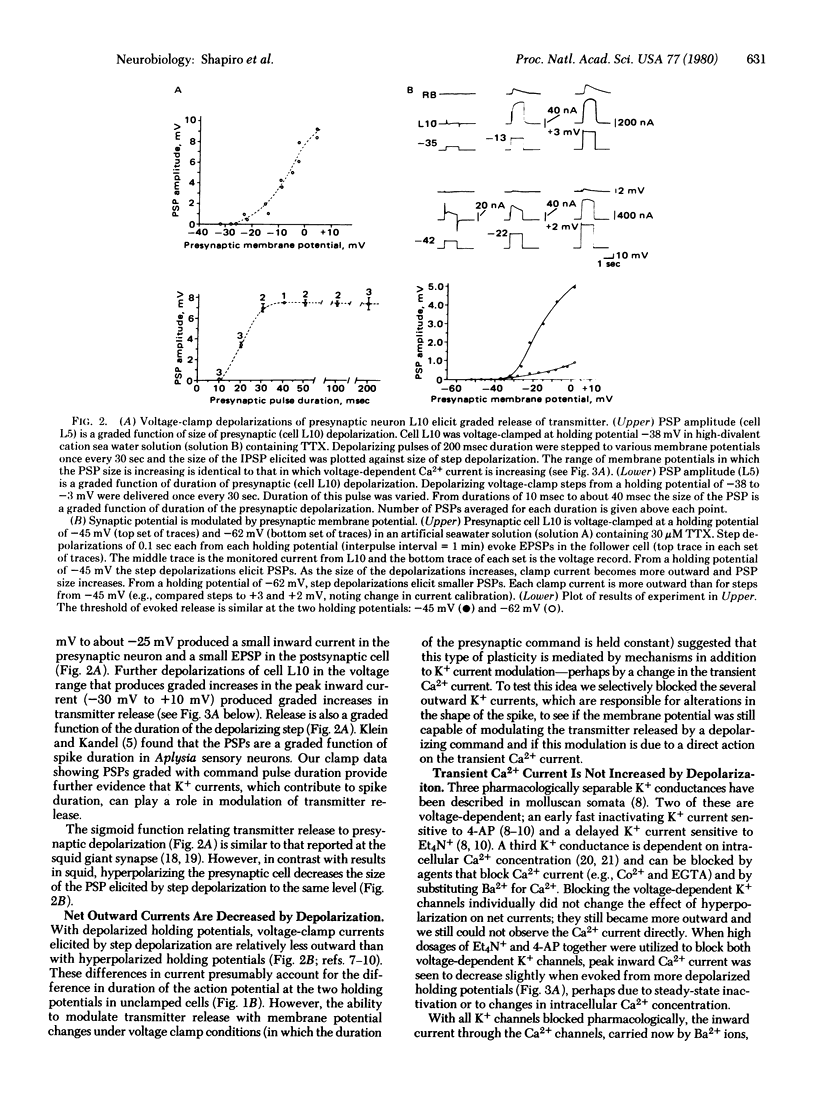
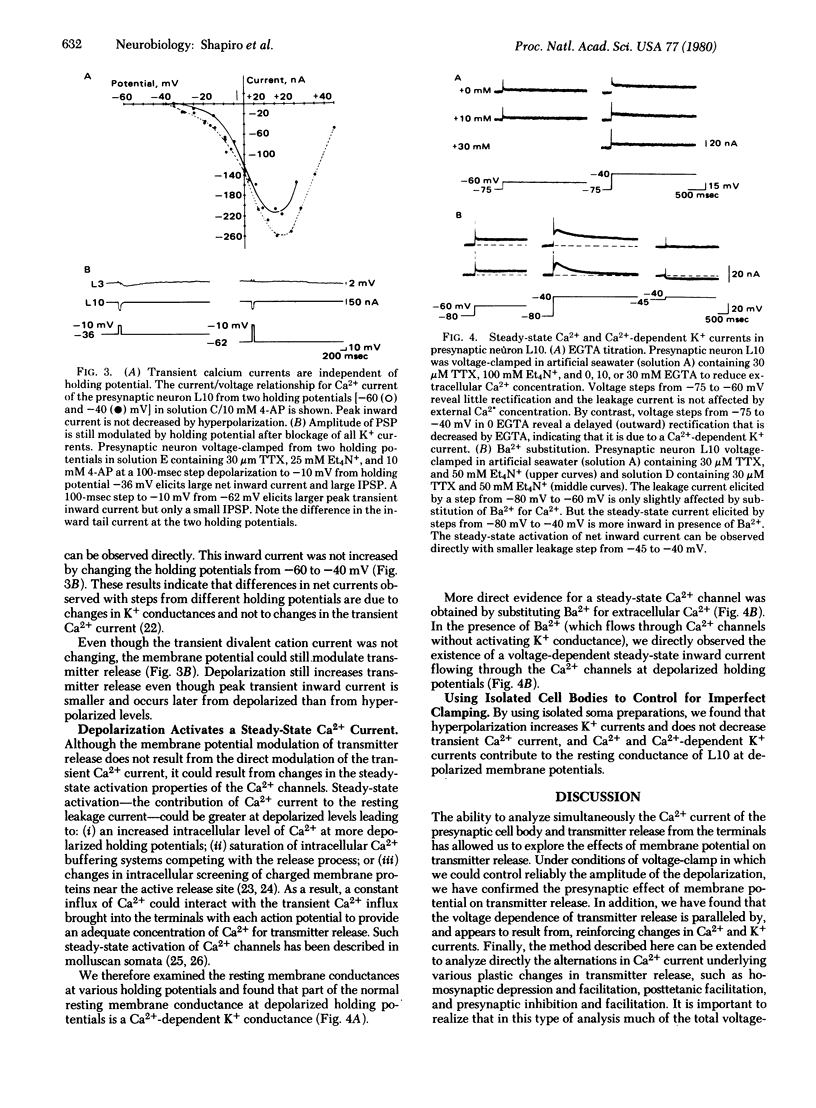
We have examined the relationships between the modulation of transmitter release and of specific ionic currents by membrane potential in the cholinergic interneuron L10 of the abdominal ganglion of Aplysia californica. The presynaptic cell body was voltage-clamped under various pharmacological conditions and transmitter release from the terminals was assayed simultaneously by recording the synaptic potentials in the postsynaptic cell. When cell L10 was voltage-clamped from a holding potential of -60 mV in the presence of tetrodotoxin, graded transmitter release was evoked by depolarizing command pulses in the membrane voltage range (-35 mV to + 10 mV) in which the Ca2+ current was also increasing. Depolarizing the holding potential of L10 results in increased transmitter output. Two ionic mechanisms contribute to this form of plasticity. First, depolarization inactivates some K+ channels so that depolarizing command pulses recruit a smaller K+ current. In unclamped cells the decreased K+ conductance causes spike-broadening and increased influx of Ca2+ during each spike. Second, small depolarizations around resting potential (-55 mV to -35 mV) activate a steady-state Ca2+ current that also contributes to the modulation of transmitter release, because, even with most presynaptic K+ currents blocked pharmacologically, depolarizing the holding potential still increases transmitter release. In contrast to the steady-state Ca2+ current, the transient inward Ca2+ current evoked by depolarizing clamp steps is relatively unchanged from various holding potentials.
Keywords: ionic current, voltage clamp, synaptic transmission
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass L., Moore W. J. Electrokinetic mechanism of miniature postsynaptic potentials. Proc Natl Acad Sci U S A. 1966 May;55(5):1214–1217. doi: 10.1073/pnas.55.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Byrne J. H., Shapiro E., Dieringer N., Koester J. Biophysical mechanisms contributing to inking behavior in Aplysia. J Neurophysiol. 1979 Sep;42(5):1233–1250. doi: 10.1152/jn.1979.42.5.1233. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Time course separation of two inward currents in molluscan neurons. Brain Res. 1977 Jan 7;119(2):487–492. doi: 10.1016/0006-8993(77)90330-4. [DOI] [PubMed] [Google Scholar]
- Eckert R., Lux H. D. A voltage-sensitive persistent calcium conductance in neuronal somata of Helix. J Physiol. 1976 Jan;254(1):129–151. doi: 10.1113/jphysiol.1976.sp011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K. Synaptic transmission without action potentials: input-output properties of a nonspiking presynaptic neuron. J Neurophysiol. 1978 Jul;41(4):1014–1025. doi: 10.1152/jn.1978.41.4.1014. [DOI] [PubMed] [Google Scholar]
- Horn R., Miller J. J. A prolonged, voltage-dependent calcium permeability revealed by tetraethylammonium in the soma and axon of Aplysia giant neuron. J Neurobiol. 1977 Sep;8(5):399–415. doi: 10.1002/neu.480080502. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Frazier W. T., Waziri R., Coggeshall R. E. Direct and common connections among identified neurons in Aplysia. J Neurophysiol. 1967 Nov;30(6):1352–1376. doi: 10.1152/jn.1967.30.6.1352. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. Three acetylcholine receptors in Aplysia neurones. J Physiol. 1972 Aug;225(1):115–146. doi: 10.1113/jphysiol.1972.sp009931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester J., Kandel E. R. Further identification of neurons in the abdominal ganglion of Aplysia using behavioral criteria. Brain Res. 1977 Jan 31;121(1):1–20. doi: 10.1016/0006-8993(77)90435-8. [DOI] [PubMed] [Google Scholar]
- Koester J., Mayeri E., Liebeswar G., Kandel E. R. Neural control of circulation in Aplysia. II. Interneurons. J Neurophysiol. 1974 May;37(3):476–496. doi: 10.1152/jn.1974.37.3.476. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents and their relation to synaptic transmission: voltage clamp study in squid giant synapse and theoretical model for the calcium gate. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2918–2922. doi: 10.1073/pnas.73.8.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeri E., Brownell P., Branton W. D. Multiple, prolonged actions of neuroendocrine bag cells on neurons in Aplysia. II. Effects on beating pacemaker and silent neurons. J Neurophysiol. 1979 Jul;42(4):1185–1197. doi: 10.1152/jn.1979.42.4.1185. [DOI] [PubMed] [Google Scholar]
- Mayeri E., Brownell P., Branton W. D., Simon S. B. Multiple, prolonged actions of neuroendocrine bag cells on neurons in Aplysia. I. Effects on bursting pacemaker neurons. J Neurophysiol. 1979 Jul;42(4):1165–1184. doi: 10.1152/jn.1979.42.4.1165. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:157–170. doi: 10.1113/jphysiol.1978.sp012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Quantal analysis of transmitter release at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:171–185. doi: 10.1113/jphysiol.1978.sp012415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimahara T., Tauc L. Multiple interneuronal afferents to the giant cells in Aplysia. J Physiol. 1975 May;247(2):299–319. doi: 10.1113/jphysiol.1975.sp010933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W., Kita H. The possible role of fixed membrane surface charges in acetylcholine release at the frog neuromuscular junction. J Membr Biol. 1973;14(4):365–382. doi: 10.1007/BF01868085. [DOI] [PubMed] [Google Scholar]
- Wachtel H., Kandel E. R. A direct synaptic connection mediating both excitation and inhibition. Science. 1967 Dec 1;158(3805):1206–1208. doi: 10.1126/science.158.3805.1206. [DOI] [PubMed] [Google Scholar]
- Waziri R. Presynaptic electrical coupling in Aplysia: effects on postsynaptic chemical transmission. Science. 1977 Feb 25;195(4280):790–792. doi: 10.1126/science.189390. [DOI] [PubMed] [Google Scholar]


