Abstract
Electronic health records in the United States currently isolate digital information in proprietary, institutional databases. Experts have identified inadequate data exchange as a leading challenge to advancements in care quality and efficiency.
Recent federal health information technology incentives adopt an extensible standard, called the Continuity of Care Document (CCD), as a new basis for digital interoperability. Although this instrument was designed for individual provider communications, the CCD can be effectively reused for population-based research and public health.
Three examples in this commentary demonstrate the potential of CCD aggregation and highlight required changes to existing public health and research practices. Transitioning to the use of this new interoperability standard should be a priority for public health investment, research, and development.
THE ADOPTION OF ELECTRONIC health records (EHRs) has focused on enhancing the delivery of individual care, but the application of digital medical data to widespread population health analysis is critically lacking. Population analysis empowers public health agencies, disease registries, medical researchers, and practicing clinicians to monitor care quality and improve disease management beyond face-to-face patient encounters. Potential applications of EHR technology to population analysis are straightforward. Health surveillance should rely on automated detection rather than manual inspection. Quality measures should be calculated and streamed directly to agencies for quality improvement. Comparative effectiveness should leverage the emerging wealth of digital data to inform decisions on care appropriateness and provide feedback to clinicians. What limits these applications is the divergence of how EHRs capture and record medical data without a standard method to exchange information between these systems. This observation led the President’s Council of Advisors on Science and Technology1 and the Institute of Medicine2 to recently identify interoperability as the major deficit of current health information technology. From their perspectives, fluid and secure data exchange has the most immediate potential to improve care quality and efficiency nationwide.
Achieving robust interoperability requires common language and structures to medical data so communication is seamless to care providers. This contrasts with current practice. Today, implementations of medical data exchange force both senders and recipients of medical data to plan in advance the content and format of exchange. This is akin to installing a unique web browser for each Web site on the Internet; the complexity and burden of such networking effectively isolates medical data at the point of care. Health information exchanges confront this same obstacle, where even successful networks note the challenge of normalizing heterogeneous EHR data.3 Information exchange is consequently the exception rather than the norm. Recent federal initiatives, however, are beginning to dismantle these barriers.
In the American Recovery and Reinvestment Act of 2009,4 Congress approved $27 billion in health information technology stimulus and placed standardized information exchange as a leading policy objective. Specific objectives for this program were released in July 2010, a majority of which focus on data structure and interoperability.5 One requirement for all providers is that an EHR must be able to create, transmit, and receive an electronic document containing key clinical information using standard terminologies. The 2 standards that may be used for this objective are the Continuity of Care Document (CCD) and the Continuity of Care Record (CCR). Although both the CCD and CCR are acceptable in federal regulation, EHR vendors have focused on the CCD because it is the newer format developed through the harmonization of the CCR with other past standards.6
THE CONTINUITY OF CARE DOCUMENT
Although it was originally designed to exchange information on individual patients, the CCD will become a powerful instrument for medical research and public health. Its enforcement of structured data and language provides the first normalized summary that can be generated from any of the 600 plus certified EHRs.7 As such, CCDs can be interpreted without previous knowledge of the source system, similar to a Web page on the Internet. The CCD uses extensible markup language to represent medical data in a consistent, tagged format. These tags, attached to every data element, identify key context descriptors such as the language being used to encode data (Figure 1). According to federal regulation, the electronic summary must include data on patient demographics, problems, medications, allergies, laboratory results, and procedures.8 Although these sections represent only a fraction of all medical data, standardization makes them available to systems beyond the originating EHR.
FIGURE 1—
A simplified example of tagged diagnosis data within the Continuity of Care Document with explanations on right.
Note. CCD = Continuity of Care Document.
Using the CCD, agencies can create tools to communicate population data that are immediately compatible with any certified EHR. Although the potential applications are many, the following three examples illustrate the promise of CCD applications to improve existing systems for population analysis.
Extending Public Health to Chronic Diseases
More than 20 million people are estimated to have diabetes in the United States, but only 20% of these patients receive the appropriate preventative services as recommended by the American Diabetes Association.9 Many persons with diabetes are consequently at high risk of complications, comorbidities, and death as a result of the disease. In 2005, the New York City Board of Health approved a novel approach to collect data about the disease and craft better public health responses. The board requires mandatory reporting of patient-identified glycosylated hemoglobin values from laboratories to the local department of public health.10 This collection format is similar to reporting systems for communicable disease, limited to patient and provider contact information and the lab date and result for glycosylated hemoglobin. As a result of this program, the city now produces quarterly reports for approximately 1600 providers and mails 400 letters each week to patients with high glucose levels.
Although local pilots demonstrate the public health benefit of this and other initiatives, key data from these efforts are missing. Relevant information on medications, preventative practices, and quality performance are not transmitted.11 An absence of total patient counts and detailed diagnoses prevents accurate incidence calculations. These limit the comprehensiveness of physician reports and patient mailings. As certified EHRs are adopted in a public health region, submitting normalized data via the CCD would enlarge the analytical power of such initiatives while minimizing the reporting burden. Moreover, it would allow expansion of such analytics to other chronic conditions, such as heart failure or hypertension, without increasing the need for new interfaces. Public health authorities exercising their statutory power to collect such information presents a significant opportunity to improve the care for chronic diseases.
Clinical Detail in Death Certificates
In 1999, the US Food and Drug Administration approved rofecoxib, commonly known by its brand name of Vioxx. Within several years, the drug had become a blockbuster for its ability to treat chronic pain without the adverse effects of gastrointestinal ulcers and bleeding. Soon after its launch, however, the safety of rofecoxib was questioned because of postapproval data on the incidence of myocardial infarction. By September 2004, Merck had voluntarily withdrawn the drug from the market because of mounting evidence of cardiovascular harm caused by rofecoxib.
One retrospective study of Kaiser Permanente members examined whether rofecoxib was associated with increased coronary events. By scanning 2.3 million person years, researchers observed an adjusted odds ratio of 3.58 for serious coronary heart disease with high-dose rofecoxib compared with similar drugs.12 With such a serious increase in risk, the drug clearly caused thousands of deaths before its withdrawal 5 years after approval. One vocal expert and researcher on the study from the US Food and Drug Administration said the agency was “incapable of protecting America against another Vioxx.”13
Transparency through data can be a valuable safeguard in researching suspected causes of death. One way to increase the power of mortality studies would be to increase data reported at the time of death. Pairing clinical detail on medications and laboratory results, as well as other known conditions, would help agencies and epidemiologists better explore vital statistics data. These data could again be aggregated by CCDs for health facilities by using certified EHR technology. With such tools, death certificate data could be scanned to test hypotheses of medication risk, similar to Kaiser’s analysis of rofecoxib. Although that may not be feasible for several years, setting expectations in advance will prepare for an eventual transition to active adverse event detection.14
Advancing Biosurveillance
Currently the Centers for Disease Control and Prevention runs a nationwide reporting system named the Influenza-like Illness Surveillance Network (ILINet). ILINet collects information weekly from approximately 1800 outpatient care sites on patients who have a fever and cough or sore throat in the absence of other known causes. Providers submit weekly patient counts stratified by age through fax or the Internet to the Centers for Disease Control and Prevention. Regional information is made publicly available and monitored for potential outbreaks. The estimated time burden for participants is less than 30 minutes per week, but collection of simple patient counts limits the depth of analysis.15 Novel approaches that electronically extract more comprehensive patient data can improve the depth, speed, and sample size of influenza analytics. One such system pioneered by the Veterans Administration uses patient diagnosis detail from all patient visits documented in its EHR.16 Using this electronic detail has detected significant shifts in condition type and patient demographics that could not be revealed through ILINet.
CCD-normalized extracts present an opportunity to rapidly scale electronic surveillance. Rather than having registries like ILINet manually collect data, simple applications could be distributed to parse and calculate statistics from certified EHR technology. The flexibility of this approach relies not on transmitting personal health information, but on locally deploying programs to analyze data within a practice’s existing infrastructure. Then, richer de-identified summaries of influenza-like encounters could be transmitted weekly, or daily, with minimal effort on the behalf of providers. Although regional pilots exist using older standards for surveillance, the CCD’s common structure and language reduces implementation cost. Future public health surveillance systems will unlock new data on health disparity, detailed symptomatology, and therapeutic regimen that will strengthen national efforts to manage infectious disease and environmental risk.
FUTURE OF CLINICAL DATA EXCHANGE AND POPULATION ANALYSIS
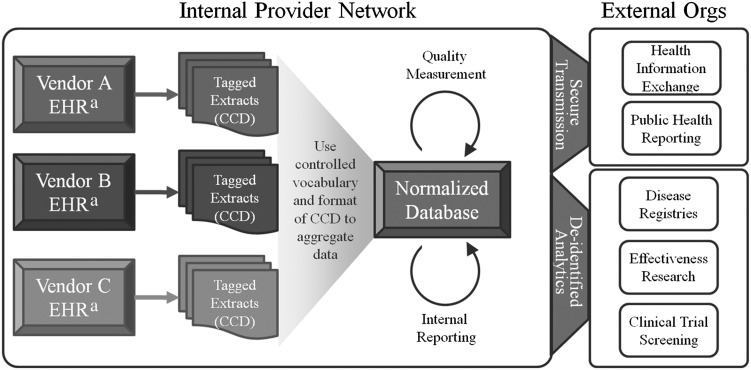
The future challenge of clinical data exchange will be to create infrastructure where information can be rapidly accessed while retaining the privacy and security expected of medical records. The previously mentioned examples illustrate how public health authorities could use the CCD to work within established practices of identified health data collection, but local CCD aggregation provides an immediate alternative for data sharing within a provider network. A normalized database that assembles CCD extracts from heterogeneous EHRs could service quality measurement and internal reporting needs for integrated group practices and health systems (Figure 2). This avoids privacy restrictions as previous rulings exempt data sharing among integrated clinicians when engaged in quality improvement and population health.17 Emerging models of care, such as the Patient Centered Medical Home, also assume such exchange as a foundation for care coordination. CCD repositories could additionally serve regional and national efforts to improve care, such as clinical trial enrollment, disease registries, and comparative effectiveness research. A prototype of such a system, called popHealth, is being piloted, but more investment and development will be required for such projects to reach sustainable scale.18
FIGURE 2—
How Continuity of Care Documents could enable normalized databases to advance medical research and public health.
Note. CCD = Continuity of Care Document; EHR = electronic health records; Orgs = organizations.
aEHRs should be certified to ensure consistent CCD format and vocabulary.
Distributed normalized repositories based on the CCD also create opportunities for existing health information exchanges and public health agencies. Although more than 190 health information exchanges initiatives exist in the United States as of 2010, the majority do not store aggregate data because of concerns about patient privacy and data ownership.19 As consequence, they provide minimal support for medical researchers or epidemiologists. De-identification of CCD repositories or distributed queries of the databases could provide valuable information about population health without compromising patient confidentiality.20 Regional and national payers may also subsidize such initiatives if they can demonstrate care coordination improvements that lower overall cost. These avenues provide strategic alternatives for health information exchanges, many of which are in financial doubt because of the unwillingness of providers to pay for transactional information exchange.21
CCD-based population analysis has clear limitations given the infancy of the standard. Until a large majority of health providers have adopted certified technology, there may be a selection bias in using CCD-data from early EHR adopters. In particular, physician practices with EHRs may underrepresent Medicaid and uninsured populations and not be representative of all providers or patients.11 Fortunately, it is estimated that by 2019, 90% of ambulatory practices will be using EHRs capable of CCD production.22 CCDs generated from different EHRs also possess heterogeneity as the standard was not widely adopted with a robust reference library before EHR developers updated their software to meet federal interoperability standards.23 In addition, CCDs do not necessarily contain standardized information on care plans, immunizations, genomics, imaging procedures, or advance directives for the first stage of the federal incentive program. The CCD format is extensible, however, which provides a natural growth trajectory for these and other data as they become routinely encoded in medical records. Even with these limitations, the CCD provides a strong basis for population health research.
A logical next step for leaders in public health will be to pilot systems for CCD-based aggregation and create a facile environment for clinicians to adopt them. This requires consideration for how the CCD could bolster new efforts for disease management and possibly replace older methods for data submission. These efforts could kindle new cooperation between public health agencies, health information exchanges, and other parties involved with medical research. For these future CCD-based initiatives, however, careful consideration of patient privacy will be required. Although public health agencies have the statutory power to collect identified medical data, broader access to de-identified data will be vital to clinical research and comparative effectiveness studies. Public health and medical informatics professionals should advocate the advancement of pilot programs, de-identification methods, and that the CCD become a common tool for medical data far beyond individual provider communications.
CONCLUSIONS
Future generations will not judge the adoption of EHRs on the elimination of paper in medical practice. Instead, they will ask whether the new digital infrastructure was effectively harnessed to break down barriers to quality improvement, effective information sharing, and the reuse of medical data. Significant opportunities exist for research and public health personnel to prepare for this transition, particularly in how CCD data can be most effectively harnessed. When clinicians can simultaneously reduce the reporting burden for public health programs and develop new channels for quality improvement, the benefits of CCD-based aggregation will become transparent. This will require the work of many over coming years, but as David Blumenthal, MD, MPP, former director of the Office of the National Coordinator for Health Information Technology, stated, “[I]nformation is the lifeblood of modern medicine. Health information technology is destined to be its circulatory system.”24(p382) The CCD can be an effective instrument to deliver on this promise.
Acknowledgments
D. F. Sittig was funded in part by a contract for Patient-Centered Cognitive Support under the Strategic Health IT Advanced Research Projects Program from the Office of the National Coordinator for Health Information Technology (ONC-10510592).
The authors would like to thank Raouf Arafat, MD, assistant director of the Houston Department of Health and Human Services, for his time in discussing both local and national program to advance public health. We acknowledge Chiehwen Ed Hsu, PhD, and Parsa Mirhaji, MD, PhD, from the University of Texas Health Science Center for their guidance and assistance in understanding current initiatives in public health. In addition, we would like to thank John Piescik and Rob McCreedy from MITRE Corporation for their time in discussing numerous initiatives demonstrating the value of the Continuity of Care Document in population health.
References
- 1. Report to the President. Realizing the full potential of health information technology to improve healthcare for Americans. Washington, DC: Executive Office of the President: President’s Council of Advisors on Science and Technology. Available at: http://www.whitehouse.gov/administration/eop/ostp/pcast. Accessed December 4, 2011.
- 2.Grossman C, Powers B, McGinnis JM, Institute of Medicine Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care. Washington, DC: National Academies Press; 2011 [PubMed] [Google Scholar]
- 3.Grannis SJ, Biondich PG, Mamlin BWet al. How disease surveillance systems can serve as practical building blocks for a health information infrastructure: the Indiana experience. AMIA Annu Symp Proc. 2005:286–290 [PMC free article] [PubMed] [Google Scholar]
- 4.American Recovery and Reinvestment Act of 2009. Washington, DC: 111th Congress of the United States of America; 2009 [Google Scholar]
- 5.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501–504 [DOI] [PubMed] [Google Scholar]
- 6.Ferranti JM, Musser RC, Kawamoto K, Hammond WE. The clinical document architecture and the continuity of care record: a critical analysis. J Am Med Inform Assoc. 2006;13(3):245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Certified Health Information Technology Product List. Office of the National Coordinator for Health IT. Available at: http://onc-chpl.force.com/ehrcert. Accessed December 4, 2011.
- 8. US Department of Health and Human Services. Health Information Technology: Initial Set of Standards, Implementation Specifications, and Certification Criteria for Electronic Health Record Technology. Washington DC: Office of the National Coordinator for Health Information Technology. Regulation Identification No. 0991–AB58.
- 9.Harris CD, Pan L, Mukhtar Q. Changes in receiving preventive care services among US adults with diabetes, 1997–2007. Prev Chronic Dis. 2010;7(3):A56. [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbrook R. Facing the diabetes epidemic—mandatory reporting of glycosylated hemoglobin values in New York City. N Engl J Med. 2006;354(6):545–548 [DOI] [PubMed] [Google Scholar]
- 11.Cebul RD, Love TE, Jain AK, Hebert CJ. Electronic health records and quality of diabetes care. N Engl J Med. 2011;365(9):825–833 [DOI] [PubMed] [Google Scholar]
- 12.Graham DJ, Campen D, Hui Ret al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365(9458):475–481 [DOI] [PubMed] [Google Scholar]
- 13.Lenzer J. FDA is incapable of protecting US “against another Vioxx.” BMJ. 2004;329(7477):1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodcock J, Behrman RE, Dal Pan GJ. Role of postmarketing surveillance in contemporary medicine. Annu Rev Med. 2011;62:1–10 [DOI] [PubMed] [Google Scholar]
- 15. New York State Department of Health. Influenza-like Illness Surveillance Program (ILINet). Available at: http://www.health.state.ny.us/diseases/communicable/influenza/surveillance/ilinet_program. Accessed December 4, 2011.
- 16.Schirmer P, Lucero C, Oda G, Lopez J, Holodniy M. Effective detection of the 2009 H1N1 influenza pandemic in U.S. Veterans Affairs medical centers using a national electronic biosurveillance system. PLoS One. 2010;5(3):e9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nass S, Institute of Medicine Beyond the HIPAA Privacy Rule: Enhancing Privacy, Improving Health Through Research. Washington, DC: National Academies Press; 2009 [PubMed] [Google Scholar]
- 18. popHealth. An open source population health reporting prototype. Available at: http://projectpophealth.org/index.html. Accessed December 4, 2011.
- 19.Adler-Milstein J, Bates DW, Jha AK. A survey of health information exchange organizations in the United States: implications for meaningful use. Ann Intern Med. 2011;154(10):666–671 [DOI] [PubMed] [Google Scholar]
- 20.Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010;48(6, Suppl):S45–S51 [DOI] [PubMed] [Google Scholar]
- 21.Sittig DF, Joe JC. Toward a statewide health information technology center (abbreviated version). South Med J. 2010;103(11):1111–1114 [DOI] [PubMed] [Google Scholar]
- 22.Elmendorf DW. January 21, 2009 letter to the Honorable Charles B. Rangel. Congressional Budget Office. Available at: http://www.cbo.gov/ftpdocs/99xx/doc9966/HITECHRangelLtr.pdf. Accessed December 4, 2011.
- 23.D’Amore JD, Sittig DF, Wright A, Iyengar MS, Ness RB. The Promise of the CCD: challenges and opportunity for quality improvement and population health. AMIA Annu Symp Proc. 2011:285–294 [PMC free article] [PubMed] [Google Scholar]
- 24.Blumenthal D. Launching HITECH. N Engl J Med. 2010;362(5):382–385 [DOI] [PubMed] [Google Scholar]