Abstract
Aspergillus fumigatus is an increasingly serious pathogen of immunocompromised patients, causing the often fatal disease invasive aspergillosis (IA). One A. fumigatus virulence determinant of IA is LaeA, a conserved virulence factor in pathogenic fungi. To further understand the role of LaeA in IA, the expression profile of laeA was compared to wild type, and several transcription factors were found significantly misregulated by LaeA loss. One of the transcription factors up-regulated over 4 fold in the laeA strain was Afu4g09710, similar in sequence to A. nidulans NosA, which is involved in sexual development. Here we assessed loss of nosA ( nosA) and over expression of nosA (OE::nosA) on A. fumigatus in both a wild type and laeA background. Based on the multiple alterations of physiological development of single and double mutants, we suggest that NosA mediates the decreased radial growth and delayed conidial germination observed in laeA strains, the former in a light dependent manner. The ΔnosA mutant showed increased virulence in the Galleria mellonella larvae model of disseminated aspergillosis, potentially due to its increased growth and germination rate. Furthermore, the A. fumigatus nosA allele was able to partially remediate sexual development in an A. nidulans ΔnosA background. Likewise, the A. nidulans nosA allele was able to restore the menadione sensitivity defect of the A. fumigatus ΔnosA strain, suggesting conservation of function of the NosA protein in these two species.
Keywords: LaeA, NosA, virulence, germination, vegetative growth, menadione, reactive oxygen species
1. Introduction
Aspergillus fumigatus is an increasingly serious pathogen which causes the majority of filamentous fungal infections of immunocompromised patients (reviewed in Willger et al., 2009). Unfortunately, the mechanisms governing the infection process of A. fumigatus are largely unknown. It is therefore of considerable interest to identify which fungal genes are responsible for successful colonization of the host, so to provide potential therapeutic targets.
A 2004 study identified a gene, laeA, involved in regulating numerous secondary metabolites in several species of Aspergillus, including A. fumigatus (Bok et al., 2004). Additional research demonstrated the A. fumigatus laeA deletion mutant (ΔlaeA) to be compromised in infection of a murine model of invasive aspergillosis (Bok et al., 2005, Sugui et al. 2007, Ben-Ami et al. 2009). Several phenotypes of the laeA mutant were suggested to be responsible for this decreased virulence, including alterations of the cell surface which led to increased uptake by pulmonary macrophages in an in vitro assay (Bok et al., 2005; Dagenais et al., 2010), decreases in iron uptake and storage (Perrin et al., 2007), and decreases in secondary metabolite production (Bok et al., 2005, Perrin et al., 2007, Sugui et al. 2007, Ben-Ami et al. 2009). The variety of phenotypes described above were supported by microarray analysis of the ΔlaeA mutant, where transcription of approximately 10% of the genome was altered (Perrin et al., 2007), 25% of which was also correlated with induction during murine infection (McDonagh et al., 2008).
Among those genes with significantly altered expression in the ΔlaeA mutant were dozens of transcription factors (Perrin et al., 2007). Modulation of the transcriptional program of ΔlaeA is likely a major contributor to its pleiotropic effects on fungal development and virulence (reviewed in Yin and Keller, 2011; Bok et al., 2006). One of the transcription factors found to be upregulated 4.4 fold in the ΔlaeA mutant was Afu4g09710, a homolog to A. nidulans NosA, which has previously been characterized in its requirement for normal sexual development in that species (Vienken and Fischer, 2006).
NosA (number of sexual spores) in the homothallic A. nidulans was originally identified as a homolog of the Sodaria macrospora developmental regulator Pro1 (Vienken and Fischer, 2006). Deletion of this gene was shown to impair development of sexual primordia, drastically reducing the number of sexual spores produced when self-mating. This deletant also showed greatly reduced expression of several genes associated with sexual development, including the glucose transporter hxtA and the catalase peroxidase cpeA (Vienken and Fischer, 2006). Little else is known about this protein in any other fungus.
Here we examined the impact of nosA deletion and overexpression in A. fumigatus as well as its ability to complement an A. nidulans ΔnosA strain. We show that heterologous complementation with A. fumigatus nosA is able to partially restore A. nidulans sexual sporulation defects, suggesting a conservation of function between these two species. In A. fumigatus loss of nosA increased vegetative growth and germination rates. This latter property was observed even in a ΔlaeA background where nosA loss restored germination of ΔlaeA to wild type levels. The OE::nosA strain mimicked the light dependent slow growth of ΔlaeA and further decreased growth and delayed germination was observed in the double ΔlaeA OE::nosA mutant. Together, this work suggests that several growth and developmental aberrancies found in ΔlaeA are mediated by NosA. Finally the ΔnosA mutant yields a more virulent strain than wild type in the Galleria model of IA.
2. Material and methods
2.1 Fungal strains, plasmids, and growth conditions
Strains and plasmids used in this study are described in Table 1. All the strains were cultured at 37 °C on glucose minimal medium (GMM) (Shimizu and Keller, 2001) unless otherwise indicated. Where necessary, 5 mM uridine and 5 mM uracil was used to supplement auxotrophs. Phylogenetic tree was constructed using the ClustalW module of MegAlign software (DNAStar Version 9) using cDNA sequences from the Aspergillus Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/).
Table 1.
Aspergillus fumigatus strains and plasmids used in this study
| Strain name | Genotype | Derivative of | Source |
|---|---|---|---|
| CEA17 KU80 pyrG- | ΔnkuB; pyrG- | A. fumigatus CEA17 | da Silva Ferreira et al., 2006 |
| TFYL19.3 | ΔnkuB; ΔlaeA::hph; pyrG- | A. fumigatus CEA17 | F.Y. Lim and N.P. Keller |
| CEA17 KU80 | ΔnkuB | A. fumigatus CEA17 | da Silva Ferreira et al., 2006 |
| TFYL20.10 | ΔnkuB; ΔlaeA::hph; A parasiticus pyrG | A. fumigatus CEA17 | F.Y. Lim and N.P. Keller |
| TAAS104 | ΔnkuB; ΔnosA::A parasiticus pyrG | A. fumigatus CEA17 | This study |
| TAAS105 | ΔnkuB; nosA(p)::A. parasiticus pyrG::A. nidulans gpdA(p)::nosA | A. fumigatus CEA17 | This study |
| TAAS107 | ΔnkuB; ΔlaeA::hph; ΔnosA::A parasiticus pyrG | A. fumigatus CEA17 | This study |
| TAAS108 | ΔnkuB; ΔlaeA::hph; nosA(p)::A. parasiticus pyrG::A. nidulans gpdA(p)::nosA | A. fumigatus CEA17 | This study |
| TAAS155.3 | ΔnkuB; ΔnosA::A parasiticus pyrG + pAAS155 | A. fumigatus TAAS104 | This study |
| TAAS156.5 | ΔnkuB; ΔnosA::A parasiticus pyrG + pAAS156 | A. fumigatus TAAS104 | This study |
| RJMP1.1 | pyrG89; pyroA4; riboB2; ΔnkuA::argB; veA1 | A. nidulans | J. Palmer and N.P. Keller |
| RJMP1.59 | pyrG89; pyroA4 | A. nidulans | Shaaban et al., 2010 |
| RJMP101.19 | pyrG89 | A. nidulans | J. Palmer and N.P. Keller |
| TAAS157.13 | ΔnosA::A parasiticus pyrG; pyrG89; pyroA4; riboB2; ΔnkuA::argB; veA1 | A. nidulans RJMP1.1 | This study |
| TAAS149.4 | pyroA::pAAS149::pyroA4; pyrG89 | A. nidulans RJMP1.59 | This study |
| TAAS150.11 | pyroA::pAAS150::pyroA4; pyrG90 | A. nidulans RJMP1.59 | This study |
| RAAS160.4 | ΔnosA::A parasiticus pyrG | TAAS157.13 x RJMP101.19 | This study |
| RAAS158.6 | ΔnosA::A parasiticus pyrG; pyroA::pAAS149::pyroA4 | TAAS157.13 x RTAAS149.4 | This study |
| RAAS159.8 | ΔnosA::A parasiticus pyrG; pyroA::pAAS150::pyroA4 | TAAS157.13 x RTAAS150.11 | This study |
| RAAS161.7 | pyroA::pAAS149::pyroA4 | TAAS149.4 x RJMP101.19 | This study |
| RAAS162.2 | pyroA::pAAS150::pyroA4 | TAAS150.11 x RJMP101.19 | This study |
| RJMP103.5 | WT | A. nidulans | Soukup et al., 2012 |
| pJW24 | A. parasitcus pyrG in pBluescript | pBluescript | Calvo et al., 2004 |
| pJMP9.1 | A. parasitcus pyrG – A. nidulans gpdA in pBluescript | pBluescript | J. Palmer and N.P. Keller |
| pUCH2.8 | hygR in pBluescript | pBluescript | Alexander et al., 1998 |
| pJW53 | 3/4 A. nidulans pyroA in pBluescript | pBluescript | Shaaban et al., 2010 |
| pAAS149 | A. fumigatus nosA and flanks in pJW53 | pJW53 | This study |
| pAAS150 | A. nidulans nosA and flanks in pJW53 | pJW53 | This study |
| pAAS155 | hygR in pAAS149 | pAAS149 | This study |
| pAAS156 | hygR in pAAS150 | pAAS150 | This study |
2.2 Construction of A. nidulans nosA mutants
Genomic DNA sequence of A. nidulans FGSCA4 AN1848 (A.n. nosA) gene was obtained from the Aspergillus Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/). Constructs to delete and overexpress nosA were created using double joint PCR (Yu et al., 2004). Primers used in this study are listed in Table 2. For the deletion cassette, 1 kb flanking regions of the nosA gene of A. nidulans were amplified using primers An nosA 5′ flank for, An nosA 5′ flank rev pyrG, An nosA 3′ for pyrG, and An nosA 3′ rev and fused to the pyrG gene of A. parasiticus from pJW24, which was previously amplified by PCR using A.p. pyrG For and A.p. pyrG Rev. The fusion PCR deletion construct was amplified with An nosA 5′ flank for and An nosA 3′ rev.
The resulting product was used to transform the auxotrophic A. nidulans strain TJMP1.1 (Table 1) to create nosA (AN1848) knockout (ΔnosA) strain TAAS157.13. Standard DNA transformation protocol was used as described previously (Szewczyk et al., 2006). Transformants were confirmed by PCR screening using An nosA 5′ flank ext and Ap pyrG int 3′, followed by Southern analysis (Sanbrook and Russell, 2001).
In order to construct complementation strains, A. fumigatus nosA (A.f. nosA) and A. nidulans nosA (A.n. nosA) genes with 1 kb flanking regions were cloned into pJW53 using NotI to create pAAS149 and pAAS150, respectively. pJW53 contains a ¾ length fragment of the pyroA gene, allowing for targeted integration of the plasmid at the pyroA locus. Strains TAAS149.4 (+ A.f. nosA) and TAAS150.11 (+A.n. nosA) were confirmed by Southern blot (Fig. S1). These strains were then crossed with either TAAS157.13 (ΔnosA) or RJMP101.19 to obtain the prototrophic strains RAAS158.6 (ΔnosA + A.f. nosA), RAAS159.8 (ΔnosA + A.n. nosA), RAAS160.4 (ΔnosA), RAAS161.7 (+ A.f. nosA), and RAAS162.2 (+ A.n. nosA).
2.3 A. fumigatus nosA mutant construction
Genomic DNA sequence of A. fumigatus AF293 Afu4g09710 (nosA) gene was obtained from the Aspergillus Comparative Sequencing Project, Broad Institute of Harvard and MIT (http://www.broadinstitute.org/). To investigate the role of nosA in A. fumigatus physiology, constructs to delete and overexpress nosA were created using double joint PCR (Yu et al., 2004). Primers used in this study are listed in Table 2. For the deletion cassette, 1.0 kb flanking regions of the nosA gene of A. fumigatus were amplified using primers NosA 5′ flank for, NosA 5′ flank Rev, NosA 3′ for, and NosA 3′ rev and fused to the pyrG gene of A. parasiticus from pJW24, which was previously amplified by PCR using A.p. pyrG For and A.p. pyrG Rev. The fusion PCR deletion construct was amplified with NosA 5′ flank for and NosA 3′ rev. In order to construct an over-expression construct for nosA, an A. parasticus pyrG – A. nidulans gpdA promoter fusion from pJMP9.1 was amplified using primers pyrG inv for pJMP9 and gpdA(p) 3′ and linked to the open reading frame (ORF) and 5′ flanking region of nosA, previously amplified using NosA 5′ flank for, NosA 5′ flank rev for pyrG & gpdA, NosA OE 3′ flank for and NosA 3′ for pyrG & gpdA. The final overexpression cassette was amplified with NosA 5′ flank for and NosA 3′ for pyrG & gpdA.
The resulting products were used to transform the auxotrophic A. fumigatus strains CEA17 KU80 pyrG- and TFYL19.3 (Table 1) to create nosA (AFUB_066820) knockout (ΔnosA) strains TAAS104 and TAAS107 and over-expression (OE::nosA) strains TAAS105 and TAAS108. These CEA10 derivatives were used as they lacked the KU80 gene thus allowing for higher probability of homologous recombination. Standard DNA transformation protocol was used as described previously (Szewczyk et al., 2006). Transformants were confirmed by PCR screening using either Af nosA 5′ flank ext and Ap pyrG int 3′ (KO) or Af nosA 3′ int 2 and gpdA(p) int 5′ (OE), followed by Southern and northern analysis (Sanbrook and Russell, 2001). Transcript intensity was quantified using ImageJ software. In order to create complementation plasmids for the prototrophic A. fumigatus strains, hph from pUCH2.8 was subsequently subcloned into pAAS149 and pAAS150 using BamHI and KpnI to create pAAS155 and pAAS156, respectively. These were transformed into TAAS104 to create strains TAAS155.3 and TAAS156.5, respectively.
2.3 Physiology tests
Conidiation and radial growth measurement tests were performed for all the strains by standard methods described previously (Tsitsigiannis et al., 2004), with either constant illumination with white light (light), or constant darkness (dark). For conidial counts, 5 x 106 conidia of designated strains were mixed in 5 mL molten GMM medium at a concentration of 0.7% agar and then overlaid on 25 mL, 1.5% agar GMM medium. Conidia from three cores (1 cm, diameter) for each plate were counted after 6 days incubation at 37 °C. Colony diameter was measured of point inoculated colonies on GMM medium every 24 h for three days 37 °C. Both assays were performed with four replicates.
For northern analysis under carbon starvation conditions, 4 replicate cultures of each strain were inoculated at 1 x 106 spores/mL in 25 mL of GMM. After 20 hours of shaking incubation at 37°C under constant light, cultures were collected and transferred to either fresh GMM, or MM (GMM – glucose) and cultured for an additional 5 hours. Mycelia were harvested, lyophilized overnight, and total RNA was extracted using Isol-RNA Lysis Reagent (5 Prime) according to manufacturer’s recommendations.
Germination was assessed in GMM liquid medium containing 0.1 % yeast extract at 37°C with a total number of 106 conidia/mL for each strain at the stationary phase (Dagenais et al, 2008). The rate germination was measured at 4, 5, 6, 7, and 8 hours by microscope examination. Spores were considered germinated when the germ tube was equal in diameter to the conidia. A total number of 100 spores were examined for each strain, time point, and replicate, with four replicates performed.
Hyphal resistance to ROS producers was tested by spotting 5 μl of a 106 conidia/mL suspension onto GMM plates containing 40 μM menadione or 4 mM H2O2. Colony diameter was observed after 72 hours in constant light at 37° C.
2.4 Sexual analysis of A. nidulans strains
Quantification was performed on overlay inoculated cultures set up by pipetting 1 x 106 conidia into CHAMPS medium with 0.75% molten agar that was subsequently poured over 1.5% solid agar petri dishes. Cultures were incubated at 37ºC in the dark for 7 days and imaged at 40X resolution. Agar cores were taken from the plates with a 1 cm cork borer. After homogenization, ascospores were quantified using a hemacytometer and represented as ascospores per square millimeter. Four replicates were performed for each strain and condition.
2.5 Virulence test using an insect model (Galleria mellonella)
Larvae of the Greater Wax Moth, G. mellonella (Northern Wax Worm Company; 250–300 mg) were used within 15 days of delivery. Larvae were inoculated with 1×106 conidia in 2.5 μl PBS through the last left pro-leg into the haemocoel (Fallon et al., 2011). Larvae were kept in petri dishes filled with wood shavings and incubated at 37ºC under dark condition. Mortality rate was recorded daily and confirmed by observing lack of movement, dehydration, and severe melanization of larvae. A solvent (PBS) control was used in all experiments. Three independent experimental replicates were performed using 30 larvae/strain.
2.6 Macrophage assay
2.6.1 Macrophage purification from human blood
Macrophage purification from human blood: Human primary macrophages were purified from human blood after informed consent in Dr. Anna Huttenlocher’s lab (University of Wisconsin – Madison); the human subject protocol was approved by the University of Wisconsin Center for Health Sciences Human Subjects Committee. 30 mL of whole blood was diluted in an equal amount of PBS (phosphate buffered saline). 10 mL of lymphoprep density gradient media (Axis-shield, Norway) was placed in 50 mL conical tubes and 20 mL of diluted blood was gently layered on the top of liquid. The suspension was centrifuged at 600 RCF for 30 minutes. The buffer coat layer was collected and immediately diluted into 50 mL conical tubes containing PBS in a ratio of no more than 10 mL of sample to 40 mL of PBS. The conical tubes were centrifuged for 3 min at 600 RCF and the cells were washed once more with 15 mL PBS and two times with 15 mL of macrophage culture media (RPMI 1640 [Invitrogen/Gibco®; Cat. No. 31870-025], 10% fetal calf serum (FCS), 100 μg/mL streptomycin, 100 U/mL penicillin, 2mM glutamine, 1% Na-pyruvate, 1% NEAA (Non-Essential Amino Acids). The washed cells were re-suspended in 15 mL culture media and placed in 10 cm diameter cell culture treated petri dishes (NUNC, Rochester NY). After 4–8 hours the non-adherent cells were aspirated and 15 mL of fresh culture media was added containing 10 μL of rh-MCSF (recombinant human macrophage colony stimulating factor) for a final concentration of 50 ng/mL. The treated petri dishes were incubated in 5% CO2 incubator at 37° C for 6–7 days. This process was repeated on days 2 and 4 (Starnes et al. 2011).
2.6.2 Macrophage harvesting and preparation
The adherent macrophages were washed with 10 mL PBS and 5 mL of trypsin-EDTA was subsequently added and incubated for 5 min at 37°C in order to lift off the cells. When all the cells were detached, trypsin was diluted in 10 mL of culture media, the cell density was measured and cell suspensions were centrifuged at 600 RCF for 3 min. The cells were re-suspended to 0.2 million cells per mL and 500 uL of cell suspension was placed in each well of a 24 well-plate for the final assay.
2.6.3 Macrophage assay for fungal spores
After allowing the macrophages to adhere for 2 hours in the incubator, the culture media was aspirated and 500 μL of fungal spore suspension was added to each well. To obtain the spore suspension, the spores were harvested in distilled water without tween, and diluted down to 1 million spores per mL in serum-free RPMI media. The spore suspension density was chosen in order to present 5 spores per macrophage. The macrophages were left for 1 hour in the incubator at 37°C in 5% CO2 to perform the phagocytosis stage of the assay. Subsequently, the media in each well was collected in an eppendorf tube, and the well was washed thoroughly twice with PBS, which was added to the collected media. For the spore survival stage of the assay, the macrophages were then incubated in an incubator at 37° C with 5% CO2 for 5 hours in 500 μl of culture media containing FBS 10% (Fetal Bovine Serum). Finally, the cell media containing FBS was collected in an eppendorf tube and the macrophages were lysed in 500 μL of lysis buffer (DI water +1% Tween 20) for 10 min in an incubator. Lysis buffer was collected into the respective tubes and the wells were washed once with 500 μL PBS, which was similarly collected. The collected suspensions at the different time points (1 and 5 hours) were diluted at a 1:20 ratio and 20 μL of the solution was plated on two plates of solid GMM medium and grown at room temperature for 4 days in order to quantify the number of colonies originated from live single spores.
2.7 Secondary metabolite analysis
Secondary metabolite production was assessed by thin-layer chromatography (TLC). For TLC, 1 × 106 spore/mL was inoculated into was point-inoculated on the center of glucose minimal medium (GMM) and cultured for 5 days at 37°C under constant light. An agar plug of the center of colonies was removed and metabolites extracted with chloroform according to the Smedsgaard’s method (Smedsgaard, 1997). Extracts (10 μl/sample) were loaded onto silica TLC plates (Whatman, PE SIL G/UV, Maidstone, Kent, England) and metabolites were separated in the developing solvent toluene: ethyl acetate: formic acid (TEF, 5:4:1). Images were taken following exposure to UV radiation at 254 and 366 nm.
2.8 Statistical analysis
Data were analyzed using Graphpad Prism software (La Jolla, CA) according to the Tukey Multiple Comparison test with a p value < 0.05.
3 Results
3.1 A. fumigatus Afu4g09710 encodes a homolog of A. nidulans NosA
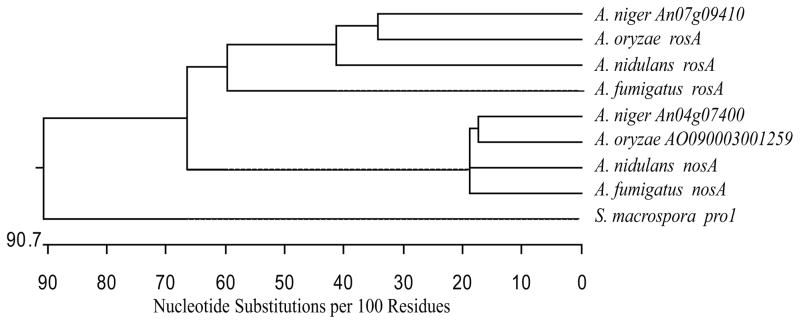
Microarray analysis of A. fumigatus AF293 laeA versus wild type strains under liquid stationary culture for 60 hours at 25°C showed the putative transcription factor Afu4g09710 to be 4.4 fold upregulated in the laeA strain (Perrin et al., 2007). BLAST searches demonstrated this protein to be a homolog of A. nidulans NosA (Vienken and Fischer, 2006), with 71% amino acid identity, and 47.4% identity to the Sordaria macrospora developmental regulator Pro1 (Masloff et al., 1999). As A. nidulans has previously been shown to encode two Pro1 paralogs with divergent functions (NosA and RosA; Vienken et al., 2005; Vienken and Fischer, 2006), ClustalW analysis of Aspergillus pro1 paralog cDNAs was performed. As shown in Figure 1, phylogenetic analysis suggests that this pro1 duplication is common to the Aspergilli, with nosA and rosA homologs each belonging to separate clades (also see Vienken and Fischer, 2006). This suggests that the function of these respective genes may be conserved within this genus.
Fig. 1.
Phylogenetic tree depicting relatedness of nosA homologs. ClustalW analysis of cDNA sequences of Aspergillus nosA homologs and the related S. macrospora pro1 shows that within the Aspergilli, all nosA sequences group within a single clade, distinct from the rosA homologs.
In A. nidulans, deletion of nosA results in several characteristic phenotypes, including inability for sexual primordia to develop, as well as defects under carbon starvation conditions in transcription of two genes associated with sexual development, hxtA and cpeA (Vienken and Fischer, 2006). We therefore sought to determine if A. fumigatus nosA could restore these defects through a heterologous complementation assay. Six strains, wild type, RAAS158.6 (ΔnosA + A.f. nosA), RAAS159.8 (ΔnosA + A.n. nosA), RAAS160.4 (ΔnosA), RAAS161.7 (+ A.f. nosA), and RAAS162.2 (+ A.n. nosA) were assessed for completion of sexual development.
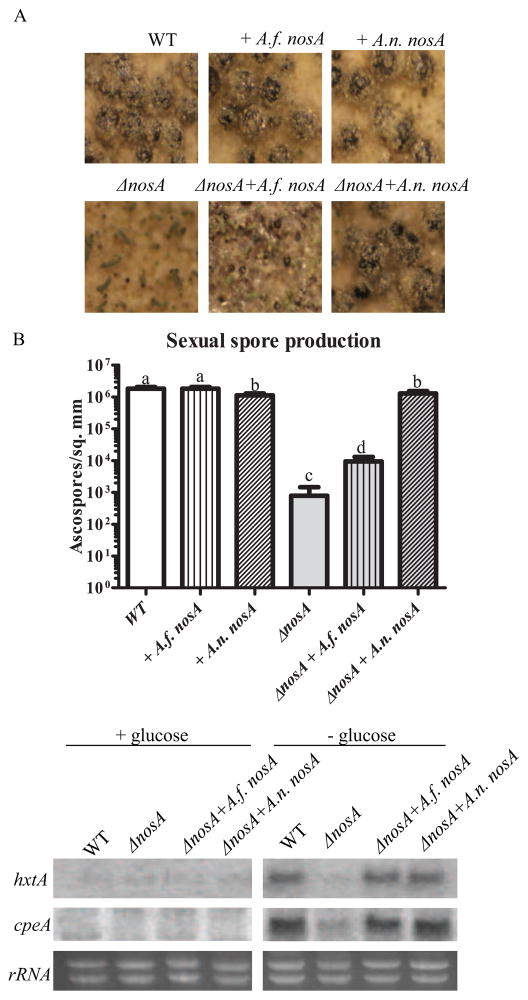
Analysis of fruiting body development showed the expected defect in the ΔnosA strain (Vienken and Fischer, 2006), yielding underdeveloped cleistothecia which appear to be arrested at the primordial stage (Fig. 2A). Addition of an ectopic copy of A.n. nosA is able to increase cleistothecia diameter to near wild type levels, as well as restore normal pigmentation. A.f. nosA addition also increases both size and pigmentation of cleistothecia in a ΔnosA background, but is unable to approach wild type levels of either. Addition of ectopic copies in a wild type background did not drastically alter physical development.
Fig. 2.
A.f. nosA can partially complement A. nidulans ΔnosA developmental defects. A. A. nidulans ΔnosA is defective in development, showing an arrest at the primordial stage of cleistothecial development (Vienken and Fischer, 2006). Introduction of an ectopic copy (ΔnosA + A.n. nosA) of wild type nosA is able to complement this defect. Heterologous complementation with A.f. nosA, in contrast, is only able to partially restore development, as seen in the increased size of cleistothecia/primordia. B. Analysis of completion of the sexual cycle by determination of ascospores produced. ΔnosA strains are severely impaired in development, producing ~1000 fold fewer ascospores than wild type (Vienken and Fischer, 2006). Introduction of an ectopic copy of A.n. nosA is able to nearly fully restore sexual spore production. Inclusion of A.f. nosA increases sexual spore production ~10 fold, but is not able to fully remediate ascospore production. Different letters denote significant differences in ascospore numbers at p < 0.05. C. ΔnosA strains also display a defect in induction of several genes under carban starvation, including the catalase peroxidase cpeA and the hexose transporter hxtA (Vienken and Fischer, 2006). Complementation with either A.n. nosA or A.f. nosA is able to restore starvation dependent induction of these genes.
In order to determine whether the apparent partial progression of sexual development in the ΔnosA + A.f. nosA strain also increased production of sexual spores, ascospore density for all strains was then determined. As shown previously (Vienken and Fischer, 2006), the ΔnosA strain is characterized by an approximately 1000 fold decrease in the number of ascospores produced (Fig. 2B). Introduction of A.n. nosA nearly completely restored development, as expected from cleistothecial morphology. Although unable to fully compensate for the sexual defect, A.f. nosA introduction increased ascospore production by approximately 10 fold in the double mutant.
In addition to development of sexual structures themselves, NosA has also been shown to be required for transcription of several genes induced during sexual development. Both the hexose transporter hxtA and the catalase peroxidase cpeA require NosA for full induction under carbon starvation conditions (Vienken and Fischer, 2006). In the presence of glucose, only very low levels of these transcripts are present (Fig. 2C). After incubation with no carbon source, these transcripts are upregulated in the wild type, but only slightly induced in the nosA mutant. Addition of either A.n. nosA or A.f. nosA was able to restore full induction of these transcripts under the conditions tested, displaying a conservation of function between these two transcription factors.
3.2 A. fumigatus nosA mutants are altered in development
To ascertain whether a subset of the differences seen in A. fumigatus ΔlaeA deletion strains are due to downstream actions of this transcription factor, deletion (ΔnosA) and overexpression (OE::nosA) alleles were constructed in both wild type and ΔlaeA backgrounds. To facilitate mutant construction, A. fumigatus CEA17 ΔakuB strains were used, containing a deletion of the KU80 homolog required for non-homologous end joining (da Silva Ferreira et al., 2006). PCR and Southern analysis confirmed an approximately 50% success rate for transformants with the correct integration (Fig. S2). A representative strain from each background was selected and nosA expression determined using northern analysis (Fig. S3), also confirming increased expression in a ΔlaeA background relative to wild type (Fig. S3). Complementation of the ΔnosA deletion was achieved by transformation of plasmids containing a hygromycin resistance cassette (hph) and either A.n. nosA + 1 kb flanks or A.f. nosA + 1 kb flanks. Strains were confirmed by Southern analysis (Fig. S4).
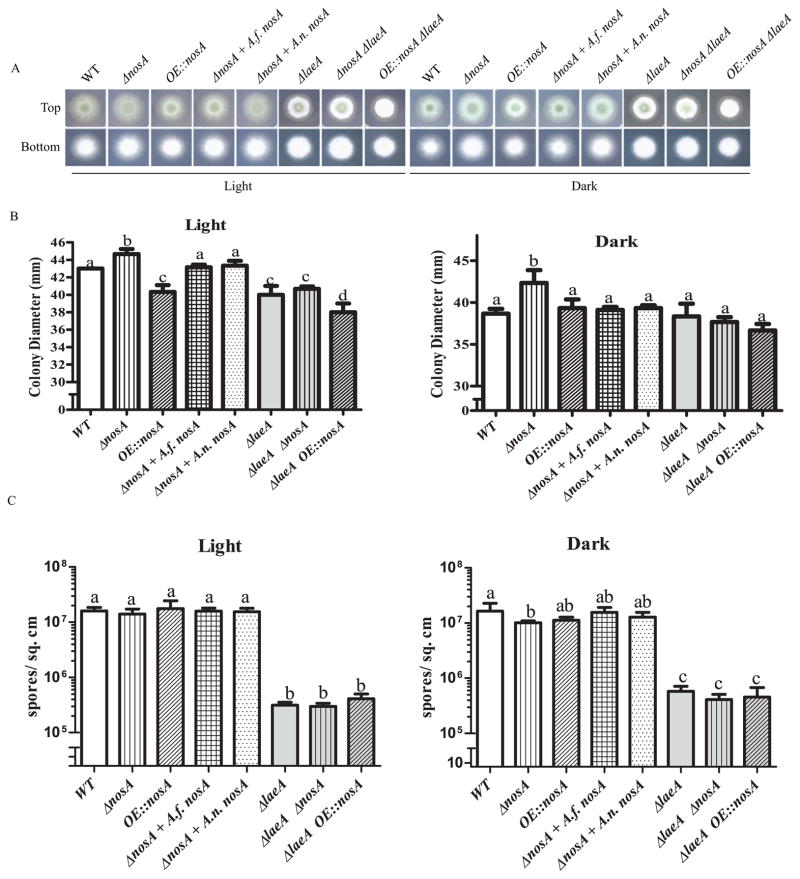
Examination of nosA mutants grown on solid media showed multiple differences relative to wild type (Fig. 3). Under both light and dark conditions, in a wild type background, the ΔnosA strain exhibited statistically significant increased radial growth. Interestingly, all ΔlaeA strains exhibited a light dependent growth defect, with decreased radial growth under constant light incubations. Overexpression of nosA in a wild type background mimicked this effect, with the OE::nosA mutant showing relatively less radial growth in light compared to dark.
Fig. 3.
Colony morphology of nosA mutants grown for 3 days on GMM at 37°C. A. Morphology of NosA mutants. B. Measurement of colony diameters reveals small but significant differences among strains. Under light conditions, the OE::nosA strain has a defect in radial growth compared to wild type. This decrease is abrogated under dark conditions and in a ΔlaeA background. Deletion of nosA increased colony diameter in a wild type background, but not in a ΔlaeA background. Error bars represent standard deviation of 4 replicates. Letters represent significant differences at p < 0.05. C. Conidia production of nosA mutants grown for 6 days on GMM at 37°C. The ΔnosA strain exhibits a dark dependent defect in conidiation relative to wild type. This difference is not seen under light conditions or in a ΔlaeA background. Error bars represent standard deviation of 4 replicates. Letters represent significant differences at p < 0.05.
As the ΔlaeA mutant had previously been shown to have various defects in spore development (Bok et al., 2005, Dagenais et al., 2010; reviewed in Bayram and Braus 2012), both spore production and germination of the nosA mutants were assessed (Fig. 3C and 4). Under dark but not light conditions, the ΔnosA strains showed a slight decrease in production of conidia. Neither overexpression nor deletion of nosA restored wild type conidiation to the ΔlaeA mutant.
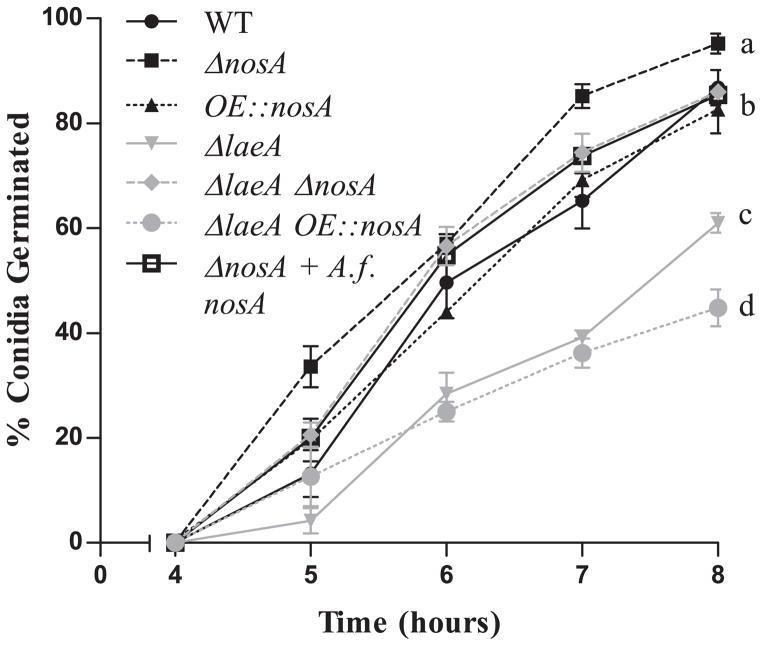
Fig. 4.
Germination rate of nosA mutants. ΔnosA displays an increased germination rate compared to wild type and restores the rate of germination to wild type levels in the ΔlaeA background (ΔlaeA ΔnosA). In contrast, overexpression of nosA does not affect germination in either a wild type but further decreases germination rate in a ΔlaeA background. Four replicates of 100 spores for each strain were counted. Letters represent significant differences at p < 0.05.
In contrast to the above, deletion of nosA restored normal germination in the ΔlaeA background. As seen in Figure 4, an assessment of germination rates of the seven strains indicated that deletion of nosA increased the rate of germination compared to wild type. This increase in germination rate was abolished by introduction of either ectopic A.f. nosA or A.n. nosA (data not shown). Furthermore, nosA loss was able to remediate the germination defect of the ΔlaeA mutant. Overexpression of nosA in the wild type background was not sufficient to delay germination, but the double OE::nosA ΔlaeA strain showed an even further drop in germination rate compared to ΔlaeA alone. After 15 hours incubation, these differences are no longer significant, confirming a delay in germination of these strains, rather than a decrease in spore viability (data not shown).
3.2 NosA impacts ROS resistance
A. nidulans nosA is required for normal induction of the catalase-peroxidase cpeA and the hexose transporter hxtA (Vienken and Fischer, 2006), which are induced during sexual development along with the NADPH oxidase noxA (Scherer et al., 2002; Lara-Ortiz et al., 2003). As we showed A.f. nosA to functionally complement the induction defect of both cpeA and hxtA in A. nidulans (Fig. 2C), we chose to determine whether A. fumigatus also showed the induction of these orthologs (the former called cat2 in A. fumigatus, Takasuka et al., 2009) under carbon limiting conditions, and whether this induction was nosA dependent, as in A. nidulans. As might be expected from the differences in timing of the sexual cycle between these two species (O’Gorman et al., 2009) transcript levels of these genes in A. fumigatus do not mirror those in A. nidulans under identical conditions (Fig. S5). A slight induction of transcript levels in medium lacking glucose is seen for all strains, but lacks both the amplitude and nosA dependence of that seen in A. nidulans.
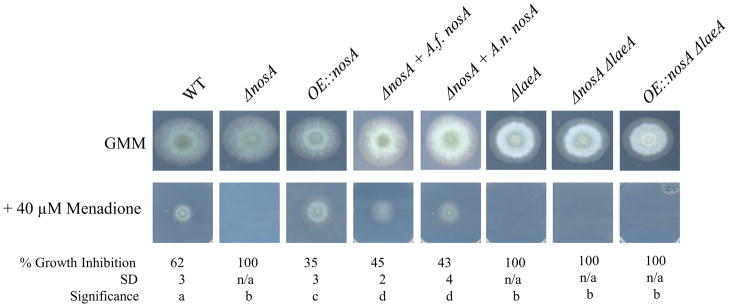
Although nosA dependent differences in cat2 expression were not detected under the condtions tested above, we next chose to examine whether A. fumigatus nosA mutants could exhibit an altered resistance to exogenous sources of ROS by other means, using hydrogen peroxide and menadione as sources. Neither deletion nor overexpression of nosA affected radial growth of fungal strains in either a wild type or ΔlaeA background when treated with 4 mM hydrogen peroxide (Fig. S6). However all ΔlaeA backgrounds were slightly less reduced in radial growth than their controls suggesting a role for laeA in protection against ROS generation by hydrogen peroxide.
Treatment with different levels of menadione, however, indicated a need for nosA and laeA for resistance to this chemical. At 20 μM, whereas there was no difference in growth of ΔnosA, OE::nosA showed a slight increase in growth compared to wild type (Fig. S6). At 40 μM menadione, deletion of nosA in a wild type background resulted in increased susceptibility to this chemical, the strain displaying no growth at this concentration (Fig. 5). Both complementation strains (ΔnosA + A.f. nosA or + A.n. nosA) restored growth. The OE::nosA strain again showed a mild resistance relative to wild type, as displayed by a significnantly decreased growth inhibition relative to wild type. All ΔlaeA strains displayed increased sensitivity to 40 μM menadione, including the ΔlaeA OE::nosA strain. Together, this data suggests that NosA contributes to resistance to menadione but cannot remediate the defect of a ΔlaeA allele. Semi-quantitative RT-PCR of transcript levels of identified catalase and superoxide dismutase genes (Takasuka et al., 2009; Lambou et al., 2010) failed to display expression patterns correlating with these differences in resistance (Fig. S6), suggesting the differences in sensitivity are due to modulation of alternative pathways.
Fig. 5.
Resistance to the ROS generator menadione. Both deletion of nosA and laeA show an increased sensitivity to menadione, evidenced by no growth on media containing 40 μM menadione. The OE::nosA strain shows a slight increase in resistance relative to wild type, as evidenced by a significantly decreased growth inhibition. Letters represent significant differences at p < 0.05.
3.3 NosA deletant is more virulent in the Galleria mellonella model of IA
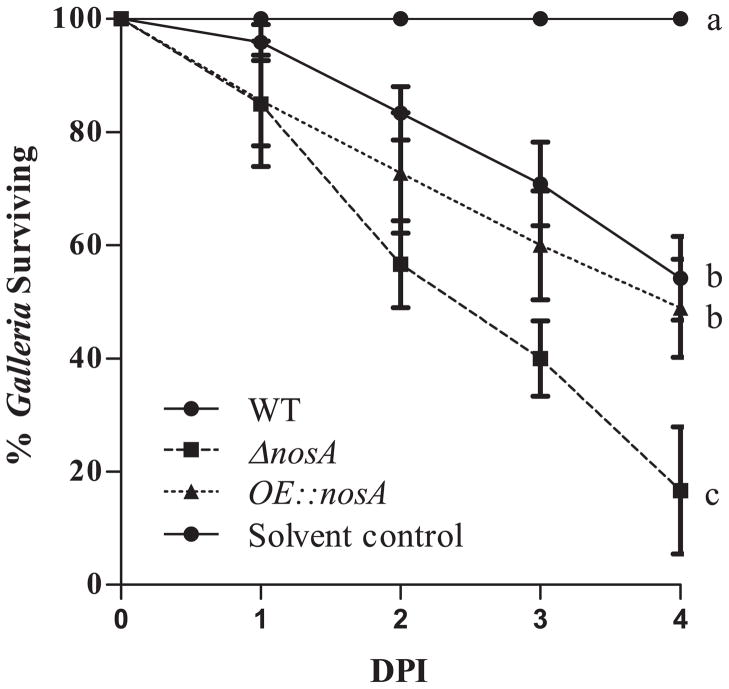
The properties exhibited by the nosA mutants, e.g. increased growth and germination in the ΔnosA strain and increased resistance to ROS in the OE::nosA strain could both be hypothesized to increase virulence in an animal model. Several insect models have recently been successfully used to screen for alterations in virulence in A. fumigatus mutants (Slater et al., 2011) and we therefore chose to examine virulence of nosA mutant strains using the Galleria model of IA. We found an increase in virulence of the nosA deletion strain, but no change in virulence of the overexpression nosA strain (Fig. 6). Nevertheless, loss of nosA did not increase virulence in a ΔlaeA strain (data not shown). These differences in virulence do not appear to be due to differential regulation of secondary metabolites, as few differences are seen by TLC analysis (Fig. S7).
Fig. 6.
Deletion of nosA increased virulence in a Galleria mellonella insect model. Mortality was significantly increased at 4 days in larvae injected with ΔnosA spores. No significance differences are seen between wild type and OE::nosA strains. Errors represent SEM of 3 independent experiments of 30 infected larvae/strain. Letters represent significant differences at p < 0.05.
As a further investigation on the potential contribution of NosA to A. fumigatus virulence, we next examined engulfment and survival of conidia by human primary alveolar macrophages (Fig. S8). However, there was no significant difference in either parameter, suggesting that the increased mortality of the ΔnosA strain in the Galleria model may not translate to changes in macrophage encounters in other systems.
2 Discussion
Phylogenetic analysis of the LaeA regulated transcription factor Afu4g09710 showed it to be closely related to A. nidulans nosA (Fig. 1), which has been characterized for its role in sexual development (Vienken and Fischer, 2006). Heterologous complementation assays showed that A.f. nosA is able to partially functionally compensate for A.n. nosA loss when introduced in A. nidulans. Examination of sexual structures showed a partial compensation of the developmental block seen in ΔnosA strains, as demonstrated by an increase in both size of cleistothecia primordia, and amount of ascospores produced (Fig. 2). Although the defect in development of sexual structures was not completely remediated, carbon starvation dependent induction of the sexual cycle induced genes cpeA and hxtA was restored to wild type levels in the A. nidulans ΔnosA + A.f. nosA strain. This suggests that the function of NosA is considerably conserved between these two species, and the lack of a full complementation maybe be due to either a slight divergence in specialization, or differences in expression, perhaps due to missing elements within the 1 kb promoter used. Both divergence (ex. CflA; Boyce et al., 2001) and promoter differences (ex. StuA; Miller et al., 1991) have previously been shown to influence the degree of complementation in closely related fungi.
We then began dissecting the action of NosA on A. fumigatus physiology, both in wild type and ΔlaeA backgrounds. As nosA is upregulated in a ΔlaeA background (Perrin et al., 2007; Fig. S2), we were particularly interested in the consequences of overexpressing nosA in a wild type background and deletion of nosA in a ΔlaeA background, to dissect which portions of the ΔlaeA phenotype may be attributed to altered expression of this transcription factor. Initial phenotypic analysis uncovered a role of nosA in growth and development under the conditions tested. Mutant response differed between light and dark conditions, further demonstrating the presence of a light sensing program in A. fumigatus related to acquiring developmental competence (Sheppard et al., 2005; Ben-Ami et al., 2010). The OE::nosA mutant displayed a distinct defect in radial growth when incubated under light conditions, mimicking the defect seen in ΔlaeA strains under this condition. Deletion of nosA, in contrast, led to increased radial growth of wild type strains under both light and dark conditions, although it failed to restore the light dependent growth defect of ΔlaeA. Increased growth of ΔnosA corresponded to a defect in production of conidia under dark conditions, suggesting a shift in morphological programming to vegetative growth. Both of these effects were negated by the introduction of an ectopic copy of either A.f nosA or A.n. nosA.
As conidia production differed among strains in our mutant series, we chose to explore whether other conidial characteristics were also altered, such as rate of germination. ΔnosA strains showed increases in germination rate in a wild type background, and loss of nosA was able to completely rescue the delayed germination of the ΔlaeA strain. Taking the radial growth and germination data together, this suggests that NosA regulates a subset of genes downstream of ΔlaeA, contributing to some of the ΔlaeA abberancies in vegetative growth and spore germination (Bok et al., 2005).
A. nidulans nosA mutants show impaired expression of the catalase-peroxidase cpeA (Vienken and Fischer, 2006). As host produced reactive oxygen species play a role in defense against pathogens (Dagenais and Keller, 2009), we examined A. fumigatus nosA mutants response to the reactive oxygen species generators H2O2 and menadione. Hyphal growth on H2O2 was unaffected by nosA mutations, although a slight resistance was seen in ΔlaeA mutants. In contrast, ΔlaeA and ΔnosA strains displayed an increased sensitivity to 40 μM menadione. While H2O2 produces peroxide radicals, menadione is believed to produce both superoxide and peroxide radicals (Farr and Kogoma, 1991). This suggests that both of these mutants may be more sensitive to superoxide, rather than peroxide radicals, although likely through different pathways. This is emphasized by the slightly increased radial growth of the OE::nosA strain on menadione, but the inability of the OE::nosA ΔlaeA strain to grow on this medium.
We were curious to test the virulence of the nosA mutants as characteristics of both the overexpression (increased resistance to menadione) and deletion (increased vegetative growth and germination rates) nosA strains have been correlated with virulence in other mutants (Ejzykowicz et al., 2009; Kim et al., 2009; reviewed in Abad et al., 2010). Because the ΔlaeA mutant (with increased nosA expression) is hypovirulent (Bok et al., 2005, Sugui et al. 2007, Ben-Ami et al. 2009), we predicted that the ΔnosA strain was more likely to show increased virulence than the OE::nosA strain. We chose to use the Galleria mellonella insect model for our virulence tests as this model has recently been shown to encode homologs of the NADPH oxidase complex required for the generation of superoxide radical ions (Bergin et al., 2005), and has been shown to yield similar results as murine models of pathogenicity for known virulence factors (Slater et al., 2011). Figure 6 shows that ΔnosA displayed increased pathogenicity in our model. Although the exact reason(s) for this remains to be elucidated, we propose that this may be due to the increased vegetative growth and germination of the ΔnosA mutant. Previous research has shown that virulence of A. fumigatus to G. mellonella larvae increases along with the germination status of the spore (Renwick et al., 2006). On the other hand, it appears that the enhanced sensitivity of ΔnosA and increased resistance of the OE::nosA to menadione was of little relevance in the insect/fungal interaction.
Taken together, our results indicate that A. fumigatus Afu4g09710 acts as a functional homolog of A. nidulans nosA. The differences in radial growth, sporulation, and germination of A. fumigatus nosA mutants show that nosA has an impact on additional aspects of fungal development and chemical testing indicates NosA contributes to resistance to menadione. Finally, the slow growth and delayed germination phenotype of ΔlaeA appear to be, at least in part, regulated by NosA. We speculate that the Galleria results shown here could be primarily attributable to the faster germination and vegetative growth rate of the ΔnosA strain.
Supplementary Material
Highlights.
LaeA regulated transcription factors effect virulence and development downstream
NosA is negatively regulated by LaeA
The ΔlaeA germination defect is remediated in the double ΔnosA ΔlaeA mutant
ΔnosA has increased pathogenicity in the Galleria model of invasive aspergillosis
NosA is involved in resistance to menadione
Acknowledgments
This research was funded by NIH 1 R01 Al065728-01 to N.P.K. and by NIH, National Research Service Award AI55397 to A.A.S.
Abbreviations
- IA
invasive aspergillosis
- WT
wild type
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abad A, et al. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol. 2010;27:155–82. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Bayram O, Braus GH. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ami R, et al. Aspergillus fumigatus inhibits angiogenesis through the production of gliotoxin and other secondary metabolites. Blood. 2009;114:5393–9. doi: 10.1182/blood-2009-07-231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R, et al. Characterization of a 5-azacytidine-induced developmental Aspergillus fumigatus variant. Virulence. 2010;1:164–73. doi: 10.4161/viru.1.3.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergin D, et al. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun. 2005;73:4161–70. doi: 10.1128/IAI.73.7.4161-4170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, et al. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell. 2005;4:1574–82. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, et al. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun. 2006;74:6761–8. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Keller NP. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot Cell. 2004;3:527–35. doi: 10.1128/EC.3.2.527-535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce KJ, et al. The CDC42 homolog of the dimorphic fungus Penicillium marneffei is required for correct cell polarization during growth but not development. J Bacteriol. 2001;183:3447–57. doi: 10.1128/JB.183.11.3447-3457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais TR, et al. Defects in conidiophore development and conidium-macrophage interactions in a dioxygenase mutant of Aspergillus fumigatus. Infect Immun. 2008;76:3214–20. doi: 10.1128/IAI.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais TR, et al. Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer. Infect Immun. 2010;78:823–9. doi: 10.1128/IAI.00980-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin Microbiol Rev. 2009;22:447–65. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejzykowicz DE, et al. The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol Microbiol. 2009;72:155–69. doi: 10.1111/j.1365-2958.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JP, et al. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology. 2011;157:1481–8. doi: 10.1099/mic.0.043786-0. [DOI] [PubMed] [Google Scholar]
- Farr SB, Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991;55:561–85. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, et al. TmpL, a transmembrane protein required for intracellular redox homeostasis and virulence in a plant and an animal fungal pathogen. PLoS Pathog. 2009;5:e1000653. doi: 10.1371/journal.ppat.1000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambou K, et al. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol Microbiol. 2010;75:910–23. doi: 10.1111/j.1365-2958.2009.07024.x. [DOI] [PubMed] [Google Scholar]
- Lara-Ortiz T, et al. Reactive oxygen species generated by microbial NADPH oxidase NoxA regulate sexual development in Aspergillus nidulans. Mol Microbiol. 2003;50:1241–55. doi: 10.1046/j.1365-2958.2003.03800.x. [DOI] [PubMed] [Google Scholar]
- Masloff S, et al. The pro1(+) gene from Sordaria macrospora encodes a C6 zinc finger transcription factor required for fruiting body development. Genetics. 1999;152:191–9. doi: 10.1093/genetics/152.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A, et al. Sub-telomere directed gene expression during initiation of invasive aspergillosis. PLoS Pathog. 2008;4:e1000154. doi: 10.1371/journal.ppat.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KY, et al. StuA is required for cell pattern formation in Aspergillus. Genes Dev. 1992;6:1770–82. doi: 10.1101/gad.6.9.1770. [DOI] [PubMed] [Google Scholar]
- O’Gorman CM, et al. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature. 2009;457:471–4. doi: 10.1038/nature07528. [DOI] [PubMed] [Google Scholar]
- Perrin RM, et al. Transcriptional regulation of chemical diversity in Aspergillus fumigatus by LaeA. PLoS Pathog. 2007;3:e50. doi: 10.1371/journal.ppat.0030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick J, et al. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia. 2006;161:377–84. doi: 10.1007/s11046-006-0021-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2001. [Google Scholar]
- Scherer M, et al. Aspergillus nidulans catalase-peroxidase gene (cpeA) is transcriptionally induced during sexual development through the transcription factor StuA. Eukaryot Cell. 2002;1:725–35. doi: 10.1128/EC.1.5.725-735.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban MI, et al. Suppressor mutagenesis identifies a velvet complex remediator of Aspergillus nidulans secondary metabolism. Eukaryot Cell. 2010;9:1816–24. doi: 10.1128/EC.00189-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, et al. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol Biol Cell. 2005;16:5866–79. doi: 10.1091/mbc.E05-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Keller NP. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater JL, et al. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med Mycol. 2011;49(Suppl 1):S107–13. doi: 10.3109/13693786.2010.523852. [DOI] [PubMed] [Google Scholar]
- Smedsgaard J. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. J Chromatogr A. 1997;760:264–70. doi: 10.1016/s0021-9673(96)00803-5. [DOI] [PubMed] [Google Scholar]
- Starnes TW, et al. Imaging podosome dynamics and matrix degradation. Methods Mol Biol. 2011;769:111–36. doi: 10.1007/978-1-61779-207-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugui JA, et al. Role of laeA in the regulation of alb1, gliP, conidial morphology, and virulence in Aspergillus fumigatus. Eukaryot Cell. 2007;6:1552–61. doi: 10.1128/EC.00140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk E, et al. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc. 2006;1:3111–20. doi: 10.1038/nprot.2006.405. [DOI] [PubMed] [Google Scholar]
- Takasuka T, et al. Aspergillus fumigatus catalases: cloning of an Aspergillus nidulans catalase B homologue and evidence for at least three catalases. FEMS Immunol Med Microbiol. 1999;23:125–33. doi: 10.1111/j.1574-695X.1999.tb01231.x. [DOI] [PubMed] [Google Scholar]
- Tsitsigiannis DI, et al. The lipid body protein, PpoA, coordinates sexual and asexual sporulation in Aspergillus nidulans. J Biol Chem. 2004;279:11344–53. doi: 10.1074/jbc.M310840200. [DOI] [PubMed] [Google Scholar]
- Vienken K, et al. The Zn(II)2Cys6 putative Aspergillus nidulans transcription factor repressor of sexual development inhibits sexual development under low-carbon conditions and in submersed culture. Genetics. 2005;169:619–30. doi: 10.1534/genetics.104.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienken K, Fischer R. The Zn(II)2Cys6 putative transcription factor NosA controls fruiting body formation in Aspergillus nidulans. Mol Microbiol. 2006;61:544–554. doi: 10.1111/j.1365-2958.2006.05257.x. [DOI] [PubMed] [Google Scholar]
- Willger SD, et al. Aspergillus fumigatus metabolism: clues to mechanisms of in vivo fungal growth and virulence. Med Mycol. 2009;47(Suppl 1):S72–9. doi: 10.1080/13693780802455313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W, Keller NP. Transcriptional regulatory elements in fungal secondary metabolism. J Microbiol. 2011;49:329–39. doi: 10.1007/s12275-011-1009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol. 2004;41:973–81. doi: 10.1016/j.fgb.2004.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.