Abstract

The efficient synthesis of enantiopure, trisubstituted cryptophane-A derivatives, organic host molecules with unusually high xenon affinity is reported. Synthesis and chromatographic separation of (+/−) tri-Mosher’s acid-substituted cryptophane diastereomers gave ready access to the enantiopure cryptophanes, which are critical components in the design of enantiomerically pure 129Xe biosensors. Hyperpolarized 129Xe NMR spectroscopy identified single resonances for both trisubstituted cryptophane diastereomers that were separated by 9.5 ppm. This highlights opportunities for using enantiopure xenon biosensors in the simultaneous detection of 129Xe in different biochemical environments.
The need for imaging agents and analytical tools that can report on the concentration and activity of various biomolecules in complex media has motivated the development of 129Xe NMR biosensors. 1 These agents have the potential to detect cancer and other diseases by localizing hyperpolarized (hp) 129Xe to a diseased tissue and/or by multiplexed detection of different protein biomarkers.1 To date, cryptophane-A organic cages, in which two cyclotriguaiacylene (CTG) units are connected by three ethylene oxide linkers, show the highest xenon binding affinity with dissociation constants ~25 μM at physiological temperature in aqueous solution.1,2 Functionalized 129Xe cryptophane biosensors can be targeted to different protein receptors, and identified by changes to the frequency of the bound 129Xe nucleus.3 The use of enantiopure cryptophanes is preferred over racemic mixtures, which have been shown to produce multiple, diastereomeric peaks upon binding to chiral protein surfaces. 4 Similarly complex hp 129Xe NMR spectra are observed when racemic cryptophanes are modified with chiral small molecules or peptides, based on diastereomeric splitting.5 For the sensitive detection of chiral biological analytes, enantiopure cryptophanes that offer well resolved “bound” and “free” 129Xe NMR peaks should offer substantial advantages. Enantiopure cryptophanes have also been employed for chiral recognition of small guests. 6 Here, we report a new method for producing enantiopure cryptophane for many different applications.
Until now, the resolution of chiral cryptophanes and hemicryptophanes has typically required expensive HPLC methods and yielded only small quantities of optically pure material.7 Another approach has been the synthesis of enantiopure cryptophanes from the optically pure CTG units, but one limitation is possible racemization of CTG during the subsequent synthetic steps.8 Recently, Dutasta and coworkers employed (−)-camphanic chloride as a chiral resolving agent to resolve mono-cryptophanol through separation of the resulting diastereomers. 9 The diastereomers were not separable by chromatography on silica gel or reversed-phase HPLC but crystallographic resolution has recently been improved to give both enantiomers in 25% yield. 10 However, this crystallographic method is time-consuming. The low yield of pure cryptophane diastereomers limits the production of enantiomerically pure cages for uses in xenon biosensors and host-guest chemistry, broadly defined.
Dutasta et al. previously demonstrated the chromatographic separation of tri-functionalized hemicryptophanes. 11 We hypothesized that a pair of cryptophane-A diastereomers substituted with three chiral auxiliaries would also result in a significant difference in polarity. Indeed, substitution with three chiral resolving groups allowed efficient separation and isolation of cryptophane diastereomers using silica gel column chromatography. Deprotection of the isolated diastereomers yielded the enantiopure trisubstituted cryptophanes, whose chemical and physical properties can be tuned at the three positions.
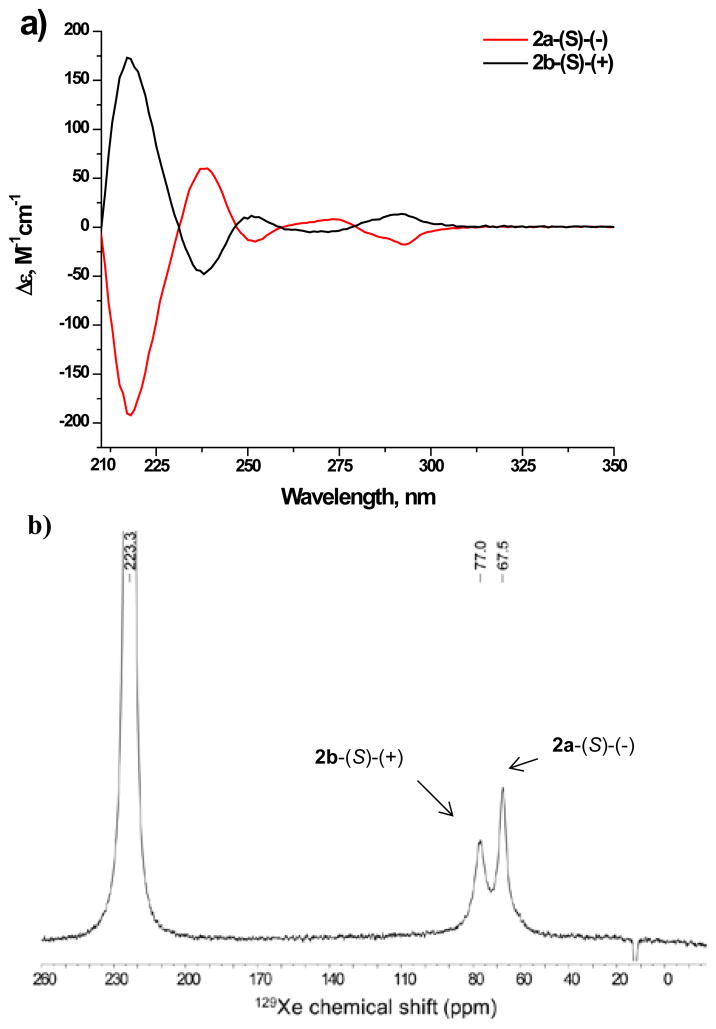
According to Scheme 1A, diastereomers 2a and 2b were synthesized from trihydroxy cryptophane 1, which was obtained by a previously published 6-step route. 12 Trihydroxy cryptophane 1 was reacted with 3.3 equiv (S)-Mosher’s acid in the presence of DMAP/Et3N. The Mosher’s acid moiety was chosen as a readily available and sterically bulky chiral resolving agent. The reaction proceeded relatively slowly and went to ~70% completion after stirring for 2 days at 70 °C in DMF solvent. The resulting cryptophane-A diastereomers 2a and 2b were successfully separated by column chromatography (silica gel, Et2O:CH2Cl2, 0.5:99.5, v/v) to give each enantiomer in 35% yield. Resolved diastereomers 2a-(S)-(−) and 2b-(S)-(+) were easily distinguished by 1H NMR spectroscopy (Scheme 1B), each showing four singlets with different chemical shift values for aromatic protons. In contrast, the aromatic region of the diastereomeric mixture exhibited eight singlets in the same region (Scheme 1B). The enantiopurity of the isolated diastereomers was confirmed by electronic circular dichroism (ECD) spectroscopy showing the same peaks with opposite sign (Fig. 1a).
Scheme 1.
(A)Synthesis of trisubstituted diastereomers of cryptophane-A from trihydroxy cryptophane 1. (B) Aromatic region of 1H NMR spectra of mixture of diastereomers, 2a and 2b.
Diastereomers 2a and 2b were isolated by column chromatography with diastereomeric excess ≥98%.
Figure 1.
(a) ECD spectra of diastereomers 2a and 2b (~0.5 mM) in 1,4-dioxane. (b) Hyperpolarized 129Xe NMR spectra of diastereomers 2a and 2b (~ 10 mM) in C2D2Cl4 at 299 ± 2 K.
The interaction between xenon and the trisubstituted cryptophane diastereomers 2a and 2b was investigated by hp 129Xe NMR spectroscopy in a non-intercalating organic solvent, 1,1,2,2-tetrachloroethane-d2 (C2D2Cl4). Hyperpolarized 129Xe was mixed with sample solution in an airtight NMR tube and spectra were taken quickly with 4 transients (Fig. 1b). Standarized by the signal from dissolved hp 129Xe in C2D2Cl4,13 hp 129Xe NMR chemical shifts for the isolated diasteromers 2a-(−) (67.5 ppm, Fig. S2a) and 2b-(+) (77.0 ppm, Fig. S2b) were recorded 9.5 ppm apart, which is the largest chemical shift difference reported for cryptophane diastereomers. Previously, for the mono-(−)-camphanic acid cryptophane diastereomers, a chemical shift difference of ~7 ppm was observed for the two diastereomers.14 Notably, for the camphanic acid derivative, the more downfield peak arose from the cryptophane-(−) diastereomer, whereas with three Mosher’s acids it was the cryptophane-(+) diastereomer. With a 1:1 mixture of diastereomers 2a and 2b, the two resonances are clearly resolvable (Fig. 2b) by hp 129Xe NMR spectroscopy.
The isolated cryptophane diastereomers are useful precursors for preparing various enantiopure functionalized cryptophanes. Removal of the Mosher moieties occurs without loss of optical activity. Diastereomers 2a and 2b were deprotected via basic hydrolysis at 70 °C, affording enantiopure trihydroxy cryptophanes 3a-(−) and 3b-(+) (Scheme 2). The recorded ECD spectra were mirror images (within experimental error) of each other, as expected for a pair of enantiomers (Fig. S1a). In the absence of an X-ray crystal structure for the isolated enantiomers, the structural assignment for the two enantiomers was made by reacting cryptophane 3b-(+) with methyl iodide to yield (+)-cryptophane-A. Its recorded ECD spectrum (Fig. S1c) was found to be opposite of the previously reported spectrum for (−)-cryptophane-A.15
Scheme 2.
Synthesis of enantiopure, trifunctionalized cryptophanes 3-(−), 3-(+), 4-(−) and 4-(+).
Similarly to (+)-cryptophane-A, various trisubstituted enantiopure cryptophane derivatives could be easily synthesized from trihydroxy cryptophane enantiomers 3a-(−) and 3b-(+). For example, reaction with excess propargyl bromide gave the enantiomerically pure tripropargyl cryptophanes 4a-(−) and 4b-(+) (Scheme 2, Fig. S1b). We previously showed that alkyl azides can react with tripropargyl cryptophane in nearly quantitative yields via the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction. 16 This route gave enantiopure tripropargyl cryptophanes 4a-(−) and 4b-(+), each in 15% overall yield starting from racemic trihydroxy cryptophane 1 (+/−).
In conclusion, an efficient synthesis of enantiopure trifunctionalized cryptophanes was developed using chromatographically resolved trisubstituted cryptophane diastereomers. ECD spectroscopy confirmed the expected chiroptical properties of the isolated diastereomeric and enantiomeric pairs. Hyperpolarized 129Xe NMR chemical shifts were recorded at 9.5 ppm apart for the cryptophane diastereomers. The potential for synthesizing gram-scale quantities of enantiomerically pure cryptophane would provide access to the various functionalized cryptophanes, precursors for various cryptophane-based enantiopure biosensors. Particularly, enantiopure Xe biosensors are desired to facilitate high-resolution X-ray crystallographic studies1b, 4b, 17 and to simplify the assignment of peaks in 129Xe NMR spectra.
Supplementary Material
Acknowledgments
I.J.D. appreciates support from the DOD (W81XWH-04-1-0657), NIH (CA110104 and GM097478), a Camille and Henry Dreyfus Teacher-Scholar Award, and UPenn Chemistry Department. We thank George Furst (Chemistry Department, the University of Pennsylvania) for NMR support.
Footnotes
Supporting Information Available: Experimental procedures and characterization data for all synthesized compounds, ECD and 129Xe NMR data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Dmochowski IJ, Taratula O. Curr Opin Chem Biol. 2010;14:97. doi: 10.1016/j.cbpa.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brotin T, Dutasta JP. Chem Rev. 2009;109:88. doi: 10.1021/cr0680437. [DOI] [PubMed] [Google Scholar]
- 2.Hill PA, Wei Q, Eckenhoff RG, Dmochowski IJ. J Am Chem Soc. 2007;129:9262. doi: 10.1021/ja072965p. [DOI] [PubMed] [Google Scholar]
- 3.(a) Berthault P, Roy V, Brotin T, Dutasta JP, Charles MH, Delair T, Mallet F, Huber G, Desvaux H, Boulard Y. ChemPhysChem. 2007;8:2082. doi: 10.1002/cphc.200700384. [DOI] [PubMed] [Google Scholar]; (b) Freund C, Schlundt A, Kilian W, Beyermann M, Sticht J, Gunther S, Hopner S, Falk K, Roetzschke O, Mitschang L. Angew Chem Int Ed. 2009;48:4142. doi: 10.1002/anie.200806149. [DOI] [PubMed] [Google Scholar]; (c) Pines A, Spence MM, Rubin SM, Dimitrov IE, Ruiz EJ, Wemmer DE, Yao SQ, Tian F, Schultz PG. Proc Natl Acad Sci USA. 2001;98:10654. doi: 10.1073/pnas.191368398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Spence MM, Ruiz EJ, Rubin SM, Lowery TJ, Winssinger N, Schultz PG, Wemmer DE, Pines A. J Am Chem Soc. 2004;126:15287. doi: 10.1021/ja0483037. [DOI] [PubMed] [Google Scholar]; (b) Chambers JM, Hill PA, Aaron JA, Han ZH, Christianson DW, Kuzma NN, Dmochowski IJ. J Am Chem Soc. 2009;131:563. doi: 10.1021/ja806092w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wei Q, Seward GK, Hill PA, Patton B, Dimitrov IE, Kuzma NN, Dmochowski IJ. J Am Chem Soc. 2006;128:13274. doi: 10.1021/ja0640501. [DOI] [PubMed] [Google Scholar]
- 5.Jameson CJ, Ruiz EJ, Sears DN, Pines A. J Am Chem Soc. 2006;128:16980. doi: 10.1021/ja066661z. [DOI] [PubMed] [Google Scholar]
- 6.(a) Canceill J, Lacombe L, Collet A. J Am Chem Soc. 1985;107:6993. [Google Scholar]; (b) Costante-Crassous J, Marrone TJ, Briggs JM, McCammon JA, Collet A. J Am Chem Soc. 1997;119:3818. [Google Scholar]; (c) Crassous J, Hediger S. J Phys Chem A. 2003;107:10233. [Google Scholar]; (d) Bouchet A, Brotin T, Linares M, Ågren H, Cavagnat D, Buffeteau T. J Org Chem. 2011;76:4178. doi: 10.1021/jo200519r. [DOI] [PubMed] [Google Scholar]; (e) Bouchet A, Brotin T, Linares M, Cavagnat D, Buffeteau T. J Org Chem. 2011;76:7816. doi: 10.1021/jo201167w. [DOI] [PubMed] [Google Scholar]
- 7.(a) Tambute A, Canceill J, Collet A. Bulletin Chem Soc Japan. 1989;62:1390. [Google Scholar]; (b) Perraud O, Raytchev PD, Martinez A, Dutasta JP. Chirality. 2010;22:885. doi: 10.1002/chir.20876. [DOI] [PubMed] [Google Scholar]
- 8.(a) Canceill J, Collet A, Gabard J, Gottarelli G, Spada GP. J Am Chem Soc. 1985;107:1299. [Google Scholar]; (b) Garcia C, Collet A. Bulletin Soc Chim Fr. 1995;132:52. [Google Scholar]
- 9.Brotin T, Barbe R, Darzac M, Dutasta JP. Chem Eur J. 2003;9:5784. doi: 10.1002/chem.200204614. [DOI] [PubMed] [Google Scholar]
- 10.Buffeteau T, Cavagnat D, Brotin T. J Org Chem. 2008;73:66. doi: 10.1021/jo701662w. [DOI] [PubMed] [Google Scholar]
- 11.Gautier A, Mulatier JC, Crassous J, Dutasta JP. Org Lett. 2005;7:1207. doi: 10.1021/ol047469+. [DOI] [PubMed] [Google Scholar]
- 12.Taratula O, Hill PA, Bai YB, Khan NS, Dmochowski IJ. Org Lett. 2011;13:1414. doi: 10.1021/ol200088f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartik K, Luhmer M, Dutasta JP, Collet A, Reisse J. J Am Chem Soc. 1998;120:784. [Google Scholar]
- 14.Huber JG, Dubois L, Desvaux H, Dutasta JP, Brotin T, Berthault P. J Phys Chem A. 2004;108:9608. [Google Scholar]
- 15.Canceill J, Collet A, Gottarelli G, Palmieri P. J Am Chem Soc. 1987;109:6454. [Google Scholar]
- 16.(a) Tornoe CW, Christensen C, Meldal MJ. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; (b) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.(a) Taratula O, Hill PA, Khan NS, Carroll PJ, Dmochowski I. J Nature Commun. 2010:148. doi: 10.1038/ncomms1151. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Roy V, Brotin T, Dutasta JP, Charles MH, Delair T, Mallet F, Huber G, Desvaux H, Boulard Y, Berthault P. ChemPhysChem. 2007;8:2082. doi: 10.1002/cphc.200700384. [DOI] [PubMed] [Google Scholar]; (c) Kotera N, Tassali N, Leonce E, Boutin C, Berthault P, Brotin T, Dutasta JP, Delacour L, Traore T, Buisson DA, Taran F, Coudert S, Rousseau B. Angew Chem Int Ed. 2012;51:4100. doi: 10.1002/anie.201109194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.