Abstract
We have previously shown that pre- and post-transplant infusions of donor splenocytes treated with 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide (ECDI-SPs) provide permanent donor-specific protection of islet allografts. The efficacy of donor ECDI-SPs in protecting vascularized cardiac allografts and mechanism(s) of protection are unknown. In the current study, we show that infusions of ECDI-SPs significantly prolong cardiac allograft survival concomitant with an impressive accumulation of CD11b+IDO+ cells in the cardiac allograft, and that the presence of this population is dependent on Gr1+ cells. Consequently, depletion of Gr1+ cells or inhibition of IDO activity abrogates graft protection by ECDI-SPs infusions. In addition, T cells from ECDI-SPs treated recipients secrete high levels of IL-10 and IL-13 upon in vitro restimulation, which are also dampened in recipients treated with the IDO inhibitor. Furthermore, combination of donor ECDI-SPs with a short course of rapamycin provides indefinite cardiac allograft survival in 100% of the recipients. These findings reveal a novel mechanism of donor ECDI-SPs in inducing cardiac transplant tolerance and provide several targets that are amenable to therapeutic manipulations for tolerance induction for cardiac transplantation.
Keywords: ECDI (ethylene carbodiimide); Cardiac transplantation; Tolerance; Rapamycin; IDO (indoleamine 2,3 dioxygenase); Gr1+ monocytes; Foxp3+ regulatory T cells; IL-10 (Interleukin 10); IL-13 (Interleukin 13)
INTRODUCTION
In clinical transplantation, chronic rejection and continuous immunosuppression with risk of opportunistic infections and other hematologic and metabolic side effects remain the major obstacles for long-term graft and patient survival (1). Therefore, inducing donor-specific immune tolerance would be an ideal solution for improving organ transplant outcomes by both eliminating rejection and obviating the need for indefinite immunosuppression.
Recently gaining attention in transplant immune tolerance studies is a suppressor cell population bearing the myeloid marker Gr1 and consequently termed myeloid-derived suppressor cells (MDSCs). In murine models of cardiac and kidney transplantation, MDSCs have been shown to accumulate in the transplanted grafts, suppress effector T cells and induce graft tolerance via production of inducible nitric oxide synthase (iNOS) or IFN-γ (2, 3). In vivo, injection of iNOS inhibitor aminoguanidine induced rejection of tolerated allografts (2). Interestingly, in cancer models, MDSCs have also been shown to either express the enzyme indoleamine 2,3 dioxygenase (IDO) themselves (4, 5), or induce other cells to express IDO as a mechanism of suppressing host anti-tumor immunity (6, 7). IDO is a tryptophan metabolizing enzyme that has been reported to play an important role in tolerance induction in various models, including cardiac transplant models (8–11). The source of IDO production in these models was primarily endothelial cells of the vascularized heart graft (12). The role of other cell types in the production of IDO and their relationship to Gr1+ cells in cardiac transplant tolerance remain largely unknown.
It has been previously shown that intravenous infusion of antigen-pulsed splenocytes fixed with 1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide (ECDI-SPs) is a powerful and safe method to induce antigen-specific tolerance in vivo (13, 14). Specifically, ECDI-SPs have been shown to prevent and treat Th1/Th17-mediated autoimmunity in disease models of experimental autoimmune encephalomyelitis (EAE) and autoimmune diabetes (15, 16). More recently, we have shown that ECDI-SPs using donor splenocytes induce donor-specific tolerance in a mouse model of islet cell transplantation (17), and the mechanisms of graft protection in this model involve deletion, anergy and regulation of T cells of direct and indirect allo-specificities (Kheradmand et al, manuscript in print).
In this study, we tested ECDI-SPs in a full MHC-mismatched murine cardiac transplant model, and showed that consistent with previous data (18), ECDI-SPs alone provided significant prolongation of graft survival, and when combined with a peri-transplant short course of rapamycin, provided indefinite cardiac graft protection in 100% of recipients. We further demonstrated that graft protection was concomitant with the presence of intragraft CD11b+ cells expressing IDO, and that induction of this CD11b+IDO+ population was dependent on the presence of Gr1+ cells. These findings reveal a novel mechanism of donor ECDI-SPs in inducing cardiac transplant tolerance, and provide important insights for future designs of therapeutic strategies for tolerance induction in cardiac transplantation.
MATERIALS AND METHODS
Mice
10- to 14-week old male BALB/c(H-2d), C57BL/6(H-2b), and SJL/J(H-2s) mice were from the Jackson Laboratory. All mice were housed under specific pathogen-free conditions at Northwestern University. Protocols were approved by NU IACUC.
Tolerance induction by donor ECDI-SPs
Donor (BALB/c) splenocytes were treated with ECDI as previously described(17). Briefly, donor mice spleens were processed into single cell suspensions. Red blood cells were lysed and splenocytes were incubated with ECDI (Calbiochem, 150 mg/ml per 3.2×108 cells) on ice for 1 hour. 108 ECDI-treated splenocytes were injected i.v. on day 7 prior to and day 1 after heart transplantation.
Heterotopic cardiac transplantation
Abdominal heart transplantation was performed as described previously (19). Briefly, the donor heart was excised en bloc via median sternotomy. The ascending aorta and pulmonary artery of the donor were anastomosed end to side to the recipient abdominal aorta and inferior vena cava, respectively. Direct abdominal palpation of heart beating was used to assess graft survival. Rejection is determined by loss of palpable cardiac impulses.
Drug or antibody treatment
Rapamycin 1mg was dissolved in 4ml 0.2% Carboxyl Methylcellulose by sonication and given at 1mg/kg/d, i.p. to recipient mice from day −1 to day +8. 1-methyl-D- tryptophan (1-MT) 1g was diluted in 40 ml 1% TMC (Methylcellulose + Tween 80) and given at 10 mg/day/i.p. from day −7 to day +7. Neutralizing anti-Gr1 (anti-mLy-6G, clone RB6-8C5, BioXcell, West Lebanon, NH) was given at 200 μg/i.p. on day −8, and additionally 100 μg on days −7, −3, −1, and +1.
Proliferation assays and cytokine detection
Splenic T cells were purified from recipient mice using T cell negative isolation kit (Miltenyi) and used as responders in in vitro mixed lymphocyte reactions (MLRs). T cell-depleted splenocytes from donor mice were irradiated at 25 Gy and used as stimulators. Responders and stimulators were co-cultured in 96-well plates at 1:5 ratios. Cultures were pulsed with 1 μCi of [3H]thymidine (PerkinElmer) per well during the last 18 hours of 96 hour cultures. Supernatants from parallel cultures were analyzed with Liquichip Mouse 22-cytokine assay kit (Millipore) for cytokine productions.
Measurement of anti-donor antibody responses
Thymocytes were harvested from donor thymus. 0.5×106 thymocytes were first blocked with 1μl Fc Blocker (BD biosciences, USA) followed by incubation with plasma samples from recipients (1:4 dilution) on ice for 1 hour. Cells were then washed and stained with FITC-labeled anti-mouse IgG antibody (eBiosciences, USA) and analyzed by FACS. Negative control was provided by incubation with naïve mouse sera.
Graft histology, immunofluorescence and immunohistochemistry
Grafts were snap frozen in OCT compound with liquid nitrogen. All sections were 5 μm thick and blocked with either 10% donkey or goat serum (Sigma-Aldrich). Sections were stained with anti-mouse CD4 mAb (1:100, rat IgG2a, κ clone H129.19, BD Biosciences), or anti-mouse CD8 (1:100, rat IgG2a, κ clone 53–6.7, BD Biosciences), followed by goat anti-rat IgG Daylight 594 (1:500, Jackson ImmunoResearch). For IDO, Foxp3, CD11b, and Gr-1 staining, frozen sections were blocked with Avidin/Biotin blocking kit (Vector Laboratories) followed by staining with anti-mouse Foxp3 mAb (1:400, rat IgG2a, κ clone FJK-16s; eBioscience), anti-mouse IDO mAb (1:100, mIDO-48, rat IgG2a; Biolegend), anti-mouse CD11b (1:200, rat IgG2b, κ clone M1/70; eBioscience), or anti-mouse Ly-6G/Ly-6C (1:2000, Rat IgG2b, clone RB6-8C5, Biolegend). Consequently, samples were stained with biotinylated goat anti-rat Ig (1:200, goat Ig clone polyclonal; BD Biosciences). Visualization of Foxp3 and IDO was performed with Vectastain ABC kit (Vector Laboratories) and DAB substrate kit (BD Biosciences). Visualization of CD11b and Gr1 was accomplished with Vectastain ABC-AP kit and Vector Blue substrate kit (Vector Laboratories).
Statistical analysis
Grafts survivals were calculated using the Kaplan-Meier method, with comparisons among groups using the log-rank test. Student’s t test and ANOVA were used for continuous variables. A P value < 0.05 was considered statistical significance. All statistical analysis was performed using SPSS 16.0 software (IBM Company, Chicago, IL).
RESULTS
ECDI-SPs in combination with short-term rapamycin lead to indefinite cardiac allograft survival across full MHC mismatched barrier
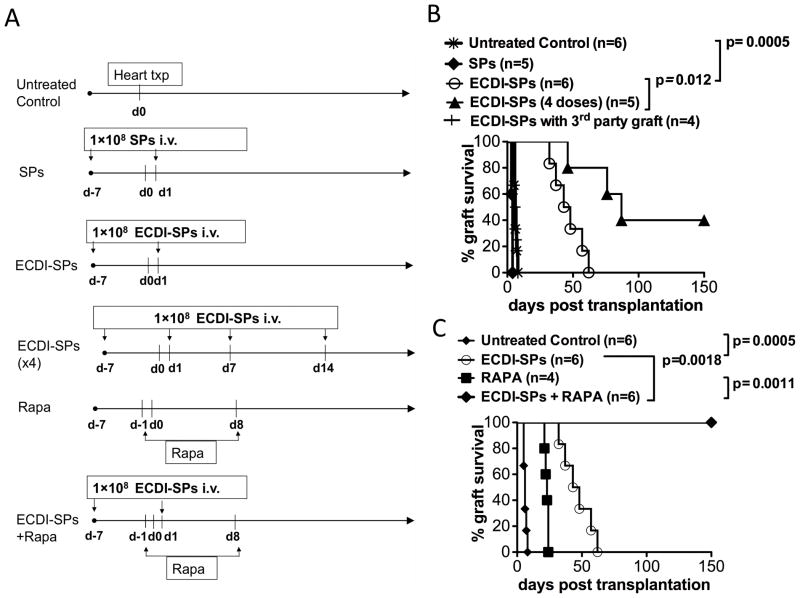
To test whether donor ECDI-SPs therapy was efficacious in protecting full MHC mismatched cardiac allografts, 1×108 BALB/c ECDI-SPs were injected intravenously into B6 recipients 7 days before (day −7) and 1 day after (day +1) heterotopic cardiac transplantation of BALB/c heart grafts (scheme shown in Fig. 1A). This treatment regimen significantly increased graft survival (Fig. 1B, mean graft survival time (MST)=45 days vs. control=9 days, P=0.0005). When two additional doses of BALB/c ECDI-SPs were given on day +7 and day +14, cardiac allograft survival was further increased (Fig. 1B, MST=85 days) with 40% recipients achieving graft functioning > 150 days. As expected, infusion of non-ECDI treated BALB/c SPs sensitized the recipients and led to accelerated graft rejection (MST=5 days). Protection by donor ECDI-SPs was donor-specific, as BALB/c (H-2d) ECDI-SPs did not protect cardiac allografts from SJL donors (H-2s) (Fig. 1B).
FIG. 1. Donor ECDI-SPs significantly prolong cardiac allograft survival, and in combination with rapamycin promote permanent cardiac allograft function.
Heterotopic heart transplant was performed using BALB/c as donors and B6 as recipients. SJL mice were used as donor for third party cardiac grafts. A: Timeline of treatment for different experimental groups. SPs: untreated donor splenocytes; ECDI-SPs: ECDI treated donor splenocytes; Rapa: rapamycin. B: Donor ECDI-SPs treatment alone prolongs cardiac allograft survival in a dose-dependent and donor-specific fashion. C: Donor ECDI-SPs treatment in combination with rapamycin allows permanent protection of cardiac allografts.
Cardiac allografts carry a significant number of passenger leukocytes which may prime donor reactive T cells via direct antigen presentation. Since ECDI-SPs mainly target the indirect antigen presentation pathway via cross-presentation by recipient APCs uptaking apoptotic ECDI-SPs (20), we hypothesized that using a pharmacological agent such as rapamycin to transiently suppress donor reactive T cells peri-transplant could synergistically enhance graft protection provided by ECDI-SPs. To test this hypothesis, we treated recipient mice with a short course of rapamycin (from day −1 to day +8). As shown in Fig. 1C, rapamycin treatment alone only transiently prolonged graft survival (MST=22 days). However, when combined with ECDI-SPs (2 doses, on days −7 and +1), the combination therapy led to long-term graft survival (>150 days) in 100% of the recipients.
Cardiac allograft protection is associated with accumulation of intra-graft IDO expressing cells
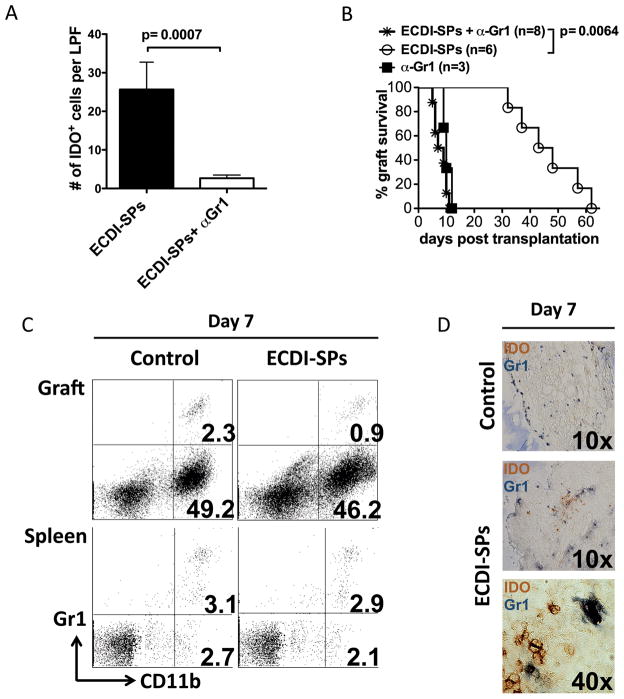
In histological profiling of cardiac infiltrating cells, we detected large numbers of cells in the cardiac allografts from ECDI-SPs treated or ECDI-SPs + rapamycin treated recipients that express the tryptophan metabolizing enzyme indoleamine 2,3-dioxygenase (IDO) early post transplant (day 7) (Fig. 2A). The presence of these cells in the protected cardiac grafts appeared to be associated with ECDI-SPs treatment, rather than rapamycin treatment, as there were very few IDO+ cells in grafts from rapamycin only treated recipients as in untreated control recipients (Fig. 2A). A trend of increase of IDO+ cells was also observed in the spleen of ECDI-SPs treated mice compared with that of control mice (Fig. 2B). To determine the importance of IDO expressing cells in the protection of cardiac allografts, we treated recipients with the IDO inhibitor 1-MT in conjunction with ECDI-SPs treatment. As shown in Fig. 2C, treatment with 1-MT abolished graft tolerance seen in the ECDI-SPs + rapamycin group (MST>150 days to MST=23 days, P = 0.0007), or graft prolongation seen in the ECDI-SPs alone group (MST=45 days to MST=10 days, P = 0.0018). These data confirmed the critical role of IDO in cardiac allograft protection induced by ECDI-SPs.
FIG. 2. Graft protection by ECDI-SPs treatment is concomitant with intra-graft IDO-expressing cells.
A: Graft histology showing immunohistochemical staining of IDO+ cells. Magnification: x10. Data shown is representative of at least three cardiac grafts obtained and sectioned from each treatment group on post transplant day 7. B: Spleen histology showing immunohistochemical staining of IDO+ cells. Magnification: x20. Data shown is representative of two spleens obtained and sectioned from each treatment group on post transplant day 7. Bar graphs show average cell numbers per high power field counted from 10–12 different sections of two different spleens from each group. C: Effect of the IDO inhibitor 1-MT on cardiac graft protection by ECDI-SPs treatment alone or ECDI-SPs treatment in combination with rapamycin. 1-MT was given at 10 mg/day i.p. from day −7 to day +7 as described in Materials and Methods. D: Dual staining of cardiac grafts with CD11b (blue) and IDO (brown). Magnification: x100. Data shown is from a cardiac graft from the ECDI-SPs alone treatment group, but is representative of 3 cardiac grafts each obtained and sectioned from the ECDI-SPs alone or ECDI-SPs + rapamycin treatment groups.
Depletion of Gr1+ cells diminishes intra-graft IDO expressing cells and abolishes cardiac allograft protection by ECDI-SPs
We next attempted to co-localize IDO expression with a number of cell surface markers to identify the cell population producing IDO in the allografts. Among tested markers, CD11c, F4/80, B220 or CD8 did not co-localize with IDO staining (data not shown), suggesting that the source of IDO was not classic dendritic cells, macrophages or B cells. Interestingly, all IDO staining strongly co-localized with CD11b (Fig. 2D), suggesting that myeloid cells among all infiltrating cells are the source of IDO production. Of note, not all CD11b+ cells were positive for IDO (Fig. 2D). As Gr1+ myeloid derived suppressor cells have been previously implicated in the production of IDO (4–7), we next examined whether Gr1+ cells played a role in graft protection provided by ECDI-SPs treatment. We used an anti-Gr1 mAb (anti-mLy-6G, clone RB6-8C5) to deplete Gr1+ cells in recipients treated with ECDI-SPs prior to receiving cardiac allografts. Cardiac grafts from these recipients had markedly diminished intra-graft IDO+ cells (Fig. 3A). Furthermore, anti-Gr1 mAb completely abolished the graft protection provided by ECDI-SPs treatment such that all grafts were rejected by day 11 (Fig. 3B). To further characterize the relationship between Gr1+ cells and CD11b+ or IDO+ cells, we analyzed cardiac infiltrating cells for Gr1/CD11b and Gr1/IDO co-expression. As shown in Fig. 3C, on day 7 post transplantation, among cardiac infiltrating cells, only 0.9–2.3% were CD11b+Gr1+ cells whereas ~45–50% were CD11b+Gr1− cells, confirming that the majority of CD11b+ cells in the heart at that time point were not Gr1+. This is in sharp contrast to the spleen from the same recipients (on day 7 post transplantation) in which the percentages of the CD11b+Gr1+ and CD11b+Gr1− cells were similar. Furthermore, co-staining of Gr1 and IDO by immunohistochemistry revealed that IDO and Gr1 staining did not co-localize (Fig. 3D). Collectively, our findings suggest that while the Gr1+ cells themselves do not produce IDO, they mediate the induction of intra-graft IDO expression by CD11b+ cells, and consequently play a critical role in the graft protection provided by ECDI-SPs treatment.
FIG. 3. Depletion of Gr1+ cells diminishes intra-graft IDO-expressing cells and abrogates the graft protection provided by ECDI-SPs treatment.
200 μg of anti-Gr1 mAb (anti-mLy-6G, clone RB6-8C5) was given i.p. on day −8, and additionally 100 μg on days −7, −3, −1, and +1. ECDI-SPs were given on day −7 and day +1. Cardiac transplant was performed on day 0. A: Heart grafts were retrieved on day 7, sectioned and stained for IDO as in FIG. 2A. IDO+ cells were enumerated from 8 sections under low power view by 2 different individuals. Results were averaged and presented as the bar graph shown. B: Effect of anti-Gr1 mAb on cardiac graft protection by ECDI-SPs treatment. Graft survival in groups treated with anti-Gr1 mAb alone or ECDI-SPs alone was shown for comparison. C: FACS analysis of cells from cardiac grafts (top panels) or the spleen (lower panels) from control vs. ECDI-SPs treated recipients (day 7 post transplantation). Plots were gated on live cells. Data presented is representative of 4 mice examined from each group in two different experiments. D: Dual staining of day 7 cardiac grafts with Gr1 (dark blue) and IDO (brown). Top panel: control graft; middle and lower panels: ECDI-SPs graft. Magnifications are shown on the micrographs.
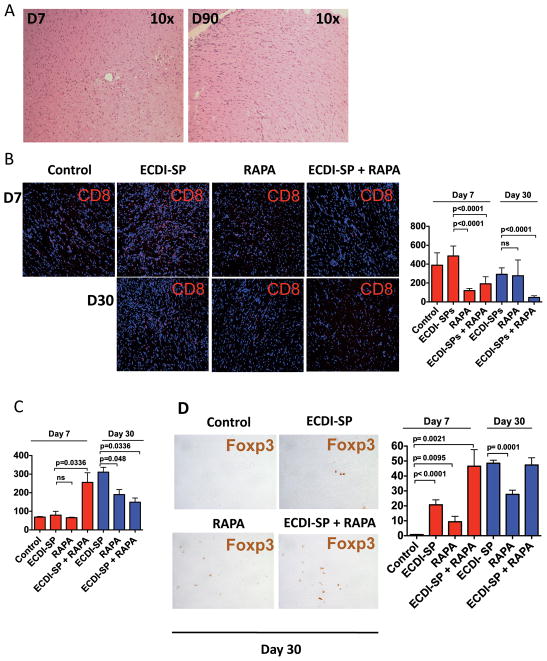
Cardiac allograft protection is associated with markedly diminished early and late graft CD8+ cell infiltration, but enhanced presence of graft Foxp3+ cells
Long-term protected cardiac grafts from ECDI-SPs + rapamycin treated recipients were examined histologically. As shown representatively in Fig. 4A, protected cardiac allografts showed intact cardiac tissues with minimal infiltrates at day 7 and minimal fibrosis at day 90. We further examined the lymphocyte populations infiltrating the cardiac allograft from groups of mice that were untreated (control), ECDI-SPs only treated, rapamycin only treated, or ECDI-SPs + rapamycin treated during early time points (day 7 and day 30). We noted that rapamycin treatment, alone or in combination with ECDI-SPs, significantly decreased the number of infiltrating CD8 cells at day 7, and this decrease persisted in ECDI-SPs + rapamycin treated recipients at day 30 (Fig. 4B). In contrast, the number of infiltrating CD4 cells at day 7 was not significantly affected by either ECDI-SPs or rapamycin treatment (Fig. 4C), and may even be increased with the combination treatment with ECDI-SPs + rapamycin. At day 30, there appeared to be an overall increase of CD4 cells in all groups compared with day 7 with the exception of the ECDI-SPs + rapamycin group. Interestingly, among the infiltrating lymphocytes at day 7 and day 30, there was an increased presence of Foxp3+ cells, most significantly in grafts from ECDI-SPs treated or ECDI-SPs + rapamycin treated recipients, and to a lesser extent in grafts from rapamycin only treated recipients (Fig. 4D). Therefore, ECDI-SPs and rapamycin appear to differentially regulate infiltrating cells in that rapamycin primarily reduces infiltrating CD8+ cells whereas ECDI-SPs preferentially increase infiltrating Foxp3+ cells. Together, these effects synergistically result in a favorable regulatory T cells to effector cell ratio that correlated with superior graft outcome.
FIG. 4. Protected cardiac grafts show intact histology, diminished graft infiltrating CD8+ cells, but increased graft infiltrating CD4+Foxp3+ cells.
A: A representative day 7 and day 90 cardiac graft retrieved from ECDI-SPs + rapamycin treated recipients. Grafts were stained by hematoxylin and eosin. For B – D, cardiac grafts from different treatment groups and control were retrieved on the indicated days post transplant, sectioned and stained for CD8 in B, CD4 in C and Foxp3 in D. Bar graphs show average cell numbers per low power field counted by two different individuals from 12 – 18 different sections from 3–4 different cardiac grafts from each group. Magnifications: A and B: x10; D: x40.
Cardiac allograft protection is associated with altered anti-donor cellular and humoral responses
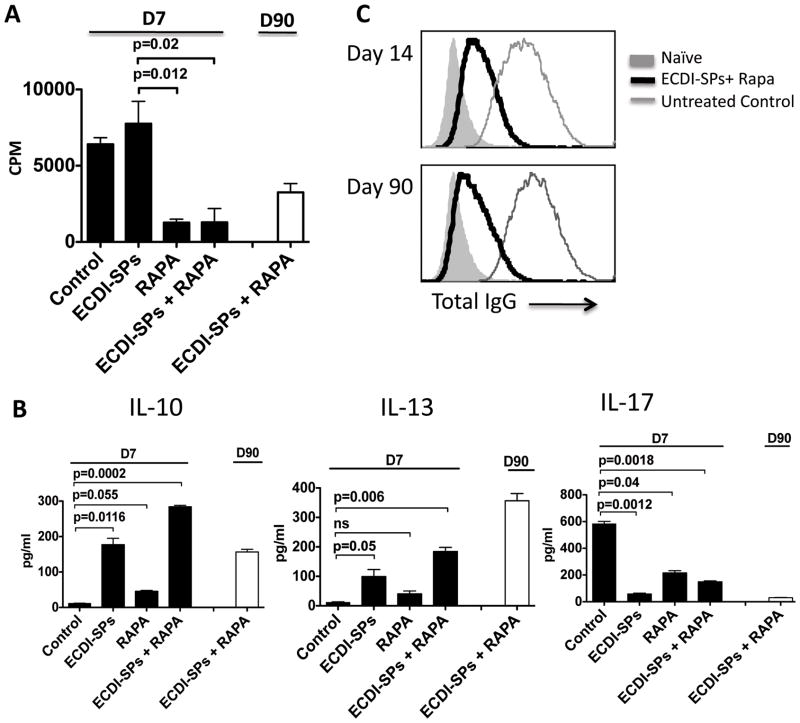
Anti-donor cellular and humoral responses were next measured. In vitro re-stimulation by MLRs were set up using donor (BALB/c) splenocytes as stimulators and T cells from recipient spleens on post-transplant day 7 as responders. As shown in Fig. 5A, T cells from recipients treated with rapamycin alone or rapamycin in combination with ECDI-SPs showed markedly diminished proliferation responses to BALB/c stimulation. In contrast, T cells from ECDI-SPs alone treated recipients showed comparable proliferations to BALB/c stimulation as those from untreated recipients. This finding is different from our previous data in allogeneic islet transplant models in which ECDI-SPs alone was sufficient to decrease recipient T cell proliferation responses to direct donor stimulation (17), and may be consistent with a heightened direct priming by passenger leukocytes from cardiac allografts than from islet allografts. MLR using splenic T cells isolated on day 90 from the ECDI-SPs + rapamycin treated recipients, the only group that still had functioning grafts, continued to show blunted proliferation (Fig. 5A).
FIG. 5. Cardiac allograft protection is associated with altered anti-donor cellular and humoral responses.
For A and B, in vitro re-stimulation by MLRs were set up using BALB/c splenocytes as stimulators and splenic T cells from different treatment groups and control (on day 7 and/or day 90 post transplant) as responders. A: Proliferation was measured by 3H-thymidine uptake. B: Cytokine production after in vitro re-stimulation was measured by multiplex cytokine assay kit as detailed in Materials and Methods. C: Serum total anti-donor IgG was measured on day 14 and day 90 from recipients treated with ECDI-SPs + rapamycin or control untreated recipients. Results shown in A and B are representative of 3 independent experiments. Results shown in C are representative of serum samples tested from 6 mice in each group.
We next examined the T cell cytokine production after in vitro re-stimulations. Splenic T cells from recipients of various groups were harvested on day 7 and stimulated in vitro with donor APCs as above. Similarly, we also used splenic T cells isolated on day 90 from the ECDI-SPs + rapamycin treated recipients and measured cytokine production after in vitro re-stimulations for comparison. At day 7, elevated levels of IL-10 and IL-13 were produced by T cells from ECDI-SPs alone treated recipients, and the levels were further increased in ECDI-SPs + rapamycin treated recipients (Fig. 5B). Rapamycin alone treated recipients, however, did not show enhanced production of either cytokine. Interestingly, at day 90, ECDI-SPs + rapamycin treated recipients continued to show sustained elevation of these cytokines (Fig. 5B), whereas ECDI-SPs alone treated recipients showed significant decline of these cytokines (data not shown) concurrent with loss of graft function in that group. Production of the inflammatory cytokine IL-17 was suppressed in all treated groups compared with the untreated group at day 7, and remained suppressed at day 90 (Fig. 5B).
Finally, anti-donor antibodies (total IgG) were measured from day 14 and day 90 serum samples. Serum from naïve mice was used as the negative control. As shown in Fig. 5C, untreated controls exhibited elevated anti-donor IgG antibodies as early as day 14 and persisted at day 90 (thin grey lines). In contrast, ECDI-SPs + rapamycin treated recipients showed markedly diminished, albeit still detectable, production of anti-donor IgG at both time points (thick black lines).
Inhibition of IDO diminishes regulatory cytokine production, and abolishes suppression of anti-donor proliferation and antibody responses
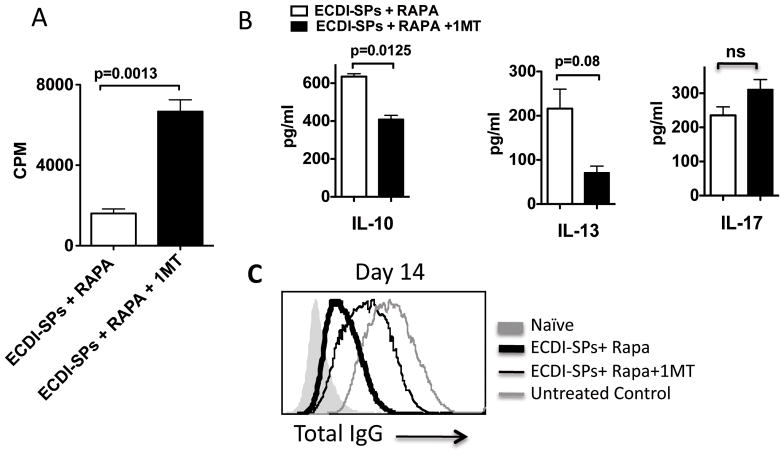
We next examined the effect of IDO inhibition on T cell proliferation, cytokine production and host anti-donor antibody production. Consistent with the shortened graft survival in ECDI-SPs + rapamycin tolerized recipients treated with 1-MT (Fig. 2C), splenic T cells from such recipients showed recovered donor-stimulated proliferation (Fig. 6A), diminished production of IL-10 (Fig. 6B) after in vitro re-stimulations, and increased production of anti-donor IgG antibodies in sera (thin black line, Fig. 6C), all compared with ECDI-SPs + rapamycin tolerized recipients without 1-MT treatment. There was also a trend towards diminished production of IL-13 (Fig. 6B). These findings suggest that IDO activity directly contributes to the diminished anti-donor proliferation and antibody responses, and to the enhanced expression of regulatory cytokines seen in tolerized recipients.
FIG. 6. Inhibition of IDO abolishes suppression of anti-donor proliferation and antibody responses.
For A and B, in vitro re-stimulation by MLRs were set up using BALB/c splenocytes as stimulators and splenic T cells from recipients treated with ECDI-SPs + rapamycin with or without 1-MT treatment (all on day 7 post transplant) as responders. A: Proliferation was measured by 3H-thumidine uptake. B: Cytokine production after in vitro re-stimulation was measured by multiplex cytokine assay kit as detailed in Materials and Methods. C: Serum total anti-donor IgG was measured on day 14 from recipients treated with ECDI-SPs + rapamycin with or without 1-MT treatment, and compared with that from control untreated recipients. Results shown in A and B are representative of 3 independent experiments. Results shown in C are representative of serum samples tested from 5–6 mice in each group.
DISCUSSION
In this study, we show that donor ECDI-SPs significantly prolongs full MHC-mismatched cardiac allograft survival, and in combination with a short course of rapamycin results in indefinite cardiac allograft survival in 100% of recipients. Mechanistically, ECDI-SPs treatment induces intra-graft accumulation of IDO+ cells and Foxp3+ cells as well as enhanced T cell production of IL-10 and IL-13. On the other hand, rapamycin significantly diminishes graft infiltrating CD8+ T cells with a concurrent small increase in Foxp3+ cells (Fig. 4). Consequently, combination of these two therapies creates a favorable graft environment that synergistically results in the indefinite survival of the cardiac allograft.
In our studies, pharmacologic inhibition of IDO results in graft rejection, confirming the obligatory role of IDO in the graft protection provided by ECDI-SPs. Co-localization studies reveal that CD11b+ cells are the cell type for IDO production. IDO is a tryptophan metabolizing enzyme reported to play an important role in tolerance induction in various models, including cardiac allograft models (8–11). It can mediate effector T cell apoptosis and induce regulatory T cell differentiation, and is expressed by cells of hematopoietic origin (21) as well as of non-hematopoietic origin, such as endothelial cells and epithelial cells (22, 23). Interestingly, in previous reports of cardiac transplantation in which IDO was shown to play a critical role in graft protection, the expression of IDO was confined to endothelial cells of the vascularized heart graft (12), different from that seen here. Our initial studies reveal that the presence of the CD11b+IDO+ population in the graft is dependent on Gr1+ cells, although the Gr1+ cells themselves are not the source of IDO production (Fig. 3). Consequently, depletion of Gr1+ cells leads to a significant decrease of intra-graft CD11b+IDO+ cells and concurrent loss of graft protection by ECDI-SPs. Cell populations that express Gr1 include neutrophils, granulocytes and myeloid-derived suppressor cells (MDSCs). Recent work in murine cardiac and kidney transplant models show that Gr1+ MDSCs accumulate in allografts, suppress effector T cells and induce tolerance via production of iNOS or IFN-γ (2, 3). Interestingly, a cross-talk between iNOS and IDO has been previously described: iNOS produces large amounts of nitric oxide (NO), which inhibits IDO production but may also be consumed in the process (24, 25). Reciprocally, a product from the IDO pathway, 3-hydroxyanthranilic acid (3-HAA), can negatively feed back to inhibit iNOS activity (26). Limited by the detection sensitivity of immunohistochemical analysis of the cardiac allografts, the nature of these CD11b+IDO+ cells was not completely determined in the present study. It remains possible that these are dendritic cells with a low level of CD11c expression or macrophages with a low level of F4/80 expression that is below detection threshold by our histological analysis. Further analysis of this cell population by cell pooling and sorting and determination of mechanism for induction and accumulation of the CD11b+IDO+ population in the protected cardiac allograft by ECDI-SPs are currently under investigation.
Heightened intra-graft Foxp3+ cells and IL-10 production are observed in ECDI-SPs treated recipients (Fig. 4D and Fig. 5B). In rodent models of cardiac allograft transplantation, intra-graft Foxp3+ regulatory T cells have been shown to cluster around IDO-expressing endothelial cells (27, 28), suggesting an intimate cell-cell interaction between these two populations. Indeed, depletion of regulatory T cells resulted in a decrease in systemic IDO production, and reciprocally Foxp3+ cells were not detected in tolerogen-treated IDO−/− recipients (27). Other studies have also shown that IDO expression in CD11c+ DCs, in particular plasmacytoid DCs, plays an important role in the generation of Foxp3+ regulatory T cells and suppression of self- and allo-reactive cells (29, 30). MDSCs have also been shown to differentiate and expand regulatory T cells in mouse and man (31–33). One possible mechanism of IDO-dependent induction of Foxp3+ cells is by activation of the aryl hydrocarbon receptor (AhR), a receptor shown to be important in the balance of regulatory T cell and Th17 differentiation (34). Kynurenine, the first breakdown product of IDO-mediated tryptophan degradation, could activate AhR leading to AhR dependent regulatory T cell induction (35, 36). Moreover, in an islet transplantation model, activation of AhR using a low molecular weight AhR agonist leads to direct induction of Foxp3+ cells, as well as indirect induction of these cells by tolerogenic DCs, concomitant with islet allograft protection (37). Similarly, IL-10 producing Tr1 cells have also been shown to be induced by AhR signaling in mouse (38, 39) and in man (36). This could also be accomplished directly (38, 39) or indirectly via DCs with altered immunogenicity (40). Our findings of heightened intra-graft Foxp3+ cells and IL-10 production seen in the context of induction of CD11b+IDO+ cells are consistent with these previous studies. The definitive role of AhR in the induction of Foxp3+ T cell and IL-10 production seen in ECDI-SPs awaits further studies.
In our studies, IL-13 was another cytokine observed to be elevated in recipients treated with ECDI-SPs (Fig. 5B), and this elevation has a trend to be dampened by IDO inhibition (Fig. 6B). Previous studies have implicated a role for IL-13 in neonatal tolerance (41) and in transplant tolerance in rodent models of cardiac and skin transplantation (42–44). Up-regulation of IL-13 has been observed by forced over-expression of CD200, a member of the Ig superfamily (43), while downstream mediators of IL-13 effects include heme oxygenase 1 (44), iNOS and IL-12 (42). A definitive role of IL-13 in the graft protection provided by ECDI-SPs awaits experiments using IL-13 deficient recipients and/or neutralizing antibodies.
In summary, our studies show that ECDI-SPs plus a short course of rapamycin induce indefinite donor-specific cardiac graft protection mediated by intra-graft CD11b+IDO+ cells and elaboration of regulatory cytokines. Tolerance induction by ECDI-SPs infusions in cardiac transplantation thus provides a unique model to further study the biology and function of this cell population that may ultimately assist the design of therapeutic strategies for tolerance induction in clinical cardiac transplantation.
Acknowledgments
This work was supported in part by the National Institute of Health (NIH) Career Award 1K08DK070029-01 (X.L.), Type 1 Diabetes Pathfinder Award DP2DK083099-01 (X.L.), and the Juvenile Diabetes Research Foundation Post-doctoral Fellowship Award 3-2010-447 (T.K.).
ABREVIATIONS
- ECDI
1-ethyl-3-(3′-dimethylaminopropyl)-carbodiimide
- SP
splenocytes
- IDO
indoleamine 2,3 dioxygenase
- MDSC
myeloid-derived suppressor cells
- IFN-γ
interferon gamma
- 1-MT
1-methyl-d-tryptophan
- MST
mean graft survival time
- MLRs
mixed lymphocyte reactions
- AhR
aryl hydrocarbon receptor
Footnotes
AUTHORSHIP
Contribution: G.C., T.K., J.B., Z.Z., X.L. designed research; G.C., T.K., J.B., S.W., J.T., J.W. performed experiments; G.C., T.K., J.B., J.T., Z.Z., X.L. analyzed data; G.C., T.K., J.B., Z.Z., X.L. wrote the manuscript.
CONFLICT OF INTEREST DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation--how much of the promise has been realized? Nat Med. 2005 Jun;11(6):605–13. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 2.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008 Jun 15;180(12):7898–906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 3.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010 Jul 1;120(7):2486–96. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jia W, Jackson-Cook C, Graf MR. Tumor-infiltrating, myeloid-derived suppressor cells inhibit T cell activity by nitric oxide production in an intracranial rat glioma + vaccination model. J Neuroimmunol. 2010 Jun;223(1–2):20–30. doi: 10.1016/j.jneuroim.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011 Nov 17;118(20):5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012 Apr;12(4):253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baban B, Chandler PR, Johnson BA, 3rd, Huang L, Li M, Sharpe ML, et al. Physiologic control of IDO competence in splenic dendritic cells. J Immunol. 2011 Sep 1;187(5):2329–35. doi: 10.4049/jimmunol.1100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010 Jul-Aug;16(4):354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood. 2009 Oct 8;114(15):3235–43. doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- 10.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010 Dec 27;90(12):1312–20. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 11.Li XL, Menoret S, Bezie S, Caron L, Chabannes D, Hill M, et al. Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J Immunol. Jul 15;185(2):823–33. doi: 10.4049/jimmunol.1000120. [DOI] [PubMed] [Google Scholar]
- 12.Guillonneau C, Hill M, Hubert FX, Chiffoleau E, Herve C, Li XL, et al. CD40Ig treatment results in allograft acceptance mediated by CD8CD45RC T cells, IFN-gamma, and indoleamine 2,3-dioxygenase. J Clin Invest. 2007 Apr;117(4):1096–106. doi: 10.1172/JCI28801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turley DM, Miller SD. Peripheral tolerance induction using ethylenecarbodiimide-fixed APCs uses both direct and indirect mechanisms of antigen presentation for prevention of experimental autoimmune encephalomyelitis. J Immunol. 2007 Feb 15;178(4):2212–20. doi: 10.4049/jimmunol.178.4.2212. [DOI] [PubMed] [Google Scholar]
- 14.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007 Sep;7(9):665–77. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MK, Tan LJ, Dal Canto MC, Miller SD. Regulation of the effector stages of experimental autoimmune encephalomyelitis via neuroantigen-specific tolerance induction. J Immunol. 1990 Jul 1;145(1):117–26. [PubMed] [Google Scholar]
- 16.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006 Nov 27;203(12):2737–47. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14527–32. doi: 10.1073/pnas.0805204105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko K, Morelli AE, Wang Z, Thomson AW. Alloantigen presentation by ethylcarbodiimide-treated dendritic cells induces T cell hyporesponsiveness, and prolongs organ graft survival. Clin Immunol. 2003 Sep;108(3):190–8. doi: 10.1016/s1521-6616(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Zhu L, Quan D, Garcia B, Ozcay N, Duff J, et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 1996 Nov 15;62(9):1267–72. doi: 10.1097/00007890-199611150-00016. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 20.Getts DR, Turley DM, Smith CE, Harp CT, McCarthy D, Feeney EM, et al. Tolerance induced by apoptotic antigen-coupled leukocytes is induced by PD-L1+ and IL-10-producing splenic macrophages and maintained by T regulatory cells. J Immunol. 2011 Sep 1;187(5):2405–17. doi: 10.4049/jimmunol.1004175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W. IDO: more than an enzyme. Nat Immunol. 2011 Sep;12(9):809–11. doi: 10.1038/ni.2088. [DOI] [PubMed] [Google Scholar]
- 22.Blaschitz A, Gauster M, Fuchs D, Lang I, Maschke P, Ulrich D, et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS One. 2011;6(7):e21774. doi: 10.1371/journal.pone.0021774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibana JA, Belland RJ, Zea AH, Schust DJ, Nagamatsu T, AbdelRahman YM, et al. Inhibition of indoleamine 2,3-dioxygenase activity by levo-1-methyl tryptophan blocks gamma interferon-induced Chlamydia trachomatis persistence in human epithelial cells. Infect Immun. 2011 Nov;79(11):4425–37. doi: 10.1128/IAI.05659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh GS, Pae HO, Choi BM, Chae SC, Lee HS, Ryu DG, et al. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem Biophys Res Commun. 2004 Aug 6;320(4):1156–62. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- 25.Ohtaki H, Ito H, Ando K, Ishikawa T, Hoshi M, Tanaka R, et al. Interaction between LPS-induced NO production and IDO activity in mouse peritoneal cells in the presence of activated Valpha14 NKT cells. Biochem Biophys Res Commun. 2009 Nov 13;389(2):229–34. doi: 10.1016/j.bbrc.2009.08.120. [DOI] [PubMed] [Google Scholar]
- 26.Samelson-Jones BJ, Yeh SR. Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry. 2006 Jul 18;45(28):8527–38. doi: 10.1021/bi060143j. [DOI] [PubMed] [Google Scholar]
- 27.Sucher R, Fischler K, Oberhuber R, Kronberger I, Margreiter C, Ollinger R, et al. IDO and Regulatory T Cell Support Are Critical for Cytotoxic T Lymphocyte-Associated Ag-4 Ig-Mediated Long-Term Solid Organ Allograft Survival. J Immunol. 2012 Jan 1;188(1):37–46. doi: 10.4049/jimmunol.1002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thebault P, Condamine T, Heslan M, Hill M, Bernard I, Saoudi A, et al. Role of IFNgamma in allograft tolerance mediated by CD4+CD25+ regulatory T cells by induction of IDO in endothelial cells. Am J Transplant. 2007 Nov;7(11):2472–82. doi: 10.1111/j.1600-6143.2007.01960.x. [DOI] [PubMed] [Google Scholar]
- 29.Gehrie E, Van der Touw W, Bromberg JS, Ochando JC. Plasmacytoid dendritic cells in tolerance. Methods Mol Biol. 2011;677:127–47. doi: 10.1007/978-1-60761-869-0_9. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trabanelli S, Ocadlikova D, Evangelisti C, Parisi S, Curti A. Induction of regulatory T Cells by dendritic cells through indoleamine 2,3-dioxygenase: a potent mechanism of acquired peripheral tolerance. Current medicinal chemistry. 2011;18(15):2234–9. doi: 10.2174/092986711795656054. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 31.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008 Jul 1;68(13):5439–49. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011 Jun 16;117(24):6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 33.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010 Jan 1;70(1):99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008 May 1;453(7191):65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 35.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010 Sep 15;185(6):3190–8. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010 Sep;11(9):846–53. doi: 10.1038/ni.1915. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauben E, Gregori S, Draghici E, Migliavacca B, Olivieri S, Woisetschlager M, et al. Activation of the aryl hydrocarbon receptor promotes allograft-specific tolerance through direct and dendritic cell-mediated effects on regulatory T cells. Blood. 2008 Aug 15;112(4):1214–22. doi: 10.1182/blood-2007-08-109843. [DOI] [PubMed] [Google Scholar]
- 38.Wu HY, Quintana FJ, da Cunha AP, Dake BT, Koeglsperger T, Starossom SC, et al. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One. 2011;6(8):e23618. doi: 10.1371/journal.pone.0023618. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010 Sep;11(9):854–61. doi: 10.1038/ni.1912. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19961–6. doi: 10.1073/pnas.1014465107. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue Y, Konieczny BT, Wagener ME, McKenzie AN, Lakkis FG. Failure to induce neonatal tolerance in mice that lack both IL-4 and IL-13 but not in those that lack IL-4 alone. J Immunol. 2001 Jul 15;167(2):1125–8. doi: 10.4049/jimmunol.167.2.1125. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 42.Davidson C, Verma ND, Robinson CM, Plain KM, Tran GT, Hodgkinson SJ, et al. IL-13 prolongs allograft survival: association with inhibition of macrophage cytokine activation. Transplant immunology. 2007 Apr;17(3):178–86. doi: 10.1016/j.trim.2006.09.035. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 43.Gorczynski RM, Chen Z, He W, Khatri I, Sun Y, Yu K, et al. Expression of a CD200 transgene is necessary for induction but not maintenance of tolerance to cardiac and skin allografts. J Immunol. 2009 Aug 1;183(3):1560–8. doi: 10.4049/jimmunol.0900200. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 44.Ke B, Shen XD, Zhai Y, Gao F, Busuttil RW, Volk HD, et al. Heme oxygenase 1 mediates the immunomodulatory and antiapoptotic effects of interleukin 13 gene therapy in vivo and in vitro. Human gene therapy. 2002 Oct 10;13(15):1845–57. doi: 10.1089/104303402760372945. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]