Abstract
Data suggest that cytokines released during the inflammatory response target subcortical structures including the basal ganglia as well as dopamine function to acutely induce behavioral changes that support fighting infection and wound healing. However, chronic inflammation and exposure to inflammatory cytokines appears to lead to persisting alterations in the basal ganglia and dopamine function reflected by anhedonia, fatigue, and psychomotor slowing. Moreover, reduced neural responses to hedonic reward, decreased dopamine metabolites in the cerebrospinal fluid and increased presynaptic dopamine uptake and decreased turnover have been described. This multiplicity of changes in the basal ganglia and dopamine function suggest fundamental effects of inflammatory cytokines on dopamine synthesis, packaging, release and/or reuptake, which may sabotage and circumvent the efficacy of current treatment approaches. Thus, examination of the mechanisms by which cytokines alter the basal ganglia and dopamine function will yield novel insights into the treatment of cytokine-induced behavioral changes and inflammatory malaise.
Keywords: inflammatory cytokines, interferon-alpha, inflammation, dopamine, tetrahydrobiopterin, kynurenine, oxidative stress, basal ganglia, fatigue, depression
1. Introduction
There has been increasing recognition that inflammatory cytokines play an important role in neuronal integrity and can exert profound effects on neurocircuitry and neurotransmitter systems in the brain, ultimately affecting behavior [86, 137, 222]. Accordingly, there has been mounting interest regarding the role of cytokines in behavioral alterations and the development and progression of neuropsychiatric disorders. Under physiologic conditions, cytokines such as TNF-alpha and IL-1 have been shown to be involved in a number of essential brain processes such as synaptic remodeling, neurogenesis and long-term potentiation [222]. However, in excess, inflammatory cytokines can act in the brain to affect monoamine neurotransmitter systems and behavior, and recent evidence indicates that dopamine function in the basal ganglia may be a primary target in this regard [32, 33, 86, 120, 206, 207]. The basal ganglia are key subcortical structures that regulate motivation and motor activity, and dopamine plays a essential modulatory role in basal ganglia function [73]. The effect of inflammatory cytokines on basal ganglia dopamine may be especially relevant to depression and fatigue as well as psychomotor disturbances and the development of neurodegenerative disorders. This review will highlight the current literature demonstrating the impact of peripheral inflammatory cytokines and local neuroinflammation on the basal ganglia and dopamine function and explore the potential mechanisms involved. In addition, cytokine interactions with other neurotransmitter systems as they relate to basal ganglia dopamine function will be described along with the implications of these findings for neuropsychiatric disease.
There is a vast literature in humans and laboratory animals describing the profound effects of inflammation and the release of inflammatory cytokines on the brain and behavior. In humans, inflammatory cytokines have been implicated in the development of behavioral disturbances including depression and fatigue in both medically ill and medically healthy individuals. For example, numerous studies have reported elevated cytokines and other inflammatory markers in depressed but otherwise healthy individuals [51, 123–125, 187], and patients exposed to increased inflammation during chronic illness experience considerably higher rates of depression and fatigue than the general population [221, 223, 224]. Evidence that inflammatory cytokines can cause behavioral alterations exists in numerous reports of the neuropsychiatric symptoms induced by chronic administration of the inflammatory cytokine, interferon (IFN)-alpha, used to treat certain cancers and viral infections. Indeed, IFN-alpha produces an array of behavioral disturbances, many of which are consistent with decreases in basal ganglia dopamine function including anhedonia, fatigue and psychomotor slowing [12, 26, 27, 126, 201]. Of note, selective serotonin reuptake inhibitors (SSRIs) have been shown to alleviate IFN-alpha-induced anxiety and some depressive symptoms. However, IFN-alpha-induced fatigue and psychomotor retardation are less responsive to SSRI therapy [27, 144, 166]. These data are consistent with findings in patients with advanced cancer undergoing chemotherapy, who also exhibit increased inflammation in association with fatigue that is not responsive to SSRIs [2, 17, 136]. In addition, fatigue is one of the primary residual symptoms in SSRI-treated medically healthy depressed patients, who, as noted above, have been shown to exhibit evidence of increased inflammation. Taken together, these findings suggest that neurotransmitter systems other than serotonin, such as dopamine, may be involved in these SSRI-resistant, inflammation-related symptoms. Nevertheless, classical stimulant medications that increase dopamine release and/or block dopamine reuptake have demonstrated limited efficacy in the treatment of fatigue in cancer patients and patients with other medical disorders associated with inflammation [22, 127, 142]. Therefore, a better understanding of the mechanisms by which inflammation and inflammatory cytokines affect dopamine function will inform strategies to improve the treatment of neuropsychiatric disturbances such as fatigue in both medically ill as well as medically healthy individuals.
2. Cytokine effects on the basal ganglia and dopamine-related behaviors
2.1 Access of peripheral cytokines and immune cells to the central nervous system
Activation of peripheral inflammation and the systemic release of inflammatory cytokines can exert profound effects on the brain and behavior as a result of communication between the periphery and brain. Of note, local tissue inflammation in the absence of a systemic immune response may activate discrete brain regions [11], however, it is unlikely that such local immune responses and their effects on the brain lead to the more global changes in neurotransmitter metabolism and neurocircuitry relevant to the development of behavioral changes, including the depression and fatigue seen during systemic inflammation. Cytokines from peripheral immune cells can access the central nervous system (CNS) by several mechanisms including 1) passage through leaky regions in the blood brain barrier such as the circumventricular organs [18, 59, 98, 103, 154], 2) activation of endothelial cells and perivascular macrophages in the cerebral vasculature to produce local inflammatory mediators such as cytokines, chemokines, prostaglandins, and nitric oxide (NO) [25, 60, 129, 139], 3) carrier-mediated transport of cytokines across the blood-brain barrier [7–9], 4) local activation of peripheral nerve afferents (e.g. the vagus) which then relay cytokine signals to relevant brain regions, including the nucleus of the solitary tract and hypothalamus (the so called ‘neural route’) [15, 59, 214], and 5) recruitment of activated immune cells such as monocytes/macrophages and T cells from the periphery to the brain, where these cells can in turn produce cytokines [44, 183].
Once in the CNS, peripheral inflammatory cytokines or activated immune cells can dramatically influence the tone of local inflammatory networks and propagate neuroinflammation by activating local production of cytokines [84, 104, 171] and inflammatory signaling pathways, such as nuclear factor (NF)-kappaB, janus kinase (JAK)-signal transducer and activator of transcription (STAT)s, and mitogen-activated protein kinases (MAPK) [97, 100, 146, 147, 212]. Cytokines and their receptors are constitutively expressed in the brain at low levels during non-pathological states and are found to be fairly ubiquitous, yet this cytokine network in the brain can be rapidly mobilized in response to inflammatory stimuli [82, 175]. Cytokines in the brain are produced primarily by microglia, but can also be produced by astrocytes [38, 119] and to some extent by neurons [18, 180] and oligodendrocytes [14, 153]. Furthermore, endothelial cells and perivascular macrophages respond to circulating cytokines to induce expression of the prostaglandin-producing enzymes cyclooxygenase-2 (COX-2) and prostaglandin E synthase (PGES) [58, 105, 114].
Following an acute inflammatory stimulus, increased CNS inflammation can confer protection to the brain [37, 101, 108], and acute changes in neurotransmitter metabolism, including increases in monoamines such as serotonin and norepinephrine in the hypothalamus, can contribute to the induction of fever, activation of the HPA axis, and transition from an anabolic to a catabolic state [53, 54]. Changes in monoamine metabolism are also believed to promote behavioral alterations including reduced locomotor activity and anhedonia that allow for shunting of energy and metabolic resources to combat infection and/or facilitate wound healing [46]. Therefore, cytokine signals from the periphery initially serve to inform the CNS of immune insult in order to prepare and protect an organism during times of sickness and injury. In contrast, under conditions of chronic inflammation such as during chronic medical illnesses or depression, CNS inflammation can exert profound and protracted changes in neurotransmitter systems, neurotrophic factors, and neuronal integrity that can have negative outcomes on behavior.
2.2. Cytokine effects on behaviors related to the basal ganglia and dopamine function
Stimulation of the immune system or the administration of inflammatory cytokines to laboratory animals and humans results in a repertoire of behavioral changes, many of which overlap with those experienced during medical illness and those that have been classically described in depression [45, 124]. Many of these symptoms are also consistent with disruption of the basal ganglia and dopamine function, including anhedonia, fatigue, psychomotor disturbance, and changes in sleep [4, 26, 29, 35, 110, 176, 190]. Typically, acute activation of the immune system or administration of inflammatory cytokines, either alone or in combination, to laboratory animals will induce an early pyrogenic response accompanied by behavioral changes termed “sickness behavior” that consist of reduced food intake and locomotor activity [41, 49]. This sickness behavior is then followed by a later onset of behavioral symptoms that persist in the absence of the febrile response. The observation that locomotor activity and food/water intake recover in a matter of hours, while anhedonic and depressive-like behaviors such as decreased sucrose consumption and immobility in the forced swim test persist for 24 to 48 hours following lipopolysaccharide (LPS) administration [64, 152], has sparked recent interest in the different phases of behavioral responses to immune activation. These persistent anhedonic and depressive-like symptoms may be indicative of cytokine-induced alterations in reward pathways and therefore dopamine neurotransmission.
In humans, a large body of data regarding the effects of cytokines on behavior has been derived from healthy volunteers acutely exposed to inflammatory stimuli or patients chronically administered cytokines, such as interferon (IFN)-alpha. IFN-alpha is an inflammatory cytokine with antiviral and antiproliferative properties, and in combination with ribivarin, is the only FDA approved treatment for hepatitis C virus (HCV). IFN-alpha potently induces other inflammatory cytokines such as interleukin (IL)-6, while also stimulating IL-1 and tumor necrosis factor (TNF)-alpha, both in vitro and in vivo [34, 62, 164, 186, 204]. Peripheral administration of IFN-alpha also increases IFN-alpha in the brain which in turn stimulates an CNS inflammatory response characterized by increases in IL-6 and monocyte chemoattractant protein-1 (MCP-1) [39, 62, 164, 189], which has been shown to attract activated monocytes to the brain [44, 195, 215]. Although IFN-alpha detected in the CNS during peripheral IFN-alpha administration is low compared to IL-6 and MCP-1 [164] and likely represents detection of exogenously administered cytokine [39, 189], microglia and astrocytes are capable of producing IFN-alpha in the CNS [3, 205, 220], as are plasmacytoid dendritic cells which reside in the meninges and can be recruited to the brain parenchyma during immune activation [43]. Moreover, microglia stimulated with IFN-alpha in vitro have been shown to increase oxidative stress (superoxide production) and IL-1 activity [40], indicating that IFN-alpha may drive microglia-induced neuroinflammation in the CNS.
Depending on the dose, up to 50% of patients administered IFN-alpha as treatment for HCV or malignant melanoma meet symptom criteria for major depression, and up to 80% experience significant fatigue [27, 28, 31, 50, 145, 163, 164, 167]. In addition to depression and fatigue, symptoms of insomnia, psychomotor slowing, and cognitive impairment are common in IFN-alpha-treated patients [29, 30]. As noted above, basal ganglia dopamine plays a pivotal role in the regulation of mood and motivation, reward, psychomotor activity, and sleep wake cycles. Therefore, changes in dopamine function may contribute to the manifestation of neuropsychiatric symptoms in IFN-alpha-treated and medically ill subjects with increased inflammation. Although it is generally recognized that other monoamines including both serotonin and norepinephrine may contribute to cytokine-induced behavioral changes [5, 6, 54, 55], this review will focus on the role of dopamine.
2.3. Cytokines and inflammation target dopamine function and the basal ganglia
Biochemical and Behavioral Studies
Evidence that inflammatory cytokines, and specifically IFN-alpha, affect basal ganglia dopamine function comes from the peripheral administration of cytokines to laboratory animals including non-human primates [62, 96, 102, 110, 179, 184]. For example, rhesus monkeys express functional type I IFN receptors that activate relevant signal transduction pathways in response to human IFN-alpha [62]. These animals also exhibit IFN-alpha-induced behavioral changes similar to those seen in humans. Relevant to dopamine, acute administration of low dose IFN-alpha to rhesus monkeys was found to decrease rapid eye movement (REM) latency [169]. REM sleep is sensitive to changes in dopamine, and reduced REM latency is observed in Parkinson’s disease (PD) [106], particularly in PD patients with co-morbid depression [107]. Furthermore, radiolabeled IFN-beta, which binds to the same receptor as IFN-alpha, delivered to the brain by the intranasal route yielded specific binding in the basal ganglia of rhesus monkeys [207], indicating that primates may have increased sensitivity to IFN and other cytokine effects on the basal ganglia and dopamine function. Decreased dopamine in the CNS has also been reported in rodents administered IFN-alpha, however results have been mixed. Some studies in rodents have reported increases [110, 179], while others have reported decreases [96, 102, 184], in brain dopamine and/or metabolites following acute or sub-chronic IFN-alpha administration. These discrepancies are likely due to differences in dosing, length of cytokine exposure, and most importantly, the fact that species-specific cytokines were variably used and rodents do not respond to human IFN-alpha with activation of classic type I IFN receptor signaling [121, 122, 212]. Moreover, human IFN-alpha administered to rodents binds to opioid receptors, which may be responsible for some of the observed changes in brain monoamines [13, 91, 213].
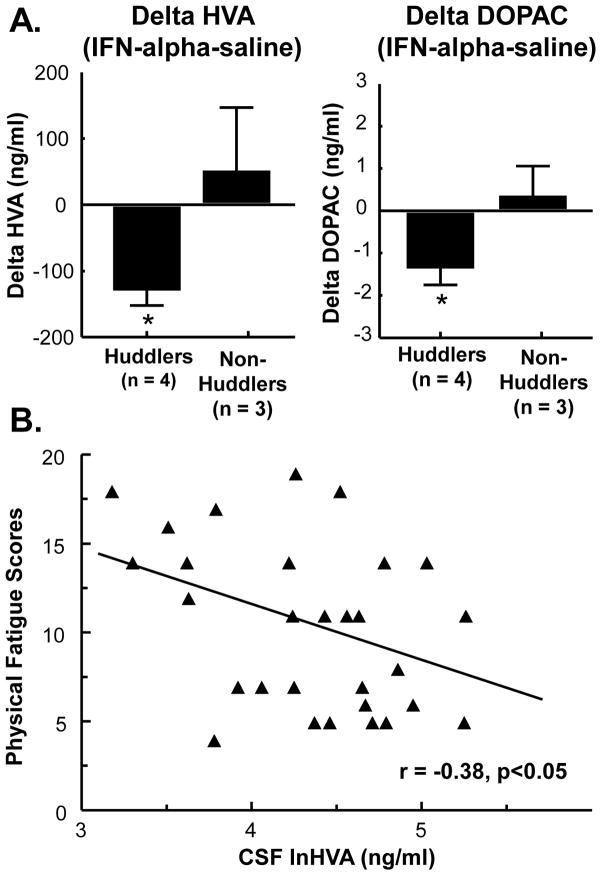
To further explore the potential impact of IFN-alpha on dopamine function and behavior, work in our laboratory has examined rhesus monkeys chronically administered recombinant human IFN-alpha for 4 weeks. Rhesus monkeys exhibit immune, neuroendocrine, and behavioral responses to IFN-alpha similar to humans, including decreases in psychomotor activity and increases in depressive-like huddling behavior (in ~50% of animals) [62]. Huddling behavior in non-human primates was first described following chronic administration of reserpine [133], a monoamine-depleting agent that also reduces REM latency [170], and has been reported in monkeys treated with dopamine receptor antagonists and partial agonists [174]. IFN-alpha-induced depressive-like huddling behavior in rhesus monkeys has been reproducible over multiple 2–4 week sessions of IFN-alpha administration separated by up to 6 months, and monkeys that display huddling behavior in response to IFN-alpha have been found to exhibit significantly lower cerebrospinal fluid (CSF) concentrations of the dopamine metabolite, homovanillic acid (HVA) as well as 3,4-dihydroxyphenylacetic acid (DOPAC) (Fig. 1A). Of note, decreased CSF HVA concentrations correlated with reduced locomotor activity in all animals (r=0.385, p<0.05, n=7), and especially in those animals that exhibited huddling behavior (r=0.726, p<.001, n=4). Similar correlations have been observed between reduced CSF HVA and physical fatigue in IFN-alpha-treated humans (r=−0.38, p<0.05, n=29) (Fig 1B) and CSF HVA and neurocognitive decline in patients infected with human immunodeficiency virus (HIV) [48]. Of note, these effects of peripherally administered IFN-alpha and HIV infection on dopamine metabolism may be secondary to activation of local immune cells in the basal ganglia. Indeed, both HIV and peripheral administration of IFN-alpha have been associated with a central inflammatory response [74, 164]. Moreover, infusion of LPS to selectively activate microglia in the striatum has been shown to reduce extracellular DOPAC as measured by in vivo microdialysis [132]. Taken together, these findings support a role for dopamine function in fatigue, psychomotor slowing, and sleep alterations associated with IFN-alpha and medical illness involving CNS inflammation and prompt further investigation into inflammation effects on dopamine function and the basal ganglia.
Fig. 1. Reduced CSF HVA concentrations during chronic IFN-alpha administration are associated with depressive-like huddling behavior in monkeys and symptoms of fatigue in humans.
Video-taped behavior and cisternal cerebrospinal (CSF) samples were collected from 7 rhesus monkeys receiving daily subcutaneous (s.c.) injections of saline or IFN-alpha (20 IU/m2 body surface area) for 2 weeks, and were analyzed for depressive-like huddling behavior and monoamine metabolite concentrations, respectively. Compared to saline, IFN-alpha produced increased huddling behavior in 4 out of 7 animals that was associated with decreased CSF homovanillic acid (HVA) and 3,4-dihydroxyphenylacetic acid (DOPAC) concentrations (A). In HCV+ human subjects treated with IFN-alpha for 11 weeks, natural log (ln) transformed concentrations of CSF HVA were correlated with fatigue scores, as measured by the physical fatigue dimension of the multidimensional fatigue inventory (MFI) (r=−0.38, p<0.05, n=29) (B). Data in A are summarized as mean+/−SE, *p<0.05
Neuroimaging Studies
Neuroimaging studies from our laboratory and others suggest disruption of the basal ganglia and the dopamine system is a major contributor to cytokine-induced behavioral changes. For example, in the first study to examine IFN-alpha effects on the brain, increased glucose metabolism in the basal ganglia, particularly in the putamen [94], was observed using positron emission tomography (PET) and fluorine-18-labeled-fluorodeoxyglucose (FDG). More recently, we have conducted a series of imaging studies examining brain activity and metabolism in patients receiving IFN-alpha therapy for malignant melanoma and HCV. Patients with malignant melanoma were imaged prior to and after receiving high dose IFN-alpha (20 MIU/m2) for 4 weeks, and FDG PET revealed increased glucose metabolism in the basal ganglia [32]. Furthermore, increased glucose metabolism in specific basal ganglia nuclei including the left putamen and left nucleus accumbens correlated significantly with reports of fatigue in these patients, as assessed by the ‘energy’ subscale of the Visual Analog Scale of Fatigue (VAS-F). This pattern of increased glucose metabolism in basal ganglia nuclei is similar to that seen in patients with PD [56, 134, 193], where it is thought to reflect increased oscillatory burst activity in relevant basal ganglia nuclei secondary to loss of inhibitory nigral dopamine input [216, 217]. Functional magnetic resonance imaging (fMRI) has also demonstrated decreased neural activation in the basal ganglia (ventral striatum) to a hedonic reward task in HCV+ patients undergoing IFN-alpha administration. Of note, administration of the cytokine-inducers LPS and typhoid vaccine to healthy volunteers has been shown to have similar effects on the basal ganglia, suggesting that findings from IFN-alpha generalize to other inflammatory stimuli [19, 57]. Indeed, typhoid vaccination produced increased evoked activity in the substantia nigra compared to controls, which was associated with both psychomotor slowing and increased peripheral blood concentrations of IL-6 [19]. Similar to IFN-alpha, LPS administration led to reduced activation in the ventral striatum during a monetary reward task that was associated with increased depressed mood, as measured by the Profile of Mood States (POMS) depression subscale [57]. In light of the role of basal ganglia dopamine in the regulation of motivated behavior and psychomotor activity, these imaging studies in humans suggest that the behavioral syndrome experienced by cytokine-treated or medically ill patients has, at least in part, a neurobiological basis in subcortical, motivation and movement-related basal ganglia regions, and in concert with the data described above also likely involves disruption of dopamine neurotransmission.
To probe the dopaminergic mechanisms of IFN-alpha effects on neural activity in the basal ganglia, a PET study was conducted using [18F]fluorodopa (FDOPA) in HCV+, IFN-alpha-treated subjects. FDOPA is taken up by dopaminergic neurons and converted by dopamine decarboxylase to dopamine, whereupon it is stored in vesicles for release. Interestingly, both increased uptake and decreased turnover of FDOPA in the caudate, putamen and ventral striatum of IFN-alpha-treated patients was found [33]. Baseline and percent change in FDOPA uptake was in turn correlated with IFN-alpha-induced behavioral alterations including depression and fatigue, as measured by the Montgomery Asberg Depression Rating Scale (MADRS) and Multidimensional-Fatigue-Inventory (MFI), respectively. Increased uptake and decreased turnover of FDOPA in the basal ganglia following IFN-alpha administration is in stark contrast to that observed in patients with PD where decreased uptake and increased FDOPA turnover is found. Decreased uptake of FDOPA in PD is believed to be a function of loss of dopaminergic neurons and/or their projections throughout the basal ganglia [95, 111, 116], and intact or increased turnover suggests that the surviving neurons are capable of normal release [111, 112]. Increased FDOPA uptake during IFN-alpha treatment suggests a potential depletion of dopamine and increased synthetic capacity. Indeed, as noted above, FDOPA is rapidly converted to dopamine by dopamine decarboxylase, whose activity can be increased in the context of reduced dopaminergic tone [83]. Alternatively, reduced signal decay, reflective of decreased turnover [111, 191], suggests that newly synthesized dopamine is not being effectively packaged and/or released or that the activity of the dopamine transporter (DAT) is increased [33]. Despite these differences in FDOPA uptake and turnover between IFN-alpha administration and PD, it is interesting to note that PD-like symptoms have been observed in some patients during IFN-alpha administration, and these PD symptoms have been responsive to treatment with levodopa, suggesting reduced dopamine neurotransmission in IFN-alpha-treated patients [12, 140, 178].
In sum, results from biochemical, behavioral and neuroimaging studies provide important clues regarding the mechanisms of cytokine-induced behavioral alterations, and strongly suggest that 1) cytokine effects on the basal ganglia are involved and 2) these effects include cytokine-induced alterations in basal ganglia dopamine function, potentially including disruptions in dopamine synthesis, packaging, release or reuptake.
3. Potential mechanisms of cytokine effects on dopamine synthesis, release, and reuptake
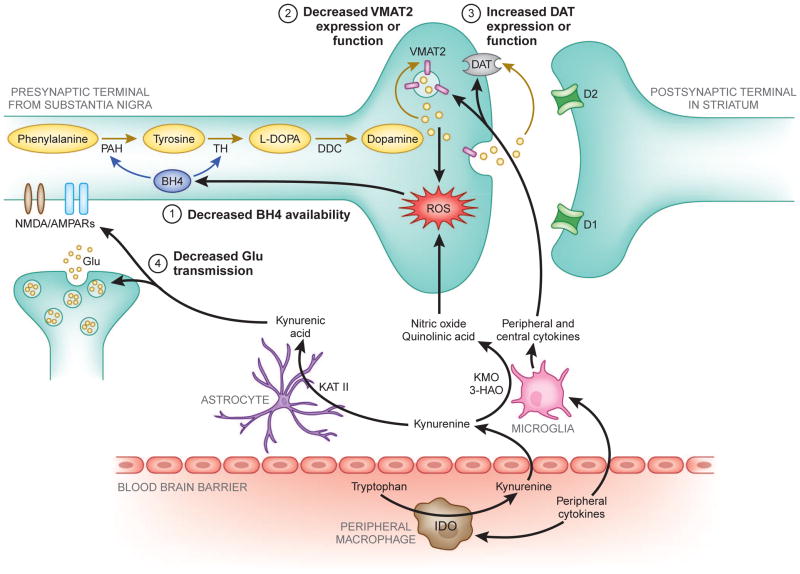
Cytokines can potentially affect multiple aspects of dopamine neurotransmission, leading to decreased synthesis, impaired packaging or release, and increased reuptake, all of which may interact to a greater or lesser extent to reduce dopamine function in the basal ganglia (see Fig. 2). The following section will discuss potential mechanisms by which cytokines may affect these aspects of dopamine function, ultimately resulting in reduced dopamine signaling in the basal ganglia.
Figure 2. Potential mechanisms of inflammatory cytokine effects on basal ganglia dopamine synthesis and release.
Evidence indicates that inflammatory cytokines from the periphery, or those produced locally by activated microglia or infiltrating macrophages, can produce nitric oxide, as well as quinolinic acid through indoleamine 2,3-dioxygenase (IDO) and kynurenine pathways, both of which contribute to oxidative stress and reactive oxygen species (ROS) generation. Increased ROS and inflammation-induced nitric oxide contribute to (1) oxidation of tetrahydrobiopterin (BH4), a cofactor required for the conversion of phenylalanine to tyrosine and tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA), which are necessary for the synthesis of dopamine. Furthermore, some evidence exists that inflammatory cytokines may (2) decrease the expression or function of the vesicular monoamine transporter 2 (VMAT2), and/or (3) increase expression or function of the dopamine transporter (DAT). Dysregulation of dopamine transport and vesicular packaging can increase cytosolic dopamine, leading to auto-oxidation and generation of ROS and neurotoxic quinones. Finally, cytokine-activation of IDO in peripheral immune cells or microglia also produces kynurenic acid from kynurenine by kynurenine aminotransferase (KAT) II activity in astrocytes. Kynurenic acid can lead to (4) reduced glutamate (glu) neurotransmission by antagonism of glu receptors and release, consequently decreasing glu-evoked dopamine release in the striatum. Although not pictured, excessive cytokine-induced release of glutamate and quinolinic acid may also contribute to increased oxidative stress and excitotoxicity.
3-HAO, 3-hydroxyanthranilic acid oxygenase; AMPAR, 2-amino-3-(5-methyl-3-oxo-1,2- oxazol-4-yl) propanoic acid receptor; BH4, tetrahydrobiopterin; D1, dopamine receptor 1; D2, dopamine receptor 2; DAT, dopamine transporter; glu, glutamate; DDC, dopamine decarboxylase; IDO, indoleamine 2,3 dioxygenase; KAT II, kynurenine aminotransferase II; KMO, kynurenine 3-monooxygenase; L-DOPA, L-3,4-dihydroxyphenylalanine; NMDAR, N-Methyl-D-aspartic acid receptor; PAH, phenylalanine hydroxylase; ROS, reactive oxygen species; TH, tyrosine hydroxylase; VMAT2, vesicular monoamine transporter 2
3.1 Cytokine effects on dopamine synthesis
Dopamine synthesis relies on the conversion of tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) by tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis. A major source of tyrosine is phenylalanine, which is converted to tyrosine by phenylalanine hydroxylase (PAH). Both of these enzymes, TH and PAH, require tetrahydrobiopterin (BH4) as an essential enzyme co-factor. BH4 is also a co-factor for NO synthases (NOS) which convert arginine to NO [42]. Additionally, BH4 is highly redox-sensitive and is readily oxidized to dihydrobiopterin (BH2), which can be regenerated to BH4, or dihydroxanthopterin (XPH2), whose oxidation is irreversible [42, 52, 86].
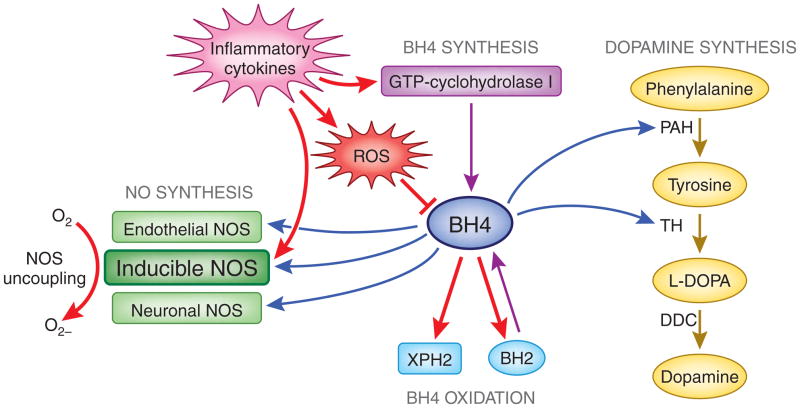
Although inflammation and cytokines have been shown to induce GTP-cyclohydrolase I, the enzyme necessary for BH4 synthesis, inflammation-induced increases in both reactive oxygen species (ROS) and inducible NOS (iNOS) activity can ultimately lead to decreased BH4 availability and thus reduced dopamine synthesis (Fig. 2, mechanism 1). One source of oxidative stress during inflammation is quinolinic acid (QUIN) [10, 177], a neurotoxic product of the kynurenine pathway [78, 90]. When dopaminergic neurons encounter oxidative stress from auto-oxidation of dopamine or the toxic products of inflammation such as QUIN, intracellular BH4 is reduced to BH2 and XPH2. Increased iNOS activity along with decreased BH4 levels results in NOS uncoupling and the preferential generation of oxygen free radicals rather than NO [42, 219]. This production of oxygen free radicals further contributes to oxidative stress and reduction of BH4 [42], conferring even less availability of BH4 for dopamine synthetic pathways (Fig. 3) [148]. Indeed, intramuscular injection of rats with IFN-alpha has been shown to decrease CNS concentrations of BH4 through stimulation of NO, and inhibition of NOS was found to reverse IFN-alpha’s inhibitory effects on brain concentrations of both BH4 and DA [102]. Of note, IL-6 treatment has also been shown to reduce BH4 content in sympathetic neurons [118].
Figure 3. Effects of inflammatory cytokines on BH4 synthesis and oxidation, and nitric oxide activity, which contribute to decreased BH4 availability for dopamine synthesis.
Dopamine synthesis relies on the conversion of tyrosine to L-DOPA by tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis. Phenylalanine is converted to tyrosine by phenylalanine hydroxylase (PAH), and both TH and PAH require BH4 as a co-factor. BH4 is also a co-factor for the NO synthases (NOS), which convert arginine to NO. BH4 is redox-sensitive and readily oxidized reversibly to dihydrobiopterin (BH2) and irreversibly to dihydroxanthopterin (XPH2). Although inflammatory cytokines induce GTP-cyclohydrolase I expression, an enzyme necessary for BH4 synthesis, they also increase both ROS and inducible NOS activity. Increased oxidation of BH4 by ROS as well as increased inducible NOS activity results in NOS uncoupling and the preferential generation of free radicals from 02 rather than NO. This production of free radicals further contributes to oxidative stress and reduction of BH4, ultimately leading to decreased BH4 availability for dopamine synthesis.
Red arrows indicate inflammatory cytokine effects on BH4 metabolism and activity. BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; GTP, guanosine-5′-triphosphate; L-DOPA, L-3,4-dihydroxyphenylalanine; NO, nitric oxide; NOS, nitric oxide synthase; PAH, phenylalanine hydroxylase; ROS, reactive oxygen species; TH, tyrosine hydroxylase; XPH2, dihydroxanthopterin
Concentrations of phenylalanine, tyrosine, BH4 and BH2 can be measured in the peripheral blood and CSF, and the BH4/BH2 and phenylalanine/tyrosine ratios have been proposed as indicators of BH4 availability and PAH activity, as well as indirect biomarkers of dopamine synthetic capacity [24, 36, 88, 148]. For example, a number of patient populations with increased inflammation, including patients with trauma, sepsis, cancer, and HIV, have been found to exhibit increased peripheral blood concentrations of phenylalanine [148]. Furthermore, increased phenylalanine concentrations in patients with cancer have been correlated with markers and mediators of inflammation including IL-6, IL-2 receptor, and soluble TNF-alpha receptor-2, as well as peripheral blood markers of oxidative stress [148]. Moreover, in a recent study of healthy elderly persons with low-grade inflammation, peripheral blood concentrations of phenylalanine, tyrosine, and an increased phenylalanine/tyrosine ratio were associated with neuropsychiatric symptoms including anhedonia and altered sleep [36], potentially reflecting decreased dopamine availability (secondary to decreased BH4 and dopamine synthesis).
3.2. Cytokine effects on dopamine packaging, release, and reuptake
Synaptic dopamine is dependent on the vesicular monoamine transporter 2 (VMAT2) to package cytosolic dopamine into vesicles for release. There is some evidence that inflammatory cytokines and inflammation may negatively affect the expression and function of VMAT2 (Fig. 2, mechanism 2). For example, the inflammatory cytokines IL-1 and TNF-alpha were found to decrease expression of VMAT2 in rat enterochromaffin-like cells, whereas transforming growth factor-beta, which is immunomodulatory and anti-inflammatory, increased VMAT2 expression [99]. Additionally, the anti-inflammatory compound, pituitary adenylate cyclase-activating polypeptides 38, administered by subcutaneous minipump, in vivo, was able to increase VMAT2 expression, reduce neuroinflammation and oxidative stress, and protect against dopamine neurotoxicity following chronic methamphetamine exposure [81].
Normal vesicular sequestration and release is particularly important in dopaminergic cells due to the risk of auto-oxidation of dopamine and the formation of free radicals and neurotoxic quinones [23, 80, 115]. Decreased VMAT2 function and increased cytosolic dopamine, such as with methamphetamine exposure, can lead to dopamine auto-oxidation and ROS formation [80, 172]. Interestingly, patients with neuroinflammation from HIV experience marked vulnerability to neurotoxicity from methamphetamine exposure [63, 185], possibly related to increased inflammation-induced oxidative stress. Therefore, decreased expression or function of the VMAT2 by inflammatory cytokines could not only reduce the amount of dopamine released into the synapse, but may also increase production of ROS that can have detrimental effects on cell viability and ultimately dopamine function.
Recent attention has been paid to the effects of cytokines and inflammatory signaling pathways on monoamine reuptake pumps, including the dopamine transporter (DAT) [143, 225–227]. Much of this work has focused on the serotonin transporter [225–227], where both in vitro and in vivo data have established that stimulation of p38 MAPK, a major signaling pathway activated by IFNAR1 and other cytokines, can increase the expression and function of the serotonin transporter, leading to increased serotonin reuptake. MAPK pathways have also been found to influence the dopamine transporter. For example, DAT-expressing cells transfected with a constitutively activate MAPK kinase (MEK) show increased DA reuptake (Vmax), whereas treatment of rat striatal synaptosomes with MEK inhibitors was associated with decreased DA reuptake in a concentration and time-dependent manner [143, 225–227]. Furthermore, subjects with neuropsychiatric disturbances as a result of HIV infection and subsequent neuroinflammation are thought to have increased expression of DAT [63, 68]. Therefore, reduced dopamine turnover secondary to inflammatory cytokines may be mediated, in part, by increased DAT expression or function (Fig. 2, mechanism 3).
3.3. Cytokine effects on glutamate neurotransmission and dopamine release
Another mechanism by which cytokines may influence the basal ganglia and dopamine function is through effects on glutamate neurotransmission. For example, there has been recent interest in the impact of cytokine stimulation of indoleamine 2,3 dioxygenase (IDO) and downstream kynurenine pathways on glutamate neurotransmission in the brain (Fig. 2, mechanism 4). IDO is expressed in multiple cell types including microglia and macrophages, and can be can be activated by cytokines, alone or in combination, through activation of cytokine-signaling pathways such as STAT-1, IFN-regulatory factor-1, p38 MAPK, and NF-kappaB [65, 77, 158]. In the brain, IDO is significantly increased at 24 hr and peaks at 48 hr in response to LPS administration, corresponding to the expression of depressive-like behaviors [117, 151]. Immune-mediated activation of IDO catabolizes tryptophan, the primary amino-acid precursor of serotonin, to kynurenine. Evidence of a role of IDO in cytokine-induced depression comes from a number of studies which have demonstrated correlations between IFN-alpha–induced depression, decreases in tryptophan, and increases in kynurenine and/or the kynurenine/tryptophan ratio [16, 31]. Although early hypotheses regarding IDO and depression were focused on the consequence of tryptophan depletion on serotonin synthesis, more recent data indicate that kynurenine administration alone is sufficient to induce depressive-like behavior in laboratory animals [151].
Kynurenine can be produced locally in the CNS or transported across the blood–brain barrier by large neutral amino acid transporters [66, 181, 188], and is further catabolized into the neuroactive metabolites kynurenic acid (KA) (in astrocytes) and QUIN (in microglia), both of which have been found to be increased in the CSF of IFN-alpha-treated patients [165, 182]. Of note, CSF QUIN significantly correlated with depressive symptoms during IFN-alpha administration, as measured by MADRS [165]. In addition to the aforementioned contribution to oxidative stress, QUIN can also directly activate the n-methyl-d-aspartate (NMDA) receptor to induce the release of glutamate [182, 202, 203]. Oxidative stress and increased glutamate release can lead to excitotoxicity in the brain, and therefore, excessive QUIN has been implicated in a number of neurodegenerative disorders, including Huntington’s disease, Amyotrophic Lateral Sclerosis, Alzheimer’s disease, and dementia secondary to infection with HIV [75, 76, 78, 79, 182]. In contrast to QUIN, KA reduces glutamate release, and has been shown to be an antagonist of NMDA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [198]. Dopamine release is in part under glutamatergic control, and thereby KA may exert downstream effects on DA [182]. Indeed, intra-striatal administration of KA to rodents leads to marked reductions in extracellular dopamine concentrations, as determined by in vivo microdialysis, that can reversed by the allosteric alpha 7 nicotinic acetylcholine receptor agonist, galantamine [218].
Finally, cytokines and inflammation have been shown to increase glutamate by effects on astrocytes. A rich literature has shown that cytokines can decrease the astrocytic expression of glutamate transporters and increase astrocytic release of glutamate [92, 208]. Of note, glutamate released by astrocytes has preferential access to extrasynaptic NMDA receptors, which lead to decreased production of trophic factors, including brain-derived neurotrophic factor, ultimately contributing to further disruption of neuronal integrity [85, 89]. Relevant to depression, increased glutamate has been found in the frontal cortex of patients with mood disorders [87, 130, 131], as well as evidence of microglial activation and increased expression of QUIN [197]. Given the sensitivity of dopamine neurons to oxidative stress and excitotoxicity, cytokine effects on astrocytic glutamate release may contribute to decreased dopamine function, and could lead to neurodegeneration. Indeed, acute and chronic (2 weeks) administration of high dose LPS (5 mg/kg) to adult mice produced ~50–70% decrease in dopaminergic neurons in the substantia nigra, thought to be mediated in part by the LPS-induced production of TNF-alpha and related increases in oxidative stress [67, 162].
4. Translational implications: potential therapeutic targets for treating cytokine effects on basal ganglia dopamine function
The data summarized herein demonstrate that inflammatory cytokines affect dopamine function and may contribute to the development of neuropsychiatric symptoms such as fatigue, anhedonia, psychomotor slowing, sleep disturbances, and depression in multiple patient populations with increased inflammation. Moreover, the prevalence of neuropsychiatric symptoms, and particularly fatigue, in patients exposed to chronic inflammation or inflammatory cytokines substantiate the need to understand the precise mechanisms of cytokine effects on dopamine function to determine optimal treatment strategies. As discussed previously, the symptom spectrum likely mediated by changes in dopamine function appears to be resistant to treatment with SSRIs [27, 144, 166]. Furthermore, as noted above, stimulants such as amphetamines and dopamine reuptake blockers to treat fatigue in medically ill and depressed populations have exhibited limited therapeutic efficacy [22, 127, 142, 161, 196, 200]. Although the use of drugs such as amantadine or modafinil that work through alternative mechanisms may have greater success in treating fatigue in medical illnesses, e.g. cancer and multiple sclerosis [109, 168, 229], their benefit still remains to be determined [161, 196]. Since stimulants act to increase dopamine release and block DAT function, these drugs may not provide long term efficacy if cytokine effects on dopamine function are primarily mediated through inhibitory effects on synthesis, packaging, or release. Therefore, consideration should be given to alternative strategies such as compounds that 1) increase dopamine synthesis or VMAT2 function, 2) inhibit potential cytokine-induced increases in DAT activity, and 3) inhibit activation of the neuroactive metabolites of the kynurenine pathway and/or glutamate. Of course, strategies that inhibit inflammation and/or the inflammatory cytokines themselves may also be considered. Indeed, administration of the TNF-alpha antagonist, etanercept, has been shown to inhibit fatigue in patients with advanced cancer [141].
Dopamine synthesis may be improved by boosting BH4 activity, thus increasing the synthetic capacity of PAH and TH. There are a number of compounds than can boost BH4 availability. Inflammation-reduced BH4 concentrations can be restored through the salvage pathway with administration of synthetic pterin following conversion by sepiapterin reductase [42, 149]. Sapropterin (Kuvan) is the first non-dietary, FDA-approved, synthetic form of BH4 for patients with phenylketonuria (PKU) that has been shown in randomized, double-blind trials to be effective in lowering blood phenylalanine levels [21]. PKU, which is characterized by excessive phenylalanine levels, is caused by reduced PAH activity from genetic mutations. Sapropterin administration increases BH4 in order to boost residual enzymatic activity that may be present in some patients by acting as a chaperone to promote normal PAH activity, and stabilizes blood phenylalanine concentrations in BH4-responsive patients [20, 209, 211]. Of note, hyperphenylalaninemia can also be caused by a deficiency of BH4 itself, and is associated with significant brain damage, mental retardation, seizures, and behavioral alterations, similar to that found in patients with PKU due to PAH deficiency. This syndrome can also be treated with sapropterin. Several other strategies are also currently available to address deficiencies in BH4, including the use of folic acid, L-methylfolate, and S-adenosyl-methionine (SAMe), all of which have a role in the synthesis and/or regeneration of BH4 [135, 138, 194]. Although BH4 administration has not been studied in the context of depression or fatigue, studies examining folic acid, L-methylfolate, and SAMe have been conducted in depression. Interestingly, low serum folate has been associated with increased risk of depression as well as non-response to antidepressant treatment and an increased likelihood of depression relapse [61, 69, 70, 156, 157]. Administration of L-methylfolate (marketed as Deplin and Zervalx) to depressed patients has been shown to augment the efficacy of standard antidepressant therapy [71, 72], and treatment with SAMe adjunctive to serotonin reuptake inhibitors leads to significantly higher rates of remission and 50% or greater decreases in depressive symptoms compared with placebo [155]. Interestingly, although the impact of inflammation and associated oxidative stress on BH4 and dopamine metabolism is well recognized, studies have yet to directly test whether strategies to augment BH4 are efficacious in restoring dopamine function, and treating fatigue and depression in patients with increased inflammation.
In terms of dopamine packaging, release, and reuptake, compounds that improve VMAT2 function or inhibit cytokine effects on DAT and VMAT2, could be considered for the treatment of cytokine-induced depression and fatigue. Activity of the VMAT2 has been found to be increased by trkB agonists, and a promising compound, 7,8-dihydroxyflavone, is currently being investigated for neuroprotective efficacy in animal models of PD [93]. Blockade of cytokine-signaling pathways that may directly affect DAT and/or VMAT2 function may also be considered, and a number of p38 MAPK inhibitors are in development for the treatment of a range of diseases, including autoimmune and inflammatory disorders, cardiovascular disease, pulmonary disorders, including asthma and COPD, as well as pain [113]. Interestingly, inhibition of p38 MAPK has been shown to reverse the development of LPS-induced behavioral changes in laboratory animals [228], in part, through reversing p38 MAPK effects on the serotonin transporter, but may also involve reversal of effects on dopamine reuptake.
Consequences of the neuroactive metabolites of kynurenine, kynurenic acid and QUIN, particularly on glutamate (as discussed above), suggest that inhibition of the IDO pathway may be important target in addressing the impact of inflammation on basal ganglia dopamine function and treating inflammation-induced depression and fatigue. Of relevance in this regard, IDO-deficient mice are resistant to behavioral changes following bacille Calmette–Guerin (BCG) infection, while showing a normal inflammatory cytokine response to BCG administration [150]. Treatment of mice with the IDO antagonist, 1-methyl tryptophan (1-MT), has been shown to abrogate the impact of LPS, as well as an attenuated form of Mycobacterium bovis, on depressive-like behavior [150, 151]. 1-MT is currently being tested in patients with advanced-stage cancers, administered alone or in combination with vaccine therapies (e.g. ClinicalTrials.gov: NCT00617422 and NCT01302821). In addition, elaboration of the crystal structure of human IDO has paved the way for the design of new IDO inhibitors [47, 173], and inhibitors of other targets in the kynurenine pathway are also being developed [182, 199]. Targeted deletion of kynurenine aminotransferase-II (the enzyme that converts kynurenine to KA) has also been shown to increase cognitive performance in association with an increase in the amplitude of long-term potentiation in vitro, while reducing extracellular kynurenic acid as measured by hippocampal in vivo microdialysis [159]. As mentioned above, dopamine release is under partial control of glutamate neurotransmission, and changes in dopamine function due to inflammation-induced KA may respond to alpha 7 nicotinic acetylcholine receptor agonists, that have been shown to reverse kynurenic acid effects on dopamine release [218].
It is important to note that administration of glutamate receptor antagonists, such as the NMDA antagonist, ketamine, have potent antidepressant effects especially in treatment resistant depressed patients who, as mentioned previously, have been shown to exhibit increased inflammation [1, 160]. Given that the neurotoxic effects of QUIN may be mediated by excessive glutamate excitotoxicity [182, 202, 203], glutamate antagonists may be useful in preventing excitotoxic effects on the highly sensitive dopamine neurons. Indeed, metabotropic glutamate receptor antagonists that modulate glutamate transmission in the basal ganglia have been successful in reducing dopamine cell loss in an animal model of PD [128]. Therefore, blockade of kynurenine pathways or modulation of glutamate neurotransmission may confer protection against inflammation and IDO-mediated effects on dopamine function, to improve behavior in medically ill and depressed individuals.
5. Future directions
Although neuroimaging and biochemical data have provided evidence of functional effects of immune activation on the basal ganglia and dopamine function, future studies to further elucidate the mechanisms of these effects are warranted. These studies will ultimately be required to determine the most appropriate treatments to target basal ganglia dopamine-mediated symptoms. Provided that monkeys experience similar behavioral changes in response to IFN-alpha as humans [62], and there is some indication that dopamine may be selectively targeted in these animals (see Fig. 1 A), in vivo microdialysis experiments in monkeys can be used to further explore the impact of inflammatory cytokines on presynaptic dopamine function. Reverse in vivo microdialysis stimulation protocols using amphetamines and high K+ to probe vesicular release as well as in vivo treatment strategies could then be used to further probe the mechanisms of inflammatory cytokine effects on dopamine synthesis, packaging and release. Furthermore, translational neuroimaging techniques can be used in non-human primates and humans, including neuroimaging with carbon-11(C11)-labeled-raclopride that can indirectly measure stimulated dopamine release in the basal ganglia [192, 210], C11-labeled diihydrotetrabenazine (DTBZ) to measure VMAT2, and F18-labeled 2β-Carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)nortropane (FECNT) to image the DAT. Moreover, in vitro models would permit investigation of cytokine effects on gene expression and protein function, such as the VMAT2 and DAT. Finally, neuroimaging of medically ill patients experiencing neuropsychiatric disorders as the result of immune activation, such as with cancer, or medically healthy subjects with depression and high inflammation, would reveal inflammation-effects on the basal ganglia and dopamine function in relation to specific symptoms experienced by these patient populations. Together, these data will provide further evidence of the mechanisms of inflammatory cytokine effects on dopamine neurotransmission, and will guide the future development and testing of novel pharmacological treatment strategies to reverse basal-ganglia-mediated behavioral changes including depression and fatigue.
6. Summary
There is strong evidence that inflammatory cytokines specifically target the basal ganglia and dopamine function to contribute to neuropsychiatric symptoms including depression and fatigue in medically ill and depressed subjects. Much of this evidence stems from biochemical and behavioral studies in humans and animals administered cytokines, such as IFN-alpha, and from neuroimaging experiments that demonstrate altered basal ganglia and dopamine function in response to inflammatory cytokines or immune activation. Increased inflammation and exposure to inflammatory cytokines produce an array of behavioral disturbances, many consistent with changes in basal ganglia dopamine function. Basal ganglia-mediated symptoms, such as fatigue and psychomotor slowing, are resistant to treatment with SSRIs in both medical illness and depression. Surprisingly, these symptoms have also been difficult to treat with classical stimulant medications that increase DAT-mediated dopamine release and/or block dopamine reuptake, indicating cytokine effects on dopamine function may sabotage or circumvent the mechanism of action of these agents. Inflammatory cytokines may affect multiple aspects of dopamine neurotransmission, leading to decreased synthesis, impaired packaging or release, and increased reuptake, all of which may interact to a greater or lesser extent to reduce dopamine function. Multiple pharmacological treatment strategies to potentially target cytokine effects on dopamine function exist, yet future studies are needed to identify the precise mechanisms of cytokine action. Further understanding of the mechanisms of inflammation and inflammatory cytokines effects on basal ganglia dopamine function will enhance our fundamental understanding of neuropsychiatric symptoms, such as fatigue, in both medically ill and medically healthy depressed individuals, and inform new approaches to the treatment of these patient populations.
Cytokines released during inflammation target the basal ganglia and dopamine
Cytokines can disrupt dopamine function by effects on synthesis, packaging, release, and reuptake
Cytokine effects on basal ganglia dopamine may cause anhedonia, fatigue, and psychomotor slowing
Cytokines may contribute to behavioral disorders associated with chronic inflammation
Acknowledgments
Financial support: This work was supported in part by grants from the National Institutes of Health to AHM (K05MH069124, R01HL073921, R01MH075102, T32MH020018, and U19 MH069056) and JCF (F32MH093054) as well as the Emory Center for AIDS Research (P30AI050409). In addition, the study was supported by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources.
Footnotes
Financial Disclosure: All authors declare that there are no conflicts of interest, and all financial disclosures are listed for each author: Andrew H Miller has served as a consultant for Abbott Laboratories, AstraZeneca, Glaxo-SmithKline, Lundbeck Research USA, F Hoffmann-La Roche, Johnson and Johnson, Schering Plough Research Institute, and Wyeth/Pfizer, and has received research support from Centocor, GlaxoSmithKline, and Schering-Plough Research Institute; Jennifer C. Felger has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Mott LA, Skalla K, Whedon MB, Bivens S, Mitchell T, Greenberg ER, Silberfarb PM. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H, Ikeda K, Katoh M, McGeer EG, McGeer PL. Expression of MRP14, 27E10, interferon-alpha and leukocyte common antigen by reactive microglia in postmortem human brain tissue. J Neuroimmunol. 1994;50:195–201. doi: 10.1016/0165-5728(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 4.Anisman H, Merali Z. Anhedonic and anxiogenic effects of cytokine exposure. Adv Exp Med Biol. 1999;461:199–233. doi: 10.1007/978-0-585-37970-8_12. [DOI] [PubMed] [Google Scholar]
- 5.Anisman H, Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 6.Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Farr SA, Morley JE. Entry of blood-borne cytokines into the central nervous system: effects on cognitive processes. Neuroimmunomodulation. 2002;10:319–327. doi: 10.1159/000071472. [DOI] [PubMed] [Google Scholar]
- 9.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 10.Behan WM, McDonald M, Darlington LG, Stone TW. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: protection by melatonin and deprenyl. British journal of pharmacology. 1999;128:1754–1760. doi: 10.1038/sj.bjp.0702940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belevych N, Buchanan K, Chen Q, Bailey M, Quan N. Location-specific activation of the paraventricular nucleus of the hypothalamus by localized inflammation. Brain Behav Immun. 2010;24:1137–1147. doi: 10.1016/j.bbi.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bersano A, Aghemo A, Rumi MG, Ballabio E, Candelise L, Colombo M. Recovery after L-DOPA treatment in peginterferon and ribavirin induced parkinsonism. Eur J Intern Med. 2008;19:370–371. doi: 10.1016/j.ejim.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Blalock JE, Smith EM. Human leukocyte interferon (HuIFN-alpha): potent endorphin-like opioid activity. Biochem Biophys Res Commun. 1981;101:472–478. doi: 10.1016/0006-291x(81)91284-5. [DOI] [PubMed] [Google Scholar]
- 14.Blasi F, Riccio M, Brogi A, Strazza M, Taddei ML, Romagnoli S, Luddi A, D’Angelo R, Santi S, Costantino-Ceccarini E, Melli M. Constitutive expression of interleukin-1beta (IL-1beta) in rat oligodendrocytes. Biol Chem. 1999;380:259–264. doi: 10.1515/BC.1999.034. [DOI] [PubMed] [Google Scholar]
- 15.Bluthe RM, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- 16.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- 19.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burlina A, Blau N. Effect of BH(4) supplementation on phenylalanine tolerance. J Inherit Metab Dis. 2009;32:40–45. doi: 10.1007/s10545-008-0947-1. [DOI] [PubMed] [Google Scholar]
- 21.Burton BK, Bausell H, Katz R, Laduca H, Sullivan C. Sapropterin therapy increases stability of blood phenylalanine levels in patients with BH4-responsive phenylketonuria (PKU) Mol Genet Metab. 2010;101:110–114. doi: 10.1016/j.ymgme.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Butler JM, Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, Lesser G, McMullen K, McQuellon R, Naughton M, Rapp S, Stieber V, Shaw EG. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69:1496–1501. doi: 10.1016/j.ijrobp.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 23.Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochemistry international. 1998;32:117–131. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- 24.Candito M, Nagatsu T, Chambon P, Chatel M. High-performance liquid chromatographic measurement of cerebrospinal fluid tetrahydrobiopterin, neopterin, homovanillic acid and 5-hydroxindoleacetic acid in neurological diseases, Journal of chromatography. B. Biomedical applications. 1994;657:61–66. doi: 10.1016/0378-4347(94)80070-7. [DOI] [PubMed] [Google Scholar]
- 25.Cao C, Matsumura K, Yamagata K, Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol. 1997;272:R1712–1725. doi: 10.1152/ajpregu.1997.272.6.R1712. [DOI] [PubMed] [Google Scholar]
- 26.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 28.Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ. Treatment of cytokine-induced depression. Brain Behav Immun. 2002;16:575–580. doi: 10.1016/s0889-1591(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 29.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 32.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 33.Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic Mechanisms of Reduced Basal Ganglia Responses to Hedonic Reward during Interferon-alpha Administration. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2011.2094. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 35.Capuron L, Ravaud A, Miller AH, Dantzer R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain Behav Immun. 2004;18:205–213. doi: 10.1016/j.bbi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Jalabi W, Shpargel KB, Farabaugh KT, Dutta R, Yin X, Kidd GJ, Bergmann CC, Stohlman SA, Trapp BD. Lipopolysaccharide-Induced Microglial Activation and Neuroprotection against Experimental Brain Injury Is Independent of Hematogenous TLR4. J Neurosci. 2012;32:11706–11715. doi: 10.1523/JNEUROSCI.0730-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 39.Collins JM, Riccardi R, Trown P, O’Neill D, Poplack DG. Plasma and cerebrospinal fluid pharmacokinetics of recombinant interferon alpha A in monkeys: comparison of intravenous, intramuscular, and intraventricular delivery. Cancer drug delivery. 1985;2:247–253. doi: 10.1089/cdd.1985.2.247. [DOI] [PubMed] [Google Scholar]
- 40.Colton CA, Yao J, Keri JE, Gilbert D. Regulation of microglial function by interferons. J Neuroimmunol. 1992;40:89–98. doi: 10.1016/0165-5728(92)90216-8. [DOI] [PubMed] [Google Scholar]
- 41.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- 42.Cunnington C, Channon KM. Tetrahydrobiopterin: pleiotropic roles in cardiovascular pathophysiology. Heart (British Cardiac Society) 2010;96:1872–1877. doi: 10.1136/hrt.2009.180430. [DOI] [PubMed] [Google Scholar]
- 43.Curtin JF, King GD, Barcia C, Liu C, Hubert FX, Guillonneau C, Josien R, Anegon I, Lowenstein PR, Castro MG. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176:3566–3577. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dantzer R, Bluthe RM, Laye S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 46.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Pucchio T, Danese S, De Cristofaro R, Rutella S. Inhibitors of indoleamine 2,3- dioxygenase: a review of novel patented lead compounds. Expert Opin Ther Pat. 2010;20:229–250. doi: 10.1517/13543770903512974. [DOI] [PubMed] [Google Scholar]
- 48.di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol. 2000;23:190–194. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Dinarello CA. Infection, fever, and exogenous and endogenous pyrogens: some concepts have changed. J Endotoxin Res. 2004;10:201–222. doi: 10.1179/096805104225006129. [DOI] [PubMed] [Google Scholar]
- 50.Donnelly S. Patient management strategies for interferon alfa-2b as adjuvant therapy of high-risk melanoma. Oncol Nurs Forum. 1998;25:921–927. [PubMed] [Google Scholar]
- 51.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 52.Dumitrescu C, Biondi R, Xia Y, Cardounel AJ, Druhan LJ, Ambrosio G, Zweier JL. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BH4. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn AJ. Systemic interleukin-1 administration stimulates hypothalamic norepinephrine metabolism parallelling the increased plasma corticosterone. Life Sci. 1988;43:429–435. doi: 10.1016/0024-3205(88)90522-x. [DOI] [PubMed] [Google Scholar]
- 54.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- 57.Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, Saper CB. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381:119–129. doi: 10.1002/(sici)1096-9861(19970505)381:2<119::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 59.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 61.Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–428. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 62.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neuroscience and biobehavioral reviews. 2008;32:883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, Seishima M. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 66.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 67.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 68.Gelman BB, Spencer JA, Holzer CE, 3rd, Soukup VM. Abnormal striatal dopaminergic synapses in National NeuroAIDS Tissue Consortium subjects with HIV encephalitis. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2006;1:410–420. doi: 10.1007/s11481-006-9030-6. [DOI] [PubMed] [Google Scholar]
- 69.Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol. 2007;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- 70.Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61:631–637. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ginsberg LD, Oubre AY, Daoud YA. L-methylfolate Plus SSRI or SNRI from Treatment Initiation Compared to SSRI or SNRI Monotherapy in a Major Depressive Episode. Innov Clin Neurosci. 2011;8:19–28. [PMC free article] [PubMed] [Google Scholar]
- 72.Godfrey PS, Toone BK, Carney MW, Flynn TG, Bottiglieri T, Laundy M, Chanarin I, Reynolds EH. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336:392–395. doi: 10.1016/0140-6736(90)91942-4. [DOI] [PubMed] [Google Scholar]
- 73.Grace AA. Dopamine. In: DKL, Charney DS, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott Williams and Wilkins; Philadelphia: 2002. pp. 119–132. [Google Scholar]
- 74.Gray F, Scaravilli F, Everall I, Chretien F, An S, Boche D, Adle-Biassette H, Wingertsmann L, Durigon M, Hurtrel B, Chiodi F, Bell J, Lantos P. Neuropathology of early HIV-1 infection. Brain Pathol. 1996;6:1–15. doi: 10.1111/j.1750-3639.1996.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 75.Guidetti P, Schwarcz R. 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington’s disease? Adv Exp Med Biol. 2003;527:137–145. doi: 10.1007/978-1-4615-0135-0_16. [DOI] [PubMed] [Google Scholar]
- 76.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 77.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 78.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 79.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Molecular neurobiology. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- 81.Guillot TS, Richardson JR, Wang MZ, Li YJ, Taylor TN, Ciliax BJ, Zachrisson O, Mercer A, Miller GW. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008;42:423–434. doi: 10.1016/j.npep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haas HS, Schauenstein K. Neuroimmunomodulation via limbic structures--the neuroanatomy of psychoimmunology. Prog Neurobiol. 1997;51:195–222. doi: 10.1016/s0301-0082(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 83.Hadjiconstantinou M, Wemlinger TA, Sylvia CP, Hubble JP, Neff NH. Aromatic L- amino acid decarboxylase activity of mouse striatum is modulated via dopamine receptors. J Neurochem. 1993;60:2175–2180. doi: 10.1111/j.1471-4159.1993.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 84.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40:140–155. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 85.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 86.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 88.Hashimoto R, Nagatsu T, Ohta T, Mizutani M, Omura I. Changes in the concentrations of tetrahydrobiopterin, the cofactor of tyrosine hydroxylase, in blood under physical stress and in depression. Annals of the New York Academy of Sciences. 2004;1018:378–386. doi: 10.1196/annals.1296.047. [DOI] [PubMed] [Google Scholar]
- 89.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 90.Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain. 1993;116(Pt 6):1425–1450. doi: 10.1093/brain/116.6.1425. [DOI] [PubMed] [Google Scholar]
- 91.Ho BT, Huo YY, Lu JG, Tansey LW, Levin VA. Opioid-dopaminergic mechanisms in the potentiation of d-amphetamine discrimination by interferon-alpha. Pharmacol Biochem Behav. 1992;42:57–60. doi: 10.1016/0091-3057(92)90446-m. [DOI] [PubMed] [Google Scholar]
- 92.Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett. 2008;432:232–236. doi: 10.1016/j.neulet.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 93.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152:383–389. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- 95.Kaasinen V, Nurmi E, Bruck A, Eskola O, Bergman J, Solin O, Rinne JO. Increased frontal [(18)F]fluorodopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- 96.Kamata M, Higuchi H, Yoshimoto M, Yoshida K, Shimizu T. Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur Neuropsychopharmacol. 2000;10:129–132. doi: 10.1016/s0924-977x(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 97.Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec (Hoboken) 2009;292:1902–1913. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- 98.Katsuura G, Arimura A, Koves K, Gottschall PE. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am J Physiol. 1990;258:E163–171. doi: 10.1152/ajpendo.1990.258.1.E163. [DOI] [PubMed] [Google Scholar]
- 99.Kazumori H, Ishihara S, Rumi MA, Ortega-Cava CF, Kadowaki Y, Kinoshita Y. Transforming growth factor-alpha directly augments histidine decarboxylase and vesicular monoamine transporter 2 production in rat enterochromaffin-like cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G508–514. doi: 10.1152/ajpgi.00269.2003. [DOI] [PubMed] [Google Scholar]
- 100.Kim HY, Park EJ, Joe EH, Jou I. Curcumin suppresses Janus kinase-STAT inflammatory signaling through activation of Src homology 2 domain-containing tyrosine phosphatase 2 in brain microglia. J Immunol. 2003;171:6072–6079. doi: 10.4049/jimmunol.171.11.6072. [DOI] [PubMed] [Google Scholar]
- 101.Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- 102.Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- 103.Komaki G, Arimura A, Koves K. Effect of intravenous injection of IL-1 beta on PGE2 levels in several brain areas as determined by microdialysis. Am J Physiol. 1992;262:E246–251. doi: 10.1152/ajpendo.1992.262.2.E246. [DOI] [PubMed] [Google Scholar]
- 104.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 105.Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 106.Kostic VS, Susic V, Covickovic-Sternic N, Marinkovic Z, Jankovic S. Reduced rapid eye movement sleep latency in patients with Parkinson’s disease. J Neurol. 1989;236:421–423. doi: 10.1007/BF00314903. [DOI] [PubMed] [Google Scholar]
- 107.Kostic VS, Susic V, Przedborski S, Sternic N. Sleep EEG in depressed and nondepressed patients with Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1991;3:176–179. doi: 10.1176/jnp.3.2.176. [DOI] [PubMed] [Google Scholar]
- 108.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 109.Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, Halper J, Johnson B, Morgante L, Grimson R. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995;45:1956–1961. doi: 10.1212/wnl.45.11.1956. [DOI] [PubMed] [Google Scholar]
- 110.Kumai T, Tateishi T, Tanaka M, Watanabe M, Shimizu H, Kobayashi S. Effect of interferon-alpha on tyrosine hydroxylase and catecholamine levels in the brain of rats. Life Sci. 2000;67:663–669. doi: 10.1016/s0024-3205(00)00660-3. [DOI] [PubMed] [Google Scholar]
- 111.Kumakura Y, Cumming P. PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist. 2009;15:635–650. doi: 10.1177/1073858409338217. [DOI] [PubMed] [Google Scholar]
- 112.Kumakura Y, Gjedde A, Danielsen EH, Christensen S, Cumming P. Dopamine storage capacity in caudate and putamen of patients with early Parkinson’s disease: correlation with asymmetry of motor symptoms. J Cereb Blood Flow Metab. 2006;26:358–370. doi: 10.1038/sj.jcbfm.9600202. [DOI] [PubMed] [Google Scholar]
- 113.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 114.Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzymes (COX-1 and COX-2) in the rat brain. J Neurochem. 1998;70:452–466. doi: 10.1046/j.1471-4159.1998.70020452.x. [DOI] [PubMed] [Google Scholar]
- 115.LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leenders KL, Palmer AJ, Quinn N, Clark JC, Firnau G, Garnett ES, Nahmias C, Jones T, Marsden CD. Brain dopamine metabolism in patients with Parkinson’s disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry. 1986;49:853–860. doi: 10.1136/jnnp.49.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lestage J, Verrier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- 118.Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;86:774–783. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- 119.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci U S A. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 121.Loftis JM, Hauser P, Macey TA, Lowe JD. Can rodents be used to model interferon-alpha-induced depressive symptoms? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1364–1365. doi: 10.1016/j.pnpbp.2006.04.004. author reply 1366. [DOI] [PubMed] [Google Scholar]
- 122.Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferon-alpha-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31:1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 123.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 124.Maes M, Bosmans E, Suy E, Vandervorst C, DeJonckheere C, Raus J. Depression-related disturbances in mitogen-induced lymphocyte responses and interleukin-1 beta and soluble interleukin-2 receptor production. Acta Psychiatr Scand. 1991;84:379–386. doi: 10.1111/j.1600-0447.1991.tb03163.x. [DOI] [PubMed] [Google Scholar]
- 125.Maes M, Lambrechts J, Bosmans E, Jacobs J, Suy E, Vandervorst C, de Jonckheere C, Minner B, Raus J. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med. 1992;22:45–53. doi: 10.1017/s0033291700032712. [DOI] [PubMed] [Google Scholar]
- 126.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mar Fan HG, Clemons M, Xu W, Chemerynsky I, Breunis H, Braganza S, Tannock IF. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 128.Masilamoni GJ, Bogenpohl JW, Alagille D, Delevich K, Tamagnan G, Votaw JR, Wichmann T, Smith Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain. 2011;134:2057–2073. doi: 10.1093/brain/awr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci. 2004;9:2819–2826. doi: 10.2741/1439. [DOI] [PubMed] [Google Scholar]
- 130.Matute C. Glutamate and ATP signalling in white matter pathology. J Anat. 2011 doi: 10.1111/j.1469-7580.2010.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- 132.Maurino R, Machado A, Santiago M. Effect of in vivo striatal perfusion of lipopolysaccharide on dopamine metabolites. Neurosci Lett. 2010;475:121–123. doi: 10.1016/j.neulet.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 133.McKinney WT, Jr, Eising RG, Moran EC, Suomi SJ, Harlow HF. Effects of reserpine on the social behavior of rhesus monkeys. Dis Nerv Syst. 1971;32:735–741. [PubMed] [Google Scholar]
- 134.Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, Edwards C, Mattis P, Eidelberg D. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. Am J Psychiatry. 2002;159:746–754. doi: 10.1176/appi.ajp.159.5.746. [DOI] [PubMed] [Google Scholar]
- 135.Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Miller AL. The methylation, neurotransmitter, and antioxidant connections between folate and depression. Altern Med Rev. 2008;13:216–226. [PubMed] [Google Scholar]
- 139.Miller DW. Immunobiology of the blood-brain barrier. J Neurovirol. 1999;5:570–578. doi: 10.3109/13550289909021286. [DOI] [PubMed] [Google Scholar]
- 140.Mizoi Y, Kaneko H, Oharazawa A, Kuroiwa H. Parkinsonism in a patient receiving interferon alpha therapy for chronic hepatitis C. Rinsho Shinkeigaku. 1997;37:54–56. [PubMed] [Google Scholar]
- 141.Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T, Caligiuri MA, Villalona-Calero MA. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852–1859. doi: 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 142.Moraska AR, Sood A, Dakhil SR, Sloan JA, Barton D, Atherton PJ, Suh JJ, Griffin PC, Johnson DB, Ali A, Silberstein PT, Duane SF, Loprinzi CL. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28:3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morrow GR, Hickok JT, Roscoe JA, Raubertas RF, Andrews PL, Flynn PJ, Hynes HE, Banerjee TK, Kirshner JJ, King DK. Differential effects of paroxetine on fatigue and depression: a randomized, double-blind trial from the University of Rochester Cancer Center Community Clinical Oncology Program. J Clin Oncol. 2003;21:4635–4641. doi: 10.1200/JCO.2003.04.070. [DOI] [PubMed] [Google Scholar]
- 145.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 146.Nadjar A, Combe C, Busquet P, Dantzer R, Parnet P. Signaling pathways of interleukin-1 actions in the brain: anatomical distribution of phospho-ERK1/2 in the brain of rat treated systemically with interleukin-1beta. Neuroscience. 2005;134:921–932. doi: 10.1016/j.neuroscience.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 147.Nadjar A, Combe C, Laye S, Tridon V, Dantzer R, Amedee T, Parnet P. Nuclear factor kappaB nuclear translocation as a crucial marker of brain response to interleukin-1. A study in rat and interleukin-1 type I deficient mouse. J Neurochem. 2003;87:1024–1036. doi: 10.1046/j.1471-4159.2003.02097.x. [DOI] [PubMed] [Google Scholar]
- 148.Neurauter G, Schrocksnadel K, Scholl-Burgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, Fuchs D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab. 2008;9:622–627. doi: 10.2174/138920008785821738. [DOI] [PubMed] [Google Scholar]
- 149.Nichol CA, Lee CL, Edelstein MP, Chao JY, Duch DS. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1546–1550. doi: 10.1073/pnas.80.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O’Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, Castanon N, Herkenham M, Dantzer R, Kelley KW. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Palma JP, Kwon D, Clipstone NA, Kim BS. Infection with Theiler’s murine encephalomyelitis virus directly induces proinflammatory cytokines in primary astrocytes via NF-kappaB activation: potential role for the initiation of demyelinating disease. J Virol. 2003;77:6322–6331. doi: 10.1128/JVI.77.11.6322-6331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pan W, Kastin AJ. Interactions of cytokines with the blood-brain barrier: implications for feeding. Curr Pharm Des. 2003;9:827–831. doi: 10.2174/1381612033455332. [DOI] [PubMed] [Google Scholar]
- 155.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 156.Papakostas GI, Petersen T, Mischoulon D, Green CH, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 2: predictors of relapse during the continuation phase of pharmacotherapy. J Clin Psychiatry. 2004;65:1096–1098. doi: 10.4088/jcp.v65n0811. [DOI] [PubMed] [Google Scholar]
- 157.Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004;65:1090–1095. doi: 10.4088/jcp.v65n0810. [DOI] [PubMed] [Google Scholar]
- 158.Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res. 1997;17:589–595. doi: 10.1089/jir.1997.17.589. [DOI] [PubMed] [Google Scholar]
- 159.Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pucci E, Branas P, D’Amico R, Giuliani G, Solari A, Taus C. Amantadine for fatigue in multiple sclerosis. Cochrane database of systematic reviews (Online) 2007:CD002818. doi: 10.1002/14651858.CD002818.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rammohan KW, Rosenberg JH, Lynn DJ, Blumenfeld AM, Pollak CP, Nagaraja HN. Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. Journal of neurology, neurosurgery, and psychiatry. 2002;72:179–183. doi: 10.1136/jnnp.72.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reite M, Laudenslager M, Jones J, Crnic L, Kaemingk K. Interferon decreases REM latency. Biol Psychiatry. 1987;22:104–107. doi: 10.1016/0006-3223(87)90136-3. [DOI] [PubMed] [Google Scholar]
- 170.Reite M, Pegram GV, Stephens LM, Bixler EC, Lewis OL. The effect of reserpine and monoamine oxidase inhibitors on paradoxical sleep in the monkey. Psychopharmacologia. 1969;14:12–17. doi: 10.1007/BF00401529. [DOI] [PubMed] [Google Scholar]
- 171.Reyes TM, Coe CL. Interleukin-1 beta differentially affects interleukin-6 and soluble interleukin-6 receptor in the blood and central nervous system of the monkey. J Neuroimmunol. 1996;66:135–141. doi: 10.1016/0165-5728(96)00038-0. [DOI] [PubMed] [Google Scholar]
- 172.Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. The AAPS journal. 2006;8:E413–418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rohrig UF, Awad L, Grosdidier A, Larrieu P, Stroobant V, Colau D, Cerundolo V, Simpson AJ, Vogel P, Van den Eynde BJ, Zoete V, Michielin O. Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem. 2010;53:1172–1189. doi: 10.1021/jm9014718. [DOI] [PubMed] [Google Scholar]
- 174.Rosenzweig-Lipson S, Hesterberg P, Bergman J. Observational studies of dopamine D1 and D2 agonists in squirrel monkeys. Psychopharmacology (Berl) 1994;116:9–18. doi: 10.1007/BF02244865. [DOI] [PubMed] [Google Scholar]
- 175.Rothwell NJ, Luheshi G, Toulmond S. Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacol Ther. 1996;69:85–95. doi: 10.1016/0163-7258(95)02033-0. [DOI] [PubMed] [Google Scholar]
- 176.Sammut S, Goodall G, Muscat R. Acute interferon-alpha administration modulates sucrose consumption in the rat. Psychoneuroendocrinology. 2001;26:261–272. doi: 10.1016/s0306-4530(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 177.Santamaria A, Flores-Escartin A, Martinez JC, Osorio L, Galvan-Arzate S, Pedraza-Chaverri J, Maldonado PD, Medina-Campos ON, Jimenez-Capdeville ME, Manjarrez J, Rios C. Copper blocks quinolinic acid neurotoxicity in rats: contribution of antioxidant systems. Free radical biology & medicine. 2003;35:418–427. doi: 10.1016/s0891-5849(03)00317-4. [DOI] [PubMed] [Google Scholar]
- 178.Sarasombath P, Sumida K, Kaku DA. Parkinsonism associated with interferon alpha therapy for chronic myelogenous leukemia. Hawaii Med J. 2002;61:48, 57. [PubMed] [Google Scholar]
- 179.Sato T, Suzuki E, Yokoyama M, Semba J, Watanabe S, Miyaoka H. Chronic intraperitoneal injection of interferon-alpha reduces serotonin levels in various regions of rat brain, but does not change levels of serotonin transporter mRNA, nitrite or nitrate. Psychiatry Clin Neurosci. 2006;60:499–506. doi: 10.1111/j.1440-1819.2006.01538.x. [DOI] [PubMed] [Google Scholar]
- 180.Schobitz B, Voorhuis DA, De Kloet ER. Localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Neurosci Lett. 1992;136:189–192. doi: 10.1016/0304-3940(92)90046-a. [DOI] [PubMed] [Google Scholar]
- 181.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 183.Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O’Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci. 2007;27:9301–9309. doi: 10.1523/JNEUROSCI.1418-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- 185.Silverstein PS, Shah A, Gupte R, Liu X, Piepho RW, Kumar S, Kumar A. Methamphetamine toxicity and its implications during HIV-1 infection. Journal of neurovirology. 2011;17:401–415. doi: 10.1007/s13365-011-0043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sissolak G, Hoffbrand AV, Mehta AB, Ganeshaguru K. Effects of interferon-alpha (IFN) on the expression of interleukin 1-beta (IL-1), interleukin 6 (IL-6), granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor-alpha (TNF) in acute myeloid leukemia (AML) blasts. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 1992;6:1155–1160. [PubMed] [Google Scholar]
- 187.Sluzewska A. Indicators of immune activation in depressed patients. Adv Exp Med Biol. 1999;461:59–73. doi: 10.1007/978-0-585-37970-8_4. [DOI] [PubMed] [Google Scholar]
- 188.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 189.Smith RA, Norris F, Palmer D, Bernhardt L, Wills RJ. Distribution of alpha interferon in serum and cerebrospinal fluid after systemic administration. Clinical pharmacology and therapeutics. 1985;37:85–88. doi: 10.1038/clpt.1985.16. [DOI] [PubMed] [Google Scholar]
- 190.Song C, Horrobin DF, Leonard BE. The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry. 2006;39:88–99. doi: 10.1055/s-2006-941557. [DOI] [PubMed] [Google Scholar]
- 191.Sossi V, Doudet DJ, Holden JE. A reversible tracer analysis approach to the study of effective dopamine turnover. J Cereb Blood Flow Metab. 2001;21:469–476. doi: 10.1097/00004647-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 192.Spencer TJ, Madras BK, Bonab AA, Dougherty DD, Clarke A, Mirto T, Martin J, Fischman AJ. A positron emission tomography study examining the dopaminergic activity of armodafinil in adults using [(1)(1)C]altropane and [(1)(1)C]raclopride. Biol Psychiatry. 2010;68:964–970. doi: 10.1016/j.biopsych.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 193.Spetsieris PG, Moeller JR, Dhawan V, Ishikawa T, Eidelberg D. Visualizing the evolution of abnormal metabolic networks in the brain using PET. Comput Med Imaging Graph. 1995;19:295–306. doi: 10.1016/0895-6111(95)00011-e. [DOI] [PubMed] [Google Scholar]
- 194.Stahl SM. Novel therapeutics for depression: L-methylfolate as a trimonoamine modulator and antidepressant-augmenting agent. CNS Spectr. 2007;12:739–744. doi: 10.1017/s1092852900015418. [DOI] [PubMed] [Google Scholar]
- 195.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 196.Stankoff B, Waubant E, Confavreux C, Edan G, Debouverie M, Rumbach L, Moreau T, Pelletier J, Lubetzki C, Clanet M. Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 2005;64:1139–1143. doi: 10.1212/01.WNL.0000158272.27070.6A. [DOI] [PubMed] [Google Scholar]
- 197.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 198.Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends in pharmacological sciences. 2000;21:149–154. doi: 10.1016/s0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- 199.Stone TW. Kynurenic acid antagonists and kynurenine pathway inhibitors. Expert Opin Investig Drugs. 2001;10:633–645. doi: 10.1517/13543784.10.4.633. [DOI] [PubMed] [Google Scholar]
- 200.Sugawara Y, Akechi T, Shima Y, Okuyama T, Akizuki N, Nakano T, Uchitomi Y. Efficacy of methylphenidate for fatigue in advanced cancer patients: a preliminary study. Palliat Med. 2002;16:261–263. doi: 10.1191/0269216302pm547xx. [DOI] [PubMed] [Google Scholar]
- 201.Sunami M, Nishikawa T, Yorogi A, Shimoda M. Intravenous administration of levodopa ameliorated a refractory akathisia case induced by interferon-alpha. Clin Neuropharmacol. 2000;23:59–61. doi: 10.1097/00002826-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 202.Tavares RG, Schmidt AP, Abud J, Tasca CI, Souza DO. In vivo quinolinic acid increases synaptosomal glutamate release in rats: reversal by guanosine. Neurochemical research. 2005;30:439–444. doi: 10.1007/s11064-005-2678-0. [DOI] [PubMed] [Google Scholar]
- 203.Tavares RG, Tasca CI, Santos CES, Alves LB, Porciuncula LO, Emanuelli T, Souza DO. Quinolinic acid stimulates synaptosomal glutamate release and inhibits glutamate uptake into astrocytes. Neurochemistry international. 2002;40:621–627. doi: 10.1016/s0197-0186(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 204.Taylor JL, Grossberg SE. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998;25:23–29. [PubMed] [Google Scholar]
- 205.Tedeschi B, Barrett JN, Keane RW. Astrocytes produce interferon that enhances the expression of H-2 antigens on a subpopulation of brain cells. J Cell Biol. 1986;102:2244–2253. doi: 10.1083/jcb.102.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Theodore S, Cass WA, Maragos WF. Involvement of cytokines in human immunodeficiency virus-1 protein Tat and methamphetamine interactions in the striatum. Exp Neurol. 2006;199:490–498. doi: 10.1016/j.expneurol.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 207.Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH., 2nd Delivery of interferon- beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152:785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 208.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. Journal of neuroscience research. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 209.Trefz FK, Scheible D, Gotz H, Frauendienst-Egger G. Significance of genotype in tetrahydrobiopterin-responsive phenylketonuria. J Inherit Metab Dis. 2009;32:22–26. doi: 10.1007/s10545-008-0940-8. [DOI] [PubMed] [Google Scholar]
- 210.Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A. Dopamine release in chronic cannabis users: a [(11)c]raclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Utz JR, Lorentz CP, Markowitz D, Rudser KD, Diethelm-Okita B, Erickson D, Whitley CB. START, a double blind, placebo-controlled pharmacogenetic test of responsiveness to sapropterin dihydrochloride in phenylketonuria patients. Mol Genet Metab. 2012;105:193–197. doi: 10.1016/j.ymgme.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 212.Wang J, Campbell IL, Zhang H. Systemic interferon-alpha regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2008;13:293–301. doi: 10.1038/sj.mp.4002013. [DOI] [PubMed] [Google Scholar]
- 213.Wang JY, Zeng XY, Fan GX, Yuan YK, Tang JS. mu- but not delta- and kappa-opioid receptor mediates the nucleus submedius interferon-alpha-evoked antinociception in the rat. Neurosci Lett. 2006;397:254–258. doi: 10.1016/j.neulet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 214.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett. 1995;183:27–31. doi: 10.1016/0304-3940(94)11105-r. [DOI] [PubMed] [Google Scholar]
- 215.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–6903. [PubMed] [Google Scholar]
- 216.Wichmann T, DeLong MR. Oscillations in the basal ganglia. Nature. 1999;400:621–622. doi: 10.1038/23148. [DOI] [PubMed] [Google Scholar]
- 217.Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv Neurol. 2003;91:9–18. [PubMed] [Google Scholar]
- 218.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 219.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. The Journal of biological chemistry. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 220.Yamada T, Horisberger MA, Kawaguchi N, Moroo I, Toyoda T. Immunohistochemistry using antibodies to alpha-interferon and its induced protein, MxA, in Alzheimer’s and Parkinson’s disease brain tissues. Neurosci Lett. 1994;181:61–64. doi: 10.1016/0304-3940(94)90560-6. [DOI] [PubMed] [Google Scholar]
- 221.Yirmiya R. Depression in medical illness: the role of the immune system. West J Med. 2000;173:333–336. doi: 10.1136/ewjm.173.5.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 223.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, Shavit Y, Ovadia H, Weidenfeld J, Morag A, Newman ME, Pollmacher T. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 224.Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv Exp Med Biol. 1999;461:283–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]
- 225.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 226.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 227.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 Receptor Activation by Systemic Lipopolysaccharide Induces Behavioral Despair Linked to MAPK Regulation of CNS Serotonin Transporters. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zifko UA, Rupp M, Schwarz S, Zipko HT, Maida EM. Modafinil in treatment of fatigue in multiple sclerosis. Results of an open-label study. Journal of neurology. 2002;249:983–987. doi: 10.1007/s00415-002-0765-6. [DOI] [PubMed] [Google Scholar]