Abstract
In this study, the effect of soil ameliorators on ectomycorrhizal (ECM) fungal communities in coal mine spoils was investigated. Organic fertilizers and slaked lime were applied as soil ameliorators in 3 abandoned coal mine spoils. One year after the initial treatment, roots of Pinus densiflora seedlings were collected and the number of ECM species, colonization rate, and species diversity were assessed. The results showed that the soil ameliorators significantly increased ECM colonization on the roots of P. densiflora. The results suggest that soil ameliorators can have a positive effect on ECM fungi in terms of growth of host plants and show the potential use of soil ameliorator treatment for revegetation with ECM-colonized pine seedlings in the coal mine spoils.
Keywords: Coal mine spoils, Colonization rate, Ectomycorrhizal fungi, Pinus densiflora, Soil ameliorator, Species diversity
Introduction
Most land plants have a symbiotic relationship with mycorrhizal fungi in their roots [1]. Mycorrhizal fungi help host plants absorb minerals (especially phosphate) and water and enable plants to develop resistance against stressors such as drought and heavy-metal contamination of soils [2]. Ectomycorrhizal (ECM) fungi have a relationship with the roots of forest woody plants and play important roles in plant growth and survival. ECM fungi canalso reduce the degree of soil contamination by decreasing the amount of heavy metals in the surrounding soil [3, 4]. The characteristics of the ECM fungal community can be influenced by numerous biotic or abiotic environmental factors, including diversity of host plants [5], soil-type [6], and forest-type [7]. Environmental changes through disturbance could also affect the ECM fungal community [8-10].
Recently, an increased level of concern regarding ecological restoration has prompted efforts to introduce ECM fungi to help the restoration of disturbed sites. Abandoned coal mines, which can be characterized as extremely disturbed sites, have serious environmental impacts associated with them, including contamination of soil and water by heavy metals. These sites are considered to be the worst for plant growth because of low nutrients, presence of toxic materials, and high temperatures. Under such conditions, however, it has been shown that mycorrhizal symbioses can help with plant establishment, growth, and nutrition [11, 12]. Marx et al. [13] demonstrated that inoculation of pine seedlings with ECM fungi improved seedling growth and establishment. One ECM fungal species, Pisolithus tinctorius, has been widely used for revegetation of abandoned mines because of its high fitness in field trials, broad host specificity, and the potential of large-scale production as an inoculum [14]. However, high levels of disturbance including accumulation of pollutant could reduce the survival of ECM fungi on their host plants, even if the plants were colonized with suitable ECM fungi. Improving soil conditions through soil ameliorators could increase the growth and survival of ECM fungi in coal mine spoils and be useful for effective restoration. In this study, the effects of soil ameliorators on ECM fungi communities in coal mine spoils were investigated.
Materials and Methods
This study was conducted at 3 sites located in Gangwon Province, Central-eastern Korea: Dogye (N 37°15', E 129°02'), Mt. Hambaek (N 37°09', E 128°56'), and Dumundongjae (N 37°12', E 128°53'). Coal mining debris was piled into terraces to ensure physical stability and was covered with forest soil and plants, according to the typical restoration procedures of coal mine spoils in Korea. At these sites, most forest soils disappeared because of erosion, and it was difficult to locate the replanted plants because most appeared to have died. Soil conditions were acidic, but pine seedlings and several grasses grew naturally at the research sites.
Organic fertilizers and slaked lime were used as soil ameliorators and evaluated on their ability to improve the soil condition. Six treatment plots (3 m × 3 m) in each site were established: control, 10 kg of organic fertilizers (OF10), 20 kg of organic fertilizers (OF20), 2.5 kg of slaked lime (LM2.5), 5 kg of slaked lime (LM5), and both organic fertilizers and slaked lime, 10 kg and 2.5 kg, respectively (OF10 + LM2.5).
One year after the initial treatment, 3 pine seedlings were randomly collected from each plot and transferred to a laboratory. Plant roots were washed with distilled water, and the ECM root tips were observed under a microscope. Morphotypes of the ECM root tips were classified according to their physical characteristics [15], and colonization rates and species diversity of ECM fungi were estimated. To identify ECM species at the molecular level, representatives of each morphotype were selected for DNA extraction and sequence analysis. Genomic DNA was extracted from each ECM root tip by using the DNeasy plant mini kit (Qiagen Science, Valencia, CA, USA). The partial internal transcribed spacer (ITS) of rDNA was amplified using ITS1F (5'-CTTGGTCATTTAGAGGAAGTAA-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3'), a fungal specific primer pair [16]. Thermocycling for PCR was conducted as follows: 94℃ for 3 min, 94℃ for 30 sec, 50℃ for 30 sec, and 72℃ for 1 min, for 30 cycles, followed by 72℃ for 5 min. The PCR products were identified using electrophoresis with 1% agarose gel that was run for 20 min in 1 × TAE buffer and visualized using a UV illuminator after ethidium bromide staining. The amplified products were then sequenced (Solgent Co. Ltd., Deajeon, Korea). DNA sequences were screened using the basic local alignment search tool algorithm made available by the National Center for Biotechnology Information to confirm that the appropriate gene was sequenced. All sequences were aligned using the software MEGA 5.0 [17], and a phylogenetic tree was constructed using the neighbor-joining method [18], with Rhizophus stolonifer selected as an outgroup.
The ECM colonization rate, number of ECM fungal species, and Shannon-Wiener diversity index (H) were calculated to compare the effects of soil ameliorators on each plot. Statistical comparisons among plots were conducted using one-way analysis of variance (ANOVA) with the statistical program SPSS ver. 18 (SPSS Inc., Chicago, IL, USA).
Results and Discussion
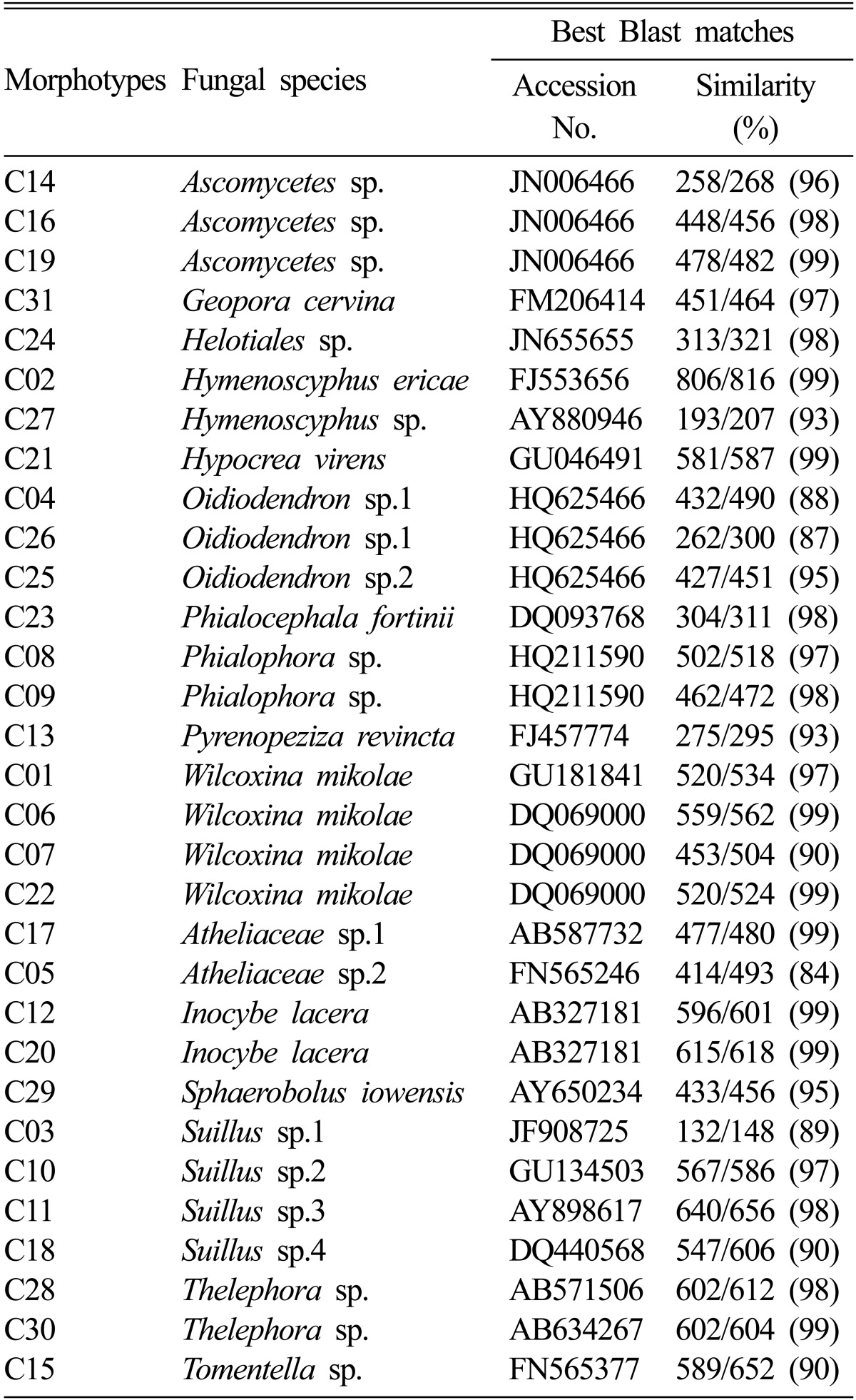
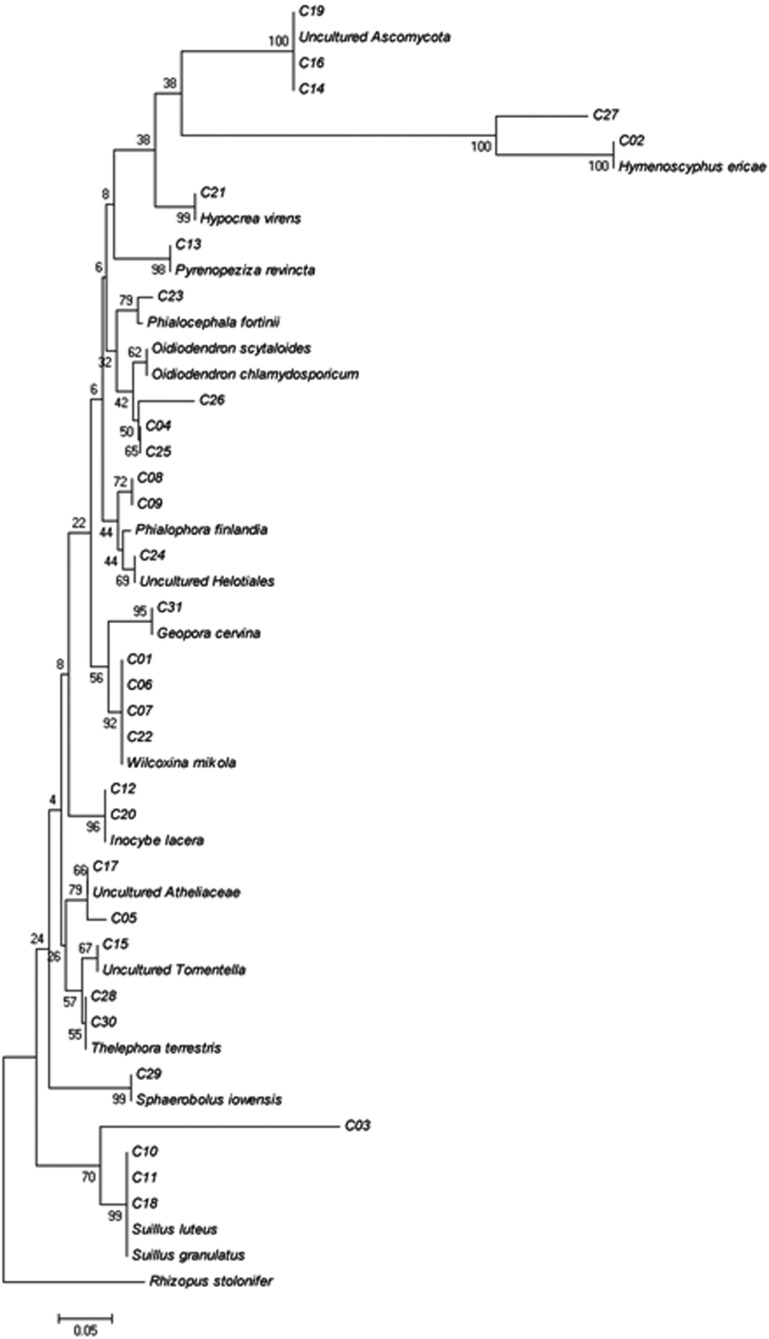
The ECM fungi were classified into 31 morphotypes (C01~C31) based on their shape and branching system. A representative of each morphotype was used for PCR and DNA sequencing to identify ECM fungal species at the molecular level. A total 22 ECM fungal species was identified. Ten and twelve species of ECM fungi belonged to Basidiomycetes and Ascomycetes, respectively (Table 1, Fig. 1).
Table 1.
The Morphological and molecular identification of the ectomycorrhizal fungi colonizing roots of pine seedlings in coal mine spoils
Fig. 1.
Neighbor-joining tree to illustrate the taxonomical topology of the ectomycorrhizal fungi in the present study. Rhizophus stolonifer was used as an outgroup.
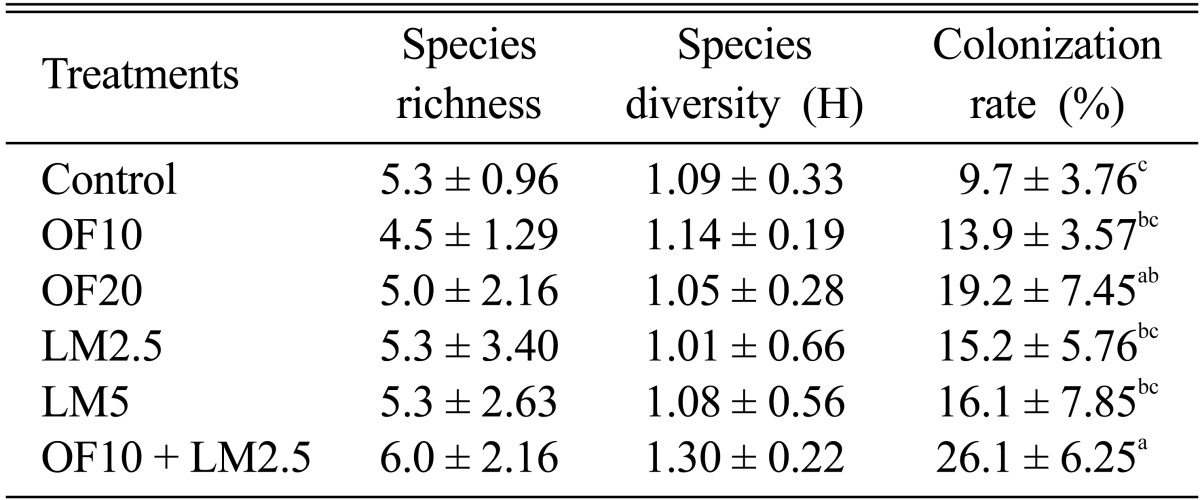
In this study, organic fertilizers and slaked lime were used as soil ameliorators to offset the low pH and lack of organic matter at the coal mine spoils. One year after the treatment, the number of ECM species, colonization rate, and species diversity at each of the six sites were compared (Table 2). In the coal mine spoils, the organic fertilizers and slaked lime did not affect the number of ECM fungal species or species diversity. However, ECM colonization rate was significantly increased in the plots treated organic fertilizers and varied among the treatments, with the highest rate in the plot treated with both organic fertilizers and slaked lime.
Table 2.
Effects of treatments of soil ameliorators on species richness, diveristy and root colonization rates of ectomycorrhizal fungi colonizing roots of Pinus densiflora in the coal mine spoils
Different letters on each column indicate significant difference between treatments according to least signifiant difference (LSD) at p < 0.05.
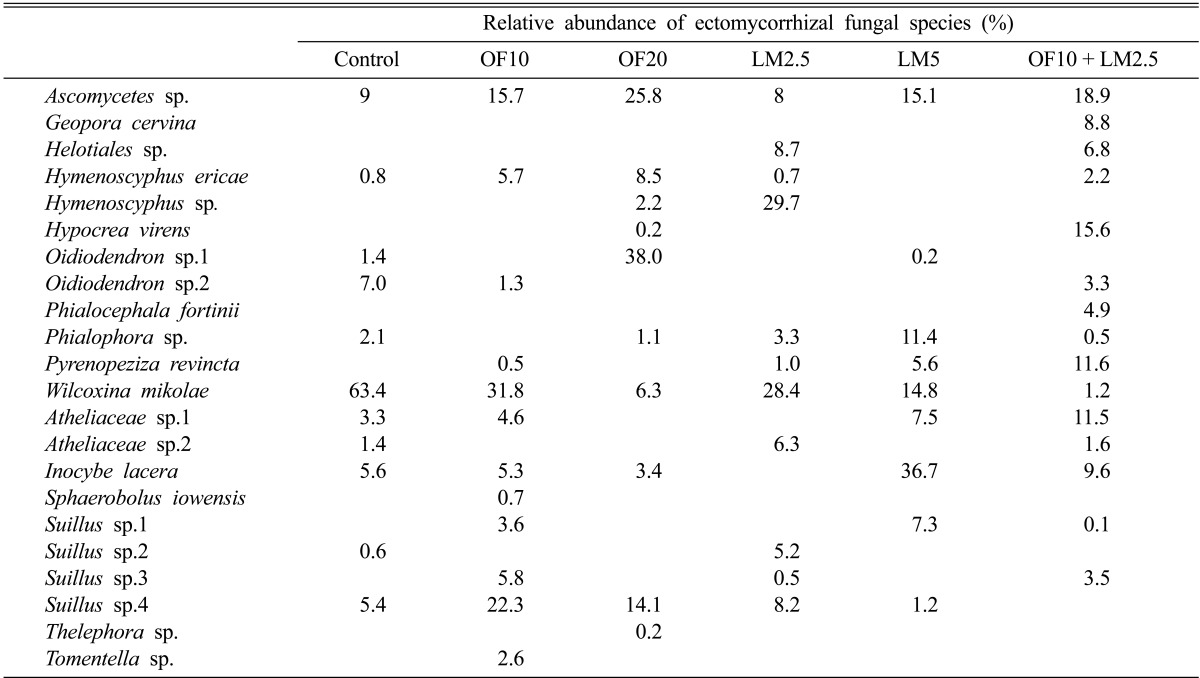
Relative abundances of ECM fungal species in each treatment are shown in Table 3. The frequency of Ascomycetes species was increased to a great extent after treatment with organic fertilizers, and notably, the colonization rate of Oidiodendron sp.1 increased remarkably after the organic fertilizer treatment. Wilcoxina mikolae was the dominant ECM species in the control plot, comprising about 63% of the relative abundance of fungal species. However, the relative abundance of W. mikolae differed across treatments (Table 3), and the colonization rate was decreased dramatically after the application of not only organic fertilizers but also slaked lime.
Table 3.
Relative abundance of ectomycorrhizal fungi in pine seedlings from coal mine spoils
Most of the Basidiomycetes identified in the coal mine spoils belonged to the genera Inocybe and Suillus, which are known as early-successional species of disturbed sites [19]. Alternative explanations could be that Inocybe and Suillus fungi are resistant to the environmental stressors found at coal mine spoils or that they are ECM fungal species specific to the pine seedlings because of symbiosis during early succession. Regardless, it appears that the extreme sterility of the soil in coal mine spoils, much like that in early-successional forests, reduced the survival and colonization of other ECM fungi, resulting in Inocybe spp. and Suillus spp. being the most abundant fungi.
This study showed that the use of slaked lime and organic fertilizers as soil ameliorators could increase the colonization rates of ECM fungi in coal mine spoils where ECM formation was previously restricted. The high ECM colonization rate in plots treated with soil ameliorators implies that ECM fungi could help improve plant growth through symbiosis with roots. Therefore, for efficient restoration of coal mine spoils, soil ameliorators like limes and organic fertilizers should be applied to recover soil conditions with plants colonized with ECM fungi. These results could be used effectively for ecological restoration by improving the growth of plants in sterile, contaminated soil. In addition, the sequential and advanced approach of using plants colonized with ECM fungi could decrease the cost and effort required to restore coal mine spoils. Additional study on ECM fungal colonization in coal mines is needed to achieve efficient revegetation of coal mine spoils
Acknowledgements
This work was conducted during a research year supported by Korea National University of Education in 2010.
References
- 1.Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd ed. London: Academic Press; 2008. [Google Scholar]
- 2.Barker SJ, Tagu D, Delp G. Regulation of root and fungal morphogenesis in mycorrhizal symbioses. Plant Physiol. 1998;116:1201–1207. doi: 10.1104/pp.116.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adriaensen K, van der Lelie D, van Laere A, Vangronsveld J, Colpaert JV. A zinc-adapted fungus protects pines from zinc stress. New Phytol. 2004;161:549–555. doi: 10.1046/j.1469-8137.2003.00941.x. [DOI] [PubMed] [Google Scholar]
- 4.Adriaensen K, Vralstad T, Noben JP, Vangronsveld J, Colpaert JV. Copper-adapted Suillus luteus, a symbiotic solution for pines colonizing Cu mine spoils. Appl Environ Microbiol. 2005;71:7279–7284. doi: 10.1128/AEM.71.11.7279-7284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molina R, Massicotte H, Trappe JM. Specificity phenomena in mycorrhizal symbioses:community-ecological consequences and practical implications. In: Allen MF, editor. Mycorrhizal functioning: an integrative plant-fungal process. New York: Chapman & Hall; 1992. pp. 357–423. [Google Scholar]
- 6.Gehring CA, Theimer TC, Whitham TG, Keim P. Ectomycorrhizal fungal community structure of pinyon pines growing in two environmental extremes. Ecology. 1998;79:1562–1572. [Google Scholar]
- 7.Goodman DM, Trofymow JA. Comparison of communities of ectomycorrhizal fungi in old-growth and mature stands of Douglas-fir at two sites on southern vancouver Island. Can J For Res. 1998;28:574–581. [Google Scholar]
- 8.Amaranthus MP, Perry DA. Effect of soil transfer on ectomycorrhiza formation and survival and growth of conifer seedlings on old, nonreforested ckear-cuts. Can J For Res. 1987;17:944–950. [Google Scholar]
- 9.Parke JL, Linderman RG, Trappe JM. Inoculum potential of ectomycorrhizal fungi in forest soils of southwest Oregon and northern California. For Sci. 1984;30:300–304. [Google Scholar]
- 10.Perry DA, Meyer MM, Egeland D, Rose SL, Pilz D. Seedling growth and mycorrhizal formation in clearcut and adjacent, undisturbed soils in montana: a green-house bioassay. For Ecol Manag. 1982;4:261–273. [Google Scholar]
- 11.Pfleger FL, Stewart EL, Noyd RK. Role of VAM fungi in mine-land revegetation. In: Pfleger FL, Linderman RG, editors. Mycorrhizae and plant health. St. Paul: APS Press; 1994. pp. 47–81. [Google Scholar]
- 12.Malajczak N, Riddell P, Brundett M. Role of ectomycorrhizal fungi in minesite reclamation. In: Pfleger FL, Linderman RG, editors. Mycorrhizae and plant health. St. Paul: APS Press; 1994. pp. 83–100. [Google Scholar]
- 13.Marx DH, Ruehle JL, Kenney DS, Cordell CE, Riffle JW, Molina RJ, Pawuk WH, Navratil S, Tinus RW, Goodwin OC. Commercial vegetative inoculum of Pisolithus tinctorius and inoculation techniques for development of ectomycorrhizae on container-grown tree seedlings. For Sci. 1982;28:373–400. [Google Scholar]
- 14.Ruehle JL, Marx DH. Fiber, food, fuel, and fungal symbionts. Science. 1979;206:419–422. doi: 10.1126/science.206.4417.419. [DOI] [PubMed] [Google Scholar]
- 15.Goodman DM, Durall DM, Trofymow JA, Berch SM. A manucal of concise descriptions of North American Ectomycorrhizae: including microscopic and molecular characterization. Victoria: Mycologue Publications; 1996-2000. [Google Scholar]
- 16.Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 17.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 19.Colpaert JV, Van Laere A, Van Assche JA. Carbon and nitrogen allocation in ectomycorrhizal and non-mycorrhizal Pinus sylvestris L. seedlings. Tree Physiol. 1996;16:787–793. doi: 10.1093/treephys/16.9.787. [DOI] [PubMed] [Google Scholar]