Abstract
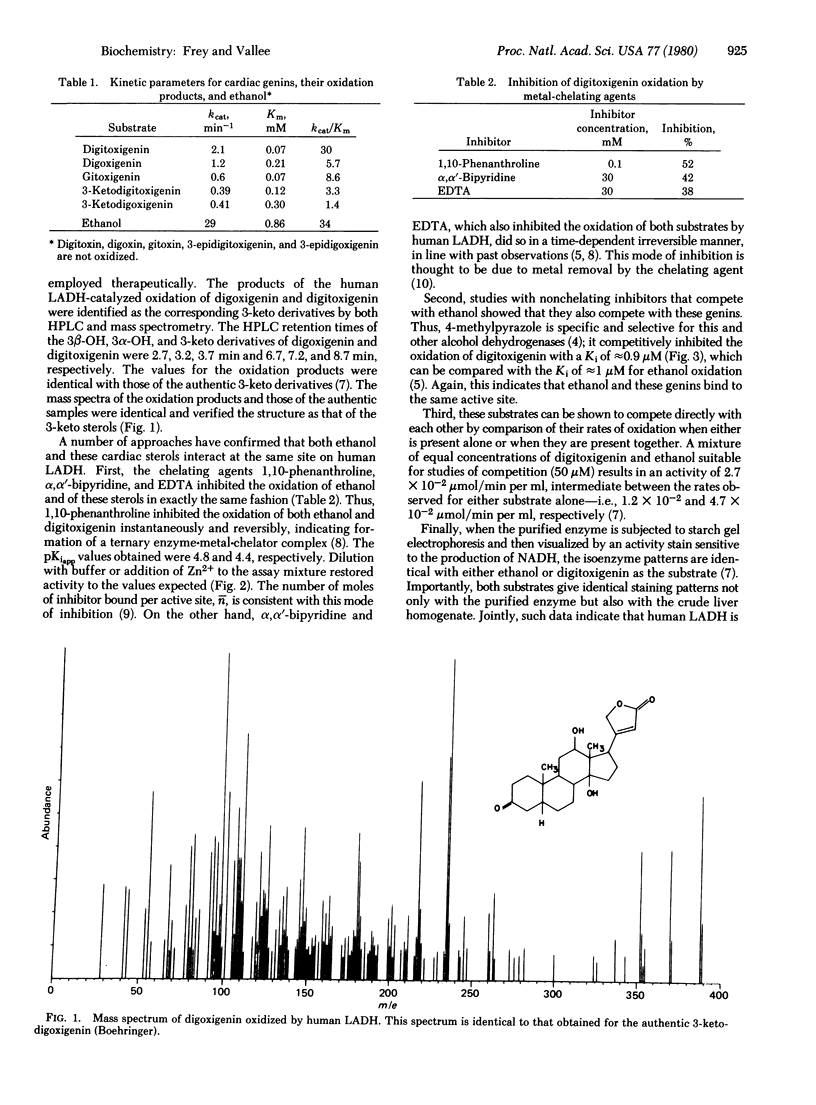
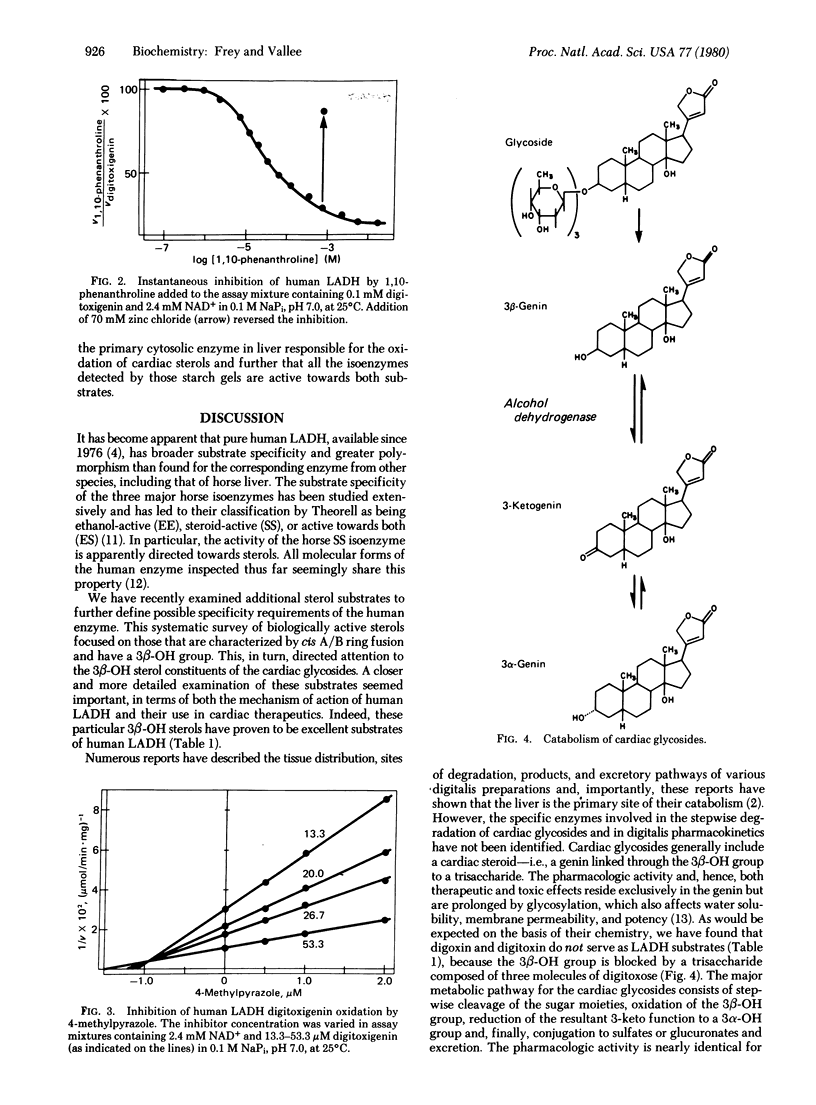
Human liver alcohol dehydrogenase (alcohol: NAD" oxidoreductase, EC 1.1.1.1) catalyzes the oxidation of the 3 beta-OH group of digitoxigenin, digoxigenin, and gitoxigenin to their 3-keto derivatives, which have been characterized by high performance liquid chromatography and mass spectrometry. These studies have identified human liver alcohol dehydrogenase as the unknown NAD(H)-dependent liver enzyme specific for the free hydroxyl group at C3 of the cardiac genins; this hydroxyl is the critical site of the genins' enzymatic oxidation and concomitant pharmacological inactivation in humans. Several kinetic approaches have demonstrated that ethanol and the pharmacologically active components of the digitalis glycosides are oxidized with closely similar kcat/Km values at the same site on human liver alcohol dehydrogenase, for which they compete. Human liver alcohol dehydrogenase thereby becomes an important biochemical link in the metabolism, pharmacology, and toxicology of ethanol and these glycosides, structurally unrelated agents that are both used widely. Both the competition of ethanol with these cardiac sterols and the narrow margin of safety in the therapeutic use of digitalis derivatives would seem to place at increased risk those individuals who receive digitalis and simultaneously consume large amounts of ethanol or whose alcohol dehydrogenase function is impaired.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blair A. H., Vallee B. L. Some catalytic properties of human liver alcohol dehydrogenase. Biochemistry. 1966 Jun;5(6):2026–2034. doi: 10.1021/bi00870a034. [DOI] [PubMed] [Google Scholar]
- Bosron W. F., Li T. K., Vallee B. L. Heterogeneity and new molecular forms of human liver alcohol dehydrogenase. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1549–1555. doi: 10.1016/0006-291x(79)91241-5. [DOI] [PubMed] [Google Scholar]
- Cronholm T., Larsén C., Jvövall J., Theorell H., Akeson A. Steroid oxidoreductase activity of alcohol dehydrogenases from horse, rat, and human liver. Acta Chem Scand B. 1975;29(5):571–576. doi: 10.3891/acta.chem.scand.29b-0571. [DOI] [PubMed] [Google Scholar]
- Frey W. A., Vallee B. L. Human liver alcohol dehydrogenase an enzyme essential to the metabolism of digitalis. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1543–1548. doi: 10.1016/0006-291x(79)91240-3. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Sytkowski A. J., Vallee B. L. Human liver alcohol dehydrogenase: purification, composition, and catalytic features. Biochemistry. 1976 Oct 19;15(21):4687–4693. doi: 10.1021/bi00666a023. [DOI] [PubMed] [Google Scholar]
- Lange L. G., Vallee B. L. Double-ternary complex affinity chromatography: preparation of alcohol dehydrogenases. Biochemistry. 1976 Oct 19;15(21):4681–4686. doi: 10.1021/bi00666a022. [DOI] [PubMed] [Google Scholar]
- Li T. K., Magnes L. J. Identification of a distinctive molecular form of alcohol dehydrogenase in human livers with high activity. Biochem Biophys Res Commun. 1975 Mar 3;63(1):202–208. doi: 10.1016/s0006-291x(75)80030-1. [DOI] [PubMed] [Google Scholar]
- REPKE K., SAMUELS L. T. ENZYMATIC BASIS FOR EPIMERIZATION OF CARDIOTONIC STEROIDS AT CARBON 3 IN RAT LIVER. Biochemistry. 1964 May;3:689–695. doi: 10.1021/bi00893a016. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L., WACKER W. E., BARTHOLOMAY A. F., HOCH F. L. Zinc metabolism in hepatic dysfunction. II. Correlation of metabolic patterns with biochemical findings. N Engl J Med. 1957 Nov 28;257(22):1055–1065. doi: 10.1056/NEJM195711282572201. [DOI] [PubMed] [Google Scholar]
- VONWARTBURG J. P., BETHUNE J. L., VALLEE B. L. HUMAN LIVER--ALCOHOL DEHYDROGENASE. KINETIC AND PHYSICOCHEMICAL PROPERTIES. Biochemistry. 1964 Nov;3:1775–1782. doi: 10.1021/bi00899a033. [DOI] [PubMed] [Google Scholar]


