Abstract
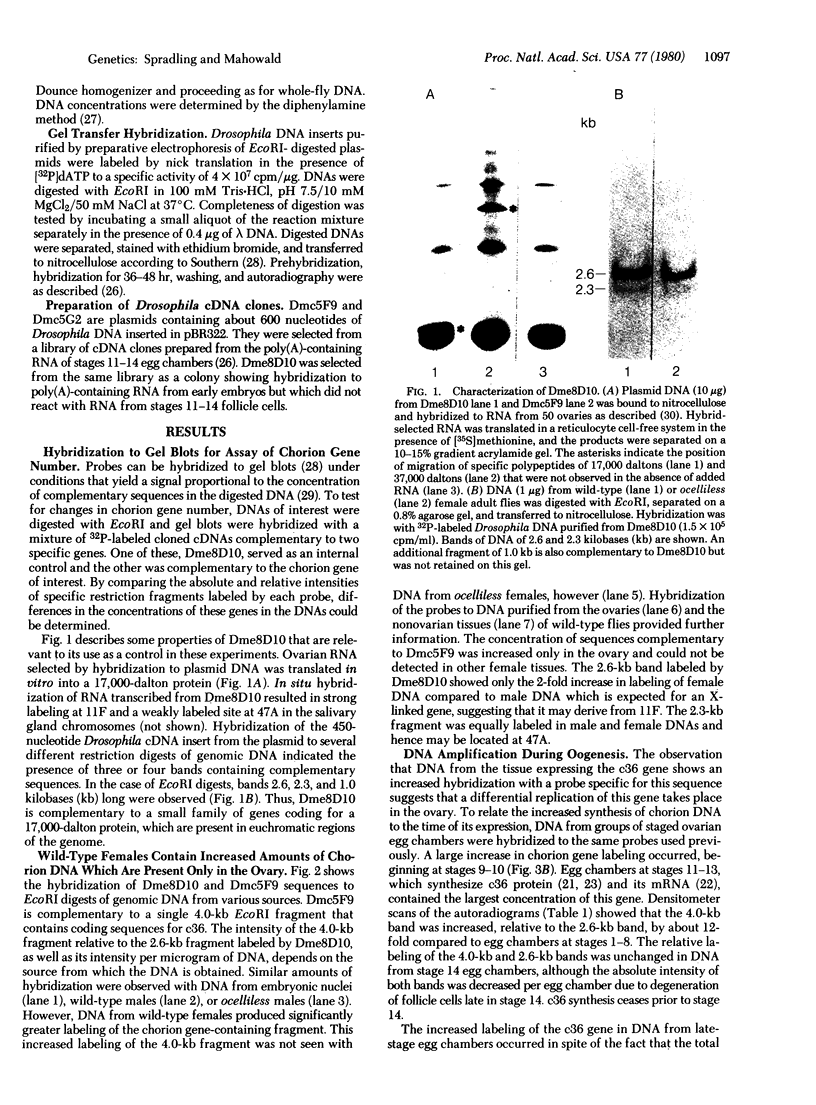
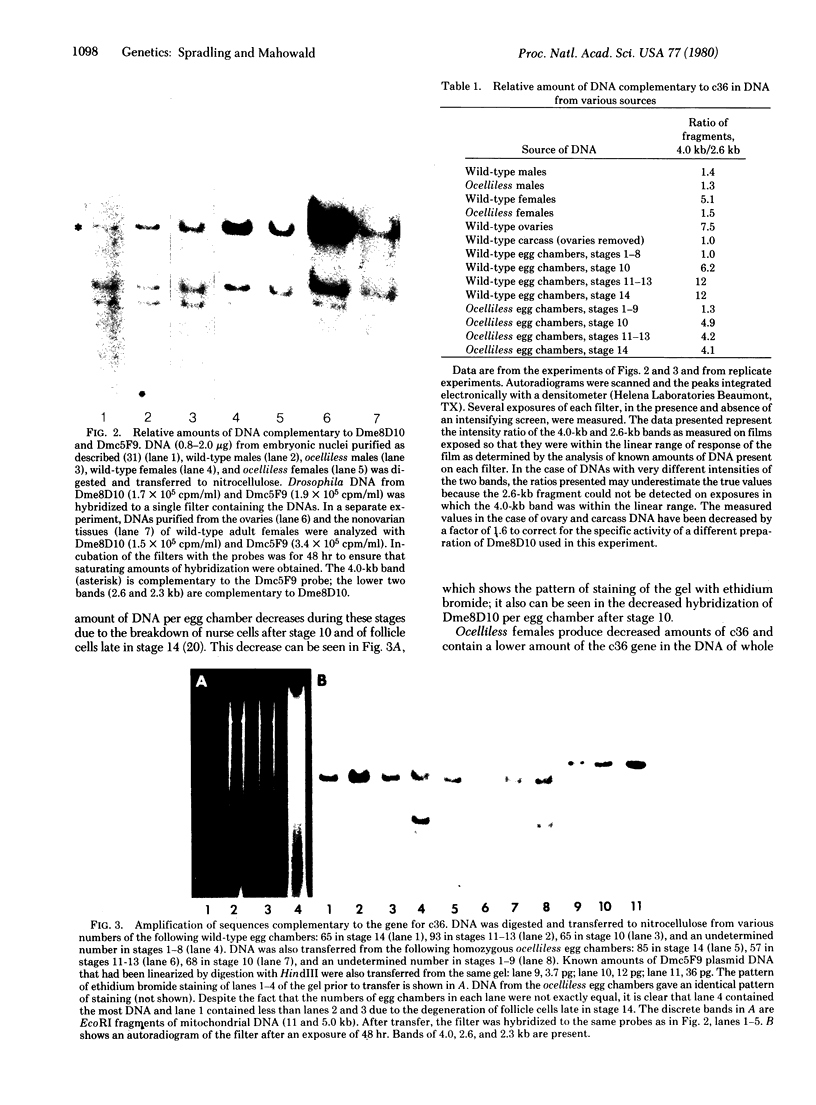
The endochorion and exochorion of Drosophila eggs are synthesized by the ovarian follicle cells during a brief period of about 5 hr. In this terminal phase of egg chamber development, the structural genes for several abundant chorion proteins are expressed at high levels according to a temporally regulated program. The female-sterile mutation ocelliless maps at the site of the genes for two of these proteins, the 36,000- and 38,000-dalton chorion proteins (c36 and c38), which are closely linked. The mutation results in a cis-acting reduction in the amounts of c36 and c38 that accumulate in late-stage egg chambers. We have investigated the mechanism that underlies this decreased production by using cDNA clones complementary to these gene sequences. Unexpectedly, it was found that, in normal females, the genes for c36, c38, and at least one other chorion protein are specifically amplified more than 10-fold in the DNA of late-stage egg chambers. The extra replication involves at least some adjacent chromosomal sequences and begins prior to the onset of mRNA and protein synthesis. The additional DNA remains stable after gene expression has ceased. The behavior of these genes is thus reminiscent of the properties of the DNA puffs that have been described in several groups of Diptera. The extent of amplification of c36 and c38, but not of the 18,000-dalton chorion protein c18 (which is unlinked), was decreased in the egg chambers of flies homozygous for ocelliless, suggesting that altered gene dosage may be responsible for the decreased synthesis of chorion proteins in the mutant.
Keywords: ocelliless mutation, polyploidization, DNA puffs, ovarian follicle cells
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- BREUER M. E., PAVAN C. Behavior of polytene chromosomes of Rhynchosciara angelae at different stages of larval development. Chromosoma. 1955;7(4):341–386. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Bonaldo M. F., Santelli R. V., Lara F. J. The transcript from a DNA puff of Rhynchosciara and its migration to the cytoplasm. Cell. 1979 Aug;17(4):827–833. doi: 10.1016/0092-8674(79)90323-4. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Gall J. G. Differential replication of satellite DNA in polyploid tissues of Drosophila virilis. Chromosoma. 1975;50(2):175–179. doi: 10.1007/BF00283238. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Manning R. F. Determination of the multiplicity of the silk fibroin gene and detection of fibroin gene-related DNA in the genome of Bombyx mori. J Mol Biol. 1976 Mar 5;101(3):327–348. doi: 10.1016/0022-2836(76)90151-0. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Differential synthesis of the genes for ribosomal RNA during amphibian oögenesis. Proc Natl Acad Sci U S A. 1968 Jun;60(2):553–560. doi: 10.1073/pnas.60.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R., Birnie G. D., Hell A., Humphries S., Young B. D., Paul J. Kinetic studies of gene frequency. I. Use of a DNA copy of reticulocyte 9 S RNA to estimate globin gene dosage in mouse tissues. J Mol Biol. 1974 Apr 25;84(4):539–554. doi: 10.1016/0022-2836(74)90115-6. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Dressler D., Wolfson J. The amplification of ribosomal RNA genes involves a rolling circle intermediate. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2926–2930. doi: 10.1073/pnas.70.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Regier J. C., Mazur G. D., Nadel M. R., Blau H. M., Petri W. H., Wyman A. R., Gelinas R. E., Moore P. B., Paul M. The eggshell of insects: differentiation-specific proteins and the control of their synthesis and accumulation during development. Results Probl Cell Differ. 1977;8:45–145. doi: 10.1007/978-3-540-37332-2_2. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C. The cocoonase zymogen cells of silk moths: a model of terminal cell differentiation for specific protein synthesis. Curr Top Dev Biol. 1972;7:125–191. doi: 10.1016/s0070-2153(08)60071-x. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Prestidge L., Hogness D. S. A novel arrangement of tandemly repeated genes at a major heat shock site in D. melanogaster. Cell. 1978 Aug;14(4):901–919. doi: 10.1016/0092-8674(78)90345-8. [DOI] [PubMed] [Google Scholar]
- Mahowald A. P., Caulton J. H., Edwards M. K., Floyd A. D. Loss of centrioles and polyploidization in follicle cells of Drosophila melanogaster. Exp Cell Res. 1979 Feb;118(2):404–410. doi: 10.1016/0014-4827(79)90167-8. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Sullivan N. L., Miller O. L., Jr Visualization of the silk fibroin transcription unit and nascent silk fibroin molecules on polyribosomes of Bombyx mori. Prog Nucleic Acid Res Mol Biol. 1976;19:313–318. doi: 10.1016/s0079-6603(08)60928-9. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packman S., Aviv H., Ross J., Leder P. A comparison of globin genes in duck reticulocytes and liver cells. Biochem Biophys Res Commun. 1972 Nov 1;49(3):813–819. doi: 10.1016/0006-291x(72)90483-4. [DOI] [PubMed] [Google Scholar]
- Petri W. H., Wyman A. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. III. The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev Biol. 1976 Mar;49(1):185–199. doi: 10.1016/0012-1606(76)90266-9. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudkin G. T. Non replicating DNA in Drosophila. Genetics. 1969;61(1 Suppl):227–238. [PubMed] [Google Scholar]
- Schachat F. H., Hogness D. S. Repetitive sequences in isolated Thomas circles from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1974;38:371–381. doi: 10.1101/sqb.1974.038.01.040. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spear B. B., Gall J. G. Independent control of ribosomal gene replication in polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1973 May;70(5):1359–1363. doi: 10.1073/pnas.70.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Mahowald A. P. Identification and genetic localization of mRNAs from ovarian follicle cells of Drosophila melanogaster. Cell. 1979 Mar;16(3):589–598. doi: 10.1016/0092-8674(79)90032-1. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Waring G. L., Mahowald A. P. Drosophila bearing the ocelliless mutation underproduce two major chorion proteins both of which map near this gene. Cell. 1979 Mar;16(3):609–616. doi: 10.1016/0092-8674(79)90034-5. [DOI] [PubMed] [Google Scholar]
- Sullivan D., Palacios R., Stavnezer J., Taylor J. M., Faras A. J., Kiely M. L., Summers N. M., Bishop J. M., Schimke R. T. Synthesis of a deoxyribonucleic acid sequence complementary to ovalbumin messenger ribonucleic acid and quantification of ovalbumin genes. J Biol Chem. 1973 Nov 10;248(21):7530–7539. [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Giza P. E. Accentuated expression of silk fibroin genes in vivo and in vitro. J Mol Biol. 1976 Nov 5;107(3):183–206. doi: 10.1016/s0022-2836(76)80001-0. [DOI] [PubMed] [Google Scholar]
- Waring G. L., Mahowald A. P. Identification and time of synthesis of chorion proteins in Drosophila melanogaster. Cell. 1979 Mar;16(3):599–607. doi: 10.1016/0092-8674(79)90033-3. [DOI] [PubMed] [Google Scholar]