Abstract
Cardiovascular disease is the leading cause of death worldwide. Unstable atherosclerotic plaques are prone to rupture followed by thrombus formation, vessel stenosis, and occlusion and frequently lead to acute myocardial infarction and brain infarction. As such, unstable plaques represent an important diagnostic target in clinical settings and the specific diagnosis of unstable plaques would enable preventive treatments for cardiovascular disease. To date, various imaging methods such as computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), and intravascular ultrasound (IVUS) have been widely used clinically. Although these methods have advantages in terms of spatial resolution and the ability to make detailed identification of morphological alterations such as calcifications and vessel stenosis, these techniques require skill or expertise to discriminate plaque instability, which is essential for early diagnosis and treatment and can present difficulties for quantitative estimation. On the other hand, nuclear imaging techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) can noninvasively collect quantitative information on the expression levels of functional molecules and metabolic activities in vivo and thus provide functional diagnoses of unstable plaques with high sensitivity. Specifically, unstable plaques are characterized by an abundance of invasive inflammatory cells (macrophages), increased oxidative stress that increases oxidized LDL and its receptor expressed on cells in the lesions, increased occurrence of apoptosis of macrophages and other cells involved in disease progression, increased protease expression and activity, and finally thrombus formation triggered by plaque rupture, which is the most important mechanism leading to the onset of infarctions and ischemic sudden death. Therefore, these characteristics can all be targets for molecular imaging by PET and SPECT. In this paper, we review the present state and future of radiolabelled probes that have been developed for detecting atherosclerotic unstable plaques with nuclear imaging techniques.
Keywords: Molecular imaging, atherosclerosis, plaque, positron emission tomography, single photon emission computed tomography, 2-[18F]Fluoro-2-deoxy-D-glucose, lectin-like oxidized low density lipoprotein receptor-1, apoptosis, matrix metalloproteinase, thrombus
Introduction
Despite recent therapeutic advances, cardiovascular disease remains the leading cause of death worldwide [1]. Since stenosis severity is reported to be a poor predictor of subsequent acute myocardial infarction (AMI) [1], methods to directly evaluate the biological properties of atherosclerotic lesions would be valuable diagnostic tools [2]. Atherosclerotic plaques formed by lipid accumulation in vessel lesions have a variety of characteristics, ranging from stable to unstable [3]. Unstable plaques are prone to rupture followed by thrombus formation, vessel stenosis, and occlusion and frequently lead to AMI and brain infarction [4,5]. Thus, the specific diagnosis of unstable plaques would enable preventive treatments for AMI and brain infarction and represents a promising diagnostic target in clinical settings.
Unstable plaques are characterized by a large, soft lipid core that contains extracellular lipids and is covered by a thin fibrous cap, as well as an abundance of invasive inflammatory cells such as macrophages. In contrast, stable plaques have a small lipid core, thick fibrous caps, and little or no macrophage invasion with the development of fibrous tissue resulting in intimal thickening of the vessel [6-9].
To date, various non-invasive imaging and invasive methods such as computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US) and intravascular ultrasound (IVUS) have been widely used clinically [10-15]. These approaches have advantages in terms of spatial resolution and the ability to identify in detail morphological alterations such as calcifications and vessel stenosis, a degree of which is retained by compensative outward dilation of the vessel, especially in the early phase of atherosclerosis, and further deteriorates after over 40% of the growth [16]. Thus, such morphological imaging techniques have disadvantages in detecting early/mild atherosclerosis. Furthermore, about 70% of diseases leading to AMI were reported to be those with less than 50% stenosis and 80-90% of the culprit lesions for infarction were those with less than 70% stenosis [17]. Therefore, the functional discrimination of plaque instability rather than the degree of stenosis is essential for early diagnosis and treatment. Although efforts have been made towards detecting unstable plaques using these morphological imaging techniques, they require skill or expertise to distinguish the plaque properties, which could make quantitative estimation challenging. On the other hand, nuclear imaging techniques such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) can noninvasively collect quantitative information on the expression levels of functional molecules and metabolic activities in vivo and thus provide functional diagnoses of unstable plaques with high sensitivity [18].
In this paper, we review the present state and future of radiolabelled probes (Figures 1 and 2) that have been developed for detecting unstable atherosclerotic plaques using nuclear imaging techniques [2,3,19-24].
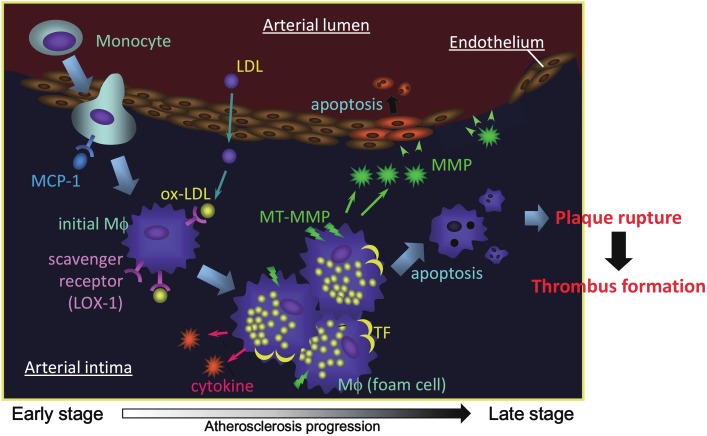
Figure 1.
Atherosclerosis progression and potential targets for molecular imaging. MCP-1: monocyte chemotactic protein-1, LDL: low density lipoprotein, Mφ: macrophage, LOX-1: lectin-like oxidized LDL receptor-1, MMP: matrix metalloproteinase, MT-MMP: membrane type MMP, TF: tissue factor.
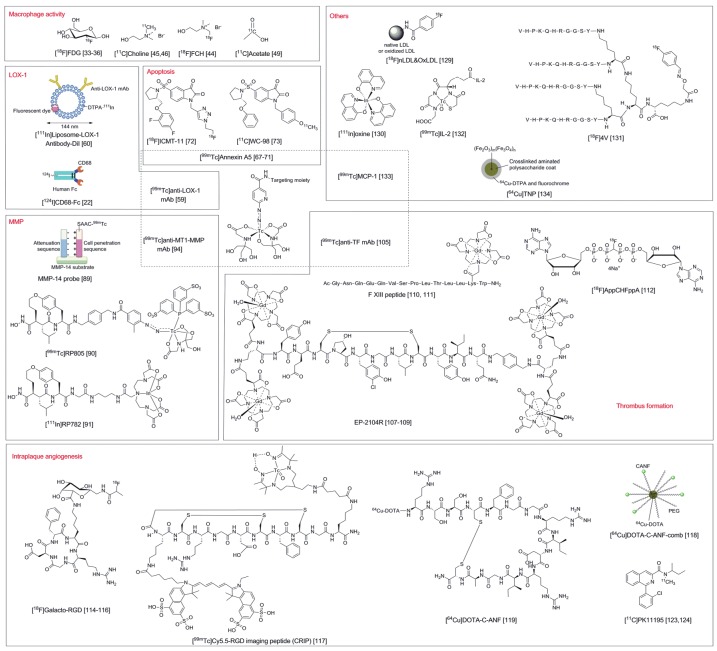
Figure 2.
Probes described in this article. DTPA: diethylenetriaminepentaacetic acid, Dil: long-chain dialkylcarbocyanine, SAAC: single amino acid chelate.
Probes to detect macrophage activity
2-[18F]Fluoro-2-deoxy-D-glucose ([18F]FDG) is taken up by cells via the glucose transporter and trapped inside cells after phosphorylation by hexokinase [25]. [18F]FDG has proven to be useful in varied clinical fields including diagnosis, prediction and measurement of treatment effectiveness in tumors, myocardial infarction, and epilepsy, making it one of the most commonly used probes in nuclear medicine [26-29]. In unstable plaques, an abundance of invading macrophages causes inflammation that makes plaques vulnerable to rupture. Therefore, specific imaging of macrophages is effective for diagnosing unstable plaques in atherosclerotic lesions. Since glucose metabolism is active in macrophages, [18F]FDG has been used widely for basic and clinical studies [27].
In a basic study using a rabbit model, myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits (WHHLMI rabbit), which present atherosclerotic lesions similar to those seen in humans [30-32], significantly higher accumulation of [18F]FDG in WHHLMI rabbit vessels than that in normal New Zealand White rabbit vessels was observed. Also, the probe accumulation in vessels of model rabbits was related to the quantity of macrophages and not intimal thickening, indicating that [18F]FDG can diagnose unstable plaques as it detects invading macrophages [33]. In fact, the usefulness of [18F]FDG to detect unstable plaques has been indicated by several clinical studies [34-36].
Since several statins reduce cholesterol levels in the blood but do not directly affect the accumulation of invading macrophages in atherosclerotic lesions [37,38], [18F]FDG imaging to diagnose the vulnerability of lesions during drug treatment should be useful for planning subsequent treatments as well as aiding drug development in clinical trials. In studies where rabbits were treated with probucol, [18F]FDG uptake in the atherosclerotic vessels decreased along with reductions in macrophages [39-41]. In clinical trials to develop atherosclerotic drugs, [18F]FDG imaging was used as an imaging biomarker [42]. Despite its usefulness as a probe, care must be taken in evaluating [18F] FDG imaging results because [18F]FDG uptake in vessels can be affected by several factors such as diet and lifestyle changes, as well as drug administration [43].
In addition, plaque-invading macrophages accompany increased construction of cell membranes with proliferative activation, which induces an increase in the uptake of choline, a constituent of the cell membrane. Thus, [11C] choline and the 18F labeled choline analog ([18F] fluoro choline ([18F]FCH)) were evaluated for their ability to detect unstable plaques and both were reported to image unstable plaques with higher sensitivity than [18F]FDG [44-46]. Fatty acids are a common constituent of atherosclerotic plaques and are synthesized in the plaque. Since a main substrate of fatty acid synthesis is acetyl-coenzyme-A, which is produced from acetate [47], [11C]acetate PET may have the potential to provide additional information for characterizing atherosclerotic plaques, similar to its current use in imaging procedures used to evaluate tumors and myocardial oxidative metabolism [48]. Indeed, the feasibility of [11C]acetate PET for imaging arterial wall alterations has been demonstrated in a cohort of asymptomatic patients [49].
Probes to detect LOX-1
Lectin-like oxidized LDL receptor-1 (LOX-1) is a receptor for oxidized LDL and is expressed on vascular endothelial cells, smooth muscle cells, monocytes, and macrophages in atherosclerotic lesions. LOX-1 is related to lesion progression and induces development of plaques and destabilization by several mechanisms: 1) induction of cell adhesion molecule and leucocyte chemotactic factor expression on the surface of vascular endothelial cells; 2) facilitation of foam cell formation from macrophages; 3) induction of apoptosis in smooth muscle cells (spontaneous cell death); and 4) induction of matrix metalloproteinases (MMP) that degrade the extracellular matrix in plaques [50-57]. Therefore, LOX-1 is an important target for nuclear imaging. In fact, the instability of atherosclerotic plaques was reported to be highly correlated with LOX-1 expression in a WHHLMI rabbit model [58].
A radiolabeled probe for LOX-1, 99mTc labeled monoclonal antibody ([99mTc]anti-LOX-1 mAb), was evaluated in WHHLMI rabbits [59]. As expected from the expression profile of LOX-1 in atherosclerotic vessels, [99mTc]anti-LOX-1 mAb, which recognizes the LOX-1 protein extracellular domain as its epitope, could detect atherosclerotic vessels in WHHLMI rabbits in planar imaging when compared with control rabbits (Figure 3). In addition, the radioactivity accumulation in vessels was correlated with the instability index of lesions estimated from immunohistochemical staining and the specificity of accumulation in unstable lesions was higher than that of [18F]FDG. Another example of the use of LOX-1 as a targeting probe was a liposome probe coated with anti-LOX-1 mAb on its surface as a targeting moiety and loaded with 111In, Gd, and fluorophores as signal emitting moieties for multimodality imaging [60]. In vivo evaluation using ApoE knock out mice revealed that this multifunctional probe could image atherosclerotic lesions by MRI, as well as with optical and nuclear imaging. In accordance with results from a previous study, radioactivity accumulation was related to LOX-1 expression, macrophage existence, apoptosis occurrence, and MMP-9 expression, indicating that LOX-1 is a promising target for evaluating unstable plaques in in vivo imaging. Another scavenger receptor, CD68, has been studied as a target for molecular imaging. 124I labeled CD68 conjugated to an Fc-fragment was evaluated as a tracer in ApoE knock out mice to indicate the enhanced radioactivity in aortic lesions by ex vivo autoradiographic analysis [22].
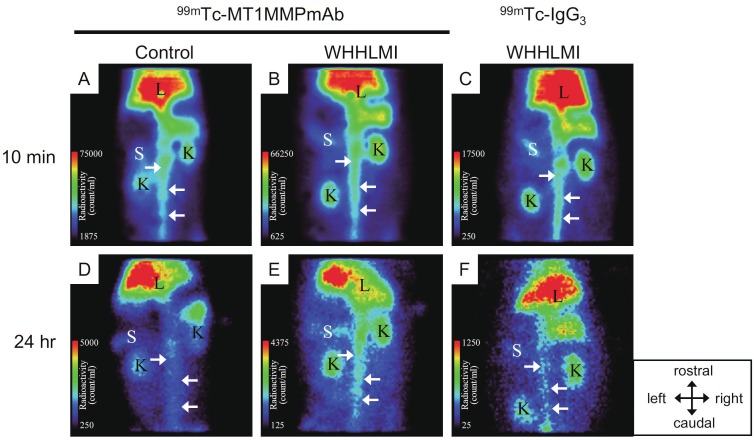
Figure 3.
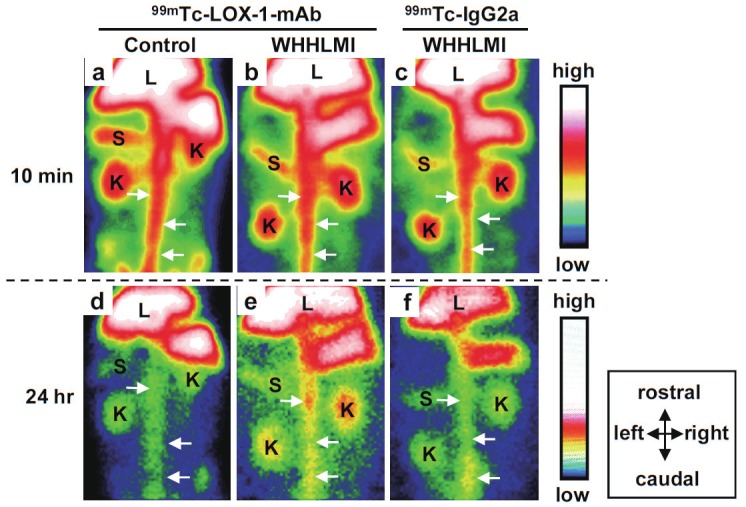
Planar images of WHHLMI and JW rabbits at 10 min and 24 hr post administration of 99mTc-LOX-1 mAb and subclass-matched control antibody (99mTc-IgG2a). The atherosclerotic abdominal aorta was clearly visible 24 hr post administration in the WHHLMI rabbits given 99mTc-LOX-1 mAb while high blood pool radioactivity in the abdominal aorta was shown in every rabbit at 10 min. Arrows = aorta; K = kidney; L = liver; S = spleen.
Probes to detect apoptosis
During atherosclerosis progression, macrophage apoptosis contributes to the formation of the lipid core of plaques while smooth muscle cell apoptosis destabilizes the plaque’s fibrous cap by suppressing extracellular matrix formation [61,62]. As such, apoptosis imaging is useful for evaluating the instability of atherosclerotic lesions. [99mTc]Annexin A5 was developed as an imaging agent for apoptosis due to its ability to bind to phosphatidyl serines that typically reside in the inner leaflet of cell membranes and become exposed on the outer surface of cell membranes during apoptosis [63-66]. [99mTc]Annexin A5 has also been widely used in atherosclerotic imaging [67-70] and reportedly can image unstable plaques more specifically than [18F]FDG [71]. The accumulation of [99mTc]Annexin A5 in atherosclerotic plaques represents the treatment efficiency of caspase inhibitors [70]. While apoptosis has been recognized as a promising target to estimate atherosclerosis in studies with [99mTc] Annexin A5, low molecular weight probes such as 18F labeled isatin derivatives have also been developed recently [72,73].
Probes to detect MMP
Unstable plaques are morphologically characterized by a thin fibrous cap that overlays a large lipid core. MMPs degrade the extracellular matrix that constitutes this fibrous cap, resulting in plaque destabilization [74-76].
MMPs can be divided into two groups: soluble and membrane-bound [77-79]. Most soluble MMPs, including MMP-2 and MMP-9, require extracellular post-translational cleavage to become biologically active following release from cells. A membrane-bound MMP, membrane type-1 matrix metalloproteinase (MT1-MMP or MMP-14), mediates activation of MMP-2 and MMP-13 on the cell surface. Increased expression of MMP-2 and MMP-9 has been observed in human atherosclerotic lesions [74,80,81] and these MMPs are known to cleave native type IV, V, VII, and X collagens and elastin, as well as the degradation products of collagens types I, II, and III after proteolysis by collagenases such as MMP-1 and MMP-13. In a recent animal study, co-distribution of MT1-MMP and MMP-2 was demonstrated in grade IV atheroma, indicating a possible role for MT1-MMP in the destabilization of atherosclerotic plaques [82], which is supported by MT1-MMP expression that was observed in human atherosclerotic plaques [83,84]. Thus, MMPs are potential targets for diagnostic imaging of atherosclerotic plaques that are at higher risk for rupture [85,86]. In the development of MMP imaging probes, the following three approaches have been pursued.
The first strategy involves radiolabelled MMP substrates that remain within the lesions after degradation by activated MMPs [87] and was used in one of the first attempts to image MMP activity in vivo for optical imaging of tumor-associated MMP activity. The resulting imaging probe was a kind of smart probe containing a MMP-2 peptide substrate with quenched near-infrared fluorochromes that are cleaved upon recognition by the MMP [88]. Recently, an activatable SPECT imaging probe specific for MMP-14 using a cell penetrating peptide as the retention moiety in the cells after MMP recognition was reported to be partly successful in in vitro experiments [89].
The second strategy uses radiolabelled MMP inhibitors such as [99mTc]RP805 (MPI) and [111In] RP782, which have been shown to have higher accumulation in vessels of apoE KO mice as compared to control mice [90,91]. The vessel accumulation of [99mTc]RP805 was reported to be correlated with the expression of MMP-2, -9 and macrophages and is effective for assessing the treatment effect of statins [92]. In addition, since MMP-2 and MMP-9 expression was higher than the rate of apoptosis in progressive atherosclerotic lesions in apoE KO mice, [99mTc] RP805 was presumed to be more useful for evaluating later stages of atherosclerosis than is [99mTc]Annexin A5 [93]. Further, low uptake of [99mTc]RP805 in the myocardium provided another advantage over [18F]FDG to yield high S/N ratios for imaging of coronary arteries [22].
The third strategy involves radiolabelled monoclonal antibodies such as 99mTc labeled monoclonal antibodies specific for membrane-bound MMPs ([99mTc]anti-MT1-MMP mAb) [94]. In a recent study comparing MMP-2 with MT1-MMP, MT1-MMP was reported to be expressed specifically in unstable lesions that are prone to rupture, indicating its potential use as a target for molecular imaging [82]. Indeed, [99mTc]anti-MT1-MMP mAb accumulated in unstable lesions (grade IV atheroma) specifically in the vessels of WHHLMI rabbits (Figure 4) [94].
Figure 4.
Planar images of WHHLMI and JW rabbits at 10 min and 24 hr post administration of 99mTc-MT1-MMP mAb and subclass-matched control antibody (99mTc-IgG3). The atherosclerotic abdominal aorta was clearly visible 24 hr post administration in the WHHLMI rabbits given 99mTc-MT1-MMP mAb while high blood pool radioactivity in the abdominal aorta was shown in every rabbit at 10 min. Arrows = aorta; K = kidney; L = liver; S = spleen.
Together with the studies described above, MMPs have long been recognized as important targets for evaluating atherosclerotic plaque instability. Other MMP subtypes such as MMP-12 have recently attracted a great deal of attention as a target for atherosclerosis treatment [95], with recent studies indicating a detrimental role for MMP-12 in plaque progression and instability in both mouse [96] and rabbit models [97,98] and an association between MMP-12 expression and advanced human atherosclerotic lesions [99,100]. Also, treatment of apoE KO mice with selective MMP-12 inhibitors could retard atherosclerosis development resulting in a more fibrous plaque phenotype [95], indicating that development of a MMP-12-selective imaging probe would be desirable for diagnosis and/or prediction of atherosclerosis and selection of drug treatments. In general, the complex relationship between MMP activity and plaque stability has made understanding the roles of each MMP subtype in atherosclerosis challenging, and the close structural similarity of the MMP active sites has made developing highly selective MMP inhibitors difficult. However, recent advances in novel peptide chemistry have made it possible to provide more selective inhibitors of zinc-proteases and this strategy could also be applied for developing selective MMP inhibitors [101].
Probes to detect thrombus formation
Thrombus formation triggered by plaque rupture is the most important mechanism leading to the onset of AMI and ischemic sudden death. Thus, thrombus-forming vulnerable plaques are a clinically important target for estimating risk and providing more effective and precise treatments. Tissue factor (TF) initiates the exogenous blood coagulation cascade that leads to thrombus formation in vivo. Although TF in atherosclerotic lesions was identified in several cell types, including endothelial cells, smooth muscle cells, monocytes, macrophages, and foam cells [102], TF expression is reported to be increased in the later stages of atheromatous progression and thus was selectively detected in atheromatous lesions in both animal and human studies [103,104]. In the blood coagulation cascade, TF initiates the cascade, while factor XIII covalently cross-links fibrin polymers and renders the thrombus more resistant to lysis.
The use of a 99mTc-labelled anti-TF monoclonal antibody as a TF imaging agent was recently reported [105]. In ex vivo experiments using WHHLMI rabbits, a close relationship between probe accumulation and TF expression in lesions and the selective accumulation of the probe in atheromatous lesions was indicated. Thus, imaging of TF has the potential to selectively detect atheromatous plaques that are at higher risk for rupture. Figure 5 illustrates the expression profiles of LOX-1, MT1-MMP, and TF that were evaluated under the same experimental conditions as those used previously with WHHLMI rabbits [59,94,105]. Although each of the three molecules is expressed mainly in atheromatous lesions, TF is the most selective in rupture-prone lesions, while the LOX-1 profile is closely related to macrophage distribution. On the other hand, a series of imaging agents have targeted fibrin and factor XIII in thrombi using antibodies or peptides [106]. EP-2104R, an 11-amino-acid peptide conjugated to four gadolinium-tetraaza-cyclododecane tetraacetic acid moieties (Gd-DOTA), binds fibrin with micromolar affinity and can detect thrombi in vivo in pigs and patients using a clinical 1.5T whole-body MRI system with high-signal amplification [107-109]. Probes that conjugate a factor XIII substrate peptide to fluorochromes or 111In-chelates were reportedly able to visualize factor XIII activity in clotted human plasma in vitro, and in acute murine thrombi induced by FeCl3 [110,111]. Since P2, P3-monochloromethylene diadenosine-5΄, 5΄΄΄-P1, P4-tetraphosphate (AppCHClppA) is a competitive inhibitor of adenosine diphosphateinduced platelet aggregation, the 18F labeled analog ([18F]AppCHFppA) has then been studied as an imaging probe that can accumulate in macrophage-rich atherosclerotic plaques in rabbit models and thus may merit further evaluation [112]. Although further studies are required to investigate which target molecule(s) in the blood coagulation cascade are the most appropriate for estimating the in vivo vulnerability of a plaque, [99mTc]anti-TF mAb will be useful for early detection of the cascade while fibrin and factor XIII imaging probes can detect later stages and thrombi themselves. Furthermore, given the great efforts that have been made in developing anti-coagulation and anti-platelet pharmaceuticals for treating atherosclerosis and hyperlipidemia, effective imaging probes that target blood coagulation cascades are also required for efficient drug development.
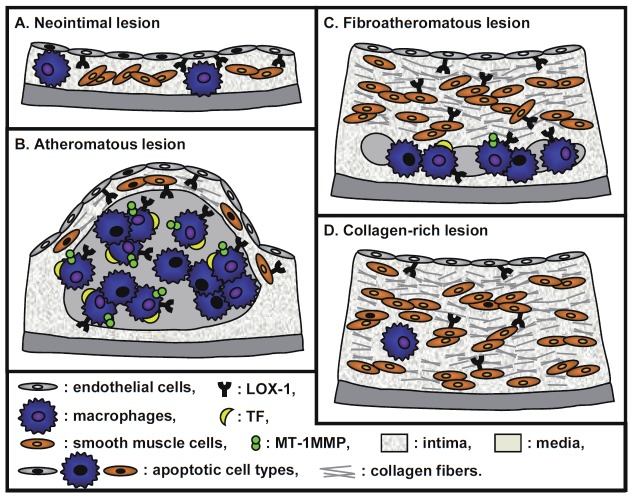
Figure 5.
Illustration of LOX-1, MT1-MMP, and TF expression in atherosclerotic lesions described in references 59, 94, and 105. Atherosclerotic lesions in model rabbits were divided into the 4 categories using a classification scheme based on the recommendations of the American Heart Association: neointimal (types I-III) A. atheromatous (type IV) B. fibroatheromatous (types Va and Vb) C. and collagen-rich (type Vc) D. Morphologic destabilization analysis showed that the atheromatous lesion was the most vulnerable to rupture.
Probes to detect intraplaque angiogenesis
While inflammation is considered to be a key feature of plaque progression, intraplaque angiogenesis mediated by proliferation of the medial vasa vasorum has also been recently implicated in rapid plaque growth and plaque rupture [113]. The fragile neovasculature structure may lead to extravasation of blood components with subsequent intraplaque hemorrhage leading to plaque rupture. Thus, angiogenesis and inflammation within atherosclerotic lesions may be an important target for molecular imaging.
[18F]Galacto-RGD is a peptide tracer that binds to αvβ3 integrin, a cell surface glycoprotein receptor that is highly expressed during angiogenesis. [18F]Galacto-RGD PET has been extensively validated for imaging of angiogenesis in tumors [114]. Dosimetry of [18F]galacto-RGD has already been evaluated in humans based on PET imaging data that indicated a radiation dose comparable to that of [18F]FDG, so that [18F]galacto-RGD can safely be used for integrin αvβ3 imaging [115]. Since both macrophages and activated endothelial cells can express high levels of αvβ3 integrin in atherosclerotic lesions, [18F]galacto-RGD PET has the potential for imaging angiogenesis in atherosclerotic lesions. In a study using hypercholesterolemic mice fed a western diet [116], [18F]galacto-RGD demonstrated specific uptake in atherosclerotic aorta lesions that was associated with macrophage density. Furthermore, an in vivo PET imaging experiment showed [18F]galacto-RGD uptake that co-localized with calcified lesions of the aortic arch as indicated by CT angiography. Thus, [18F]galacto-RGD is a potential tracer for noninvasive imaging of atherosclerotic lesions. A 99mTc labeled Cy5.5-RGD imaging peptide (CRIP) [117] was also developed for assessing cardiac remodeling after myocardial infarction and its responsiveness to anti-angiotensin treatment. In addition, atrial natriuretic peptide and C-type natriuretic peptide have recently been demonstrated to attenuate angiogenesis and have been widely investigated for their therapeutic potential. Thus, the clearance receptor (NPR-C) has been recognized as an ideal target for imaging the antiangiogenic effect of NPs, followed by the development of imaging probes such as 64Cu labeled peptide probe and 64Cu labeled peptide conjugate nanoprobe [118,119], which have been used in mice and rabbit models for imaging NPR-C in angiogenesis.
Intraplaque inflammation plays an important role in the progression and destabilization of atherosclerotic lesions [120]. [11C]PK11195 is a specific ligand of the translocator protein (TSPO), which is highly expressed in activated cells of the mononuclear phagocyte lineage [121]. [3H]PK11195 was recently reported to show specific binding to macrophages in human carotid endarterectomy samples [122] and [11C]PK11195 can be used with CT angiography in humans to assess vascular inflammation in carotid atherosclerotic plaques in vivo [123,124]. In addition, [67Ga]gallium has traditionally been used to image inflammation with gamma cameras, while [68Ga]gallium has been applied for imaging macrophage-rich areas in inflammatory lesions in mice [125]. Although that study indicated a moderate uptake in the plaques, especially at the sites rich in macrophages, the slow blood clearance may limit this probe’s usefulness for clinical imaging of atherosclerotic plaques.
Probes for other targets
In addition to the probes described above, 99mTc, 111In, or 18F labeled LDL [126-129], which exploit the important role of LDL in plaque progression especially in the early phase, 111In-oxine labeled monocytes [130], 18F labeled small vascular cell adhesion molecule (VCAM)-1 affinity ligand ([18F]4V) [131], and radiolabeled cytokines such as IL-2 [132] and MCP-1 [133], which rely on the close relationship between the occurrence of vessel inflammation and plaque progression have also been studied. Furthermore, researchers have recently been investigating the use of nanoparticles as a fundamental part of molecular probes for MRI and optical imaging, as well as for nuclear medicine [134].
Conclusion
Although a variety of molecular probes have been developed for molecular imaging of atherosclerotic lesions, only [18F]FDG and [99mTc] annexin A5 have had successful clinical applications. One possible obstacle for probe development would be low signal levels in the lesion that may be due to inefficient probe delivery to the lesion. Drug delivery systems that use nanocarriers such as liposomes, micelles, and monoclonal antibodies as well as multimerization of targeting moieties like RGD probes typically used in tumor imaging [114] can be beneficial strategies for facilitating development of atherosclerotic lesion probes. In a clinical setting, a large variety of imaging strategies have been utilized for imaging of atherosclerosis, such as nuclear medical techniques (PET and SPECT), MRI, US, and optical imaging. Nuclear medical techniques are advantageous owing to their high sensitivity and high quantitative capacity to noninvasively provide biological information on molecules that deteriorate in atherosclerotic processes deep within the human body. In addition, results from PET and SPECT noninvasive whole body imaging in animals can be translated to use in humans while optical imaging can only be used in animal imaging and may be difficult to quantify. Furthermore, PET is more sensitive than SPECT and probes in tracer amounts can be detected by in vivo PET imaging, which may minimize the possibility of pharmacological effects and target saturation and in turn be important for molecular imaging in atherosclerosis because the expression of target molecules in lesions is usually low. Continued progress in probe development, especially for PET, is urgently needed for successful disease prevention and patient treatment strategies.
References
- 1.Alsheikh-Ali AA, Kitsios GD, Balk EM, Lau J, Ip S. The vulnerable atherosclerotic plaque: scope of the literature. Ann Intern Med. 2010;153:387–395. doi: 10.7326/0003-4819-153-6-201009210-00272. [DOI] [PubMed] [Google Scholar]
- 2.Riou LM, Broisat A, Dimastromatteo J, Pons G, Fagret D, Ghezzi C. Pre-clinical and clinical evaluation of nuclear tracers for the molecular imaging of vulnerable atherosclerosis: an overview. Curr Med Chem. 2009;16:1499–1511. doi: 10.2174/092986709787909596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47:1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscl Throm Vas. 2010;30:1282–1292. doi: 10.1161/ATVBAHA.108.179739. [DOI] [PubMed] [Google Scholar]
- 5.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Coronary artery injury and the biology of atherosclerosis: inflammation, thrombosis, and stabilization. Am J Cardiol. 2000;86:3J–8J. doi: 10.1016/s0002-9149(00)01339-4. discussion 8J-9J. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the unstable plaque. Prog Cardiovasc Dis. 2002;44:349–356. doi: 10.1053/pcad.2002.122475. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Chew M, Ravn HB, Falk E. Plaque pathology and coronary thrombosis in the pathogenesis of acute coronary syndromes. Scand J Clin Lab Invest Suppl. 1999;230:3–11. [PubMed] [Google Scholar]
- 10.Fayad ZA, Fuster V. Clinical imaging of the high-risk or vulnerable atherosclerotic plaque. Circ Res. 2001;89:305–316. doi: 10.1161/hh1601.095596. [DOI] [PubMed] [Google Scholar]
- 11.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 12.Kylintireas I, Shirodaria C, Lee JM, Cunningon C, Lindsay A, Francis J, Robson MD, Neubauer S, Channon KM, Choudhury RP. Multimodal cardiovascular magnetic resonance quantifies regional variation in vascular structure and function in patients with coronary artery disease: Relationships with coronary disease severity. J Cardiovasc Magn Reson. 2011;13:61. doi: 10.1186/1532-429X-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollet NR, Cademartiri F, Nieman K, Saia F, Lemos PA, McFadden EP, Pattynama PM, Serruys PW, Krestin GP, de Feyter PJ. Multislice spiral computed tomography coronary angiography in patients with stable angina pectoris. J Am Coll Cardiol. 2004;43:2265–2270. doi: 10.1016/j.jacc.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 14.Nissen SE, Yock P. Intravascular ultrasound: novel pathophysiological insights and current clinical applications. Circulation. 2001;103:604–616. doi: 10.1161/01.cir.103.4.604. [DOI] [PubMed] [Google Scholar]
- 15.Wilensky RL, Song HK, Ferrari VA. Role of magnetic resonance and intravascular magnetic resonance in the detection of vulnerable plaques. J Am Coll Cardiol. 2006;47:C48–56. doi: 10.1016/j.jacc.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 16.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 17.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 18.Westera G, Schubiger PA. Functional imaging of physiological processes by positron emission tomography. News Physiol Sci. 2003;18:175–178. doi: 10.1152/nips.01420.2002. [DOI] [PubMed] [Google Scholar]
- 19.Elkhawad M, Rudd JH. Radiotracer imaging of atherosclerotic plaque biology. Cardiol Clin. 2009;27:345–354. doi: 10.1016/j.ccl.2008.12.006. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 20.Fox JJ, Strauss HW. One step closer to imaging vulnerable plaque in the coronary arteries. J Nucl Med. 2009;50:497–500. doi: 10.2967/jnumed.108.056325. [DOI] [PubMed] [Google Scholar]
- 21.Glaudemans AW, Slart RH, Bozzao A, Bonanno E, Arca M, Dierckx RA, Signore A. Molecular imaging in atherosclerosis. Eur J Nucl Med Mol Imaging. 2010;37:2381–2397. doi: 10.1007/s00259-010-1406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer HF, Haubner R, Pichler BJ, Gawaz M. Radionuclide imaging: a molecular key to the atherosclerotic plaque. J Am Coll Cardiol. 2008;52:1–12. doi: 10.1016/j.jacc.2008.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsay AC, Choudhury RP. Form to function: current and future roles for atherosclerosis imaging in drug development. Nat Rev Drug Discov. 2008;7:517–529. doi: 10.1038/nrd2588. [DOI] [PubMed] [Google Scholar]
- 24.Sadeghi MM, Glover DK, Lanza GM, Fayad ZA, Johnson LL. Imaging atherosclerosis and vulnerable plaque. J Nucl Med. 2010;51(Suppl 1):51S–65S. doi: 10.2967/jnumed.109.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kearfott KJ, Elmaleh DR, Goodman M, Correia JA, Alpert NM, Ackerman RH, Brownell GL, Strauss WH. Comparison of 2- and 3-18F-fluoro-deoxy-D-glucose for studies of tissue metabolism. Int J Nucl Med Biol. 1984;11:15–22. doi: 10.1016/0047-0740(84)90023-8. [DOI] [PubMed] [Google Scholar]
- 26.Drake B, Cook GJ. Positron emission tomography computed tomography in oncology. Br J Hosp Med (Lond) 2011;72:631–637. doi: 10.12968/hmed.2011.72.11.631. [DOI] [PubMed] [Google Scholar]
- 27.Leuschner F, Nahrendorf M. Molecular imaging of coronary atherosclerosis and myocardial infarction: considerations for the bench and perspectives for the clinic. Circ Res. 2011;108:593–606. doi: 10.1161/CIRCRESAHA.110.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taheri MR, Krauthamer A, Otjen J, Khanna PC, Ishak GE. Neuroimaging of migrational disorders in pediatric epilepsy. Curr Probl Diagn Radiol. 2012;41:11–19. doi: 10.1067/j.cpradiol.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Vach W, Hoilund-Carlsen PF, Gerke O, Weber WA. Generating evidence for clinical benefit of PET/CT in diagnosing cancer patients. J Nucl Med. 2011;52(Suppl 2):77S–85S. doi: 10.2967/jnumed.110.085704. [DOI] [PubMed] [Google Scholar]
- 30.Shiomi M, Ito T, Shiraishi M, Watanabe Y. Inheritability of atherosclerosis and the role of lipoproteins as risk factors in the development of atherosclerosis in WHHL rabbits: risk factors related to coronary atherosclerosis are different from those related to aortic atherosclerosis. Atherosclerosis. 1992;96:43–52. doi: 10.1016/0021-9150(92)90036-g. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit) Atherosclerosis. 1980;36:261–268. doi: 10.1016/0021-9150(80)90234-8. [DOI] [PubMed] [Google Scholar]
- 32.Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit) Arterioscl Throm Vas. 2003;23:1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50. [DOI] [PubMed] [Google Scholar]
- 33.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, Kudomi N, Shiomi M, Magata Y, Iida H, Saji H. 18F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–1250. [PubMed] [Google Scholar]
- 34.Bural GG, Torigian DA, Chamroonrat W, Houseni M, Chen W, Basu S, Kumar R, Alavi A. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008;35:562–569. doi: 10.1007/s00259-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 35.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. 18Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Wykrzykowska J, Lehman S, Williams G, Parker JA, Palmer MR, Varkey S, Kolodny G, Laham R. Imaging of inflamed and vulnerable plaque in coronary arteries with 18F-FDG PET/CT in patients with suppression of myocardial uptake using a low-carbohydrate, high-fat preparation. J Nucl Med. 2009;50:563–568. doi: 10.2967/jnumed.108.055616. [DOI] [PubMed] [Google Scholar]
- 37.Fukumoto Y, Libby P, Rabkin E, Hill CC, Enomoto M, Hirouchi Y, Shiomi M, Aikawa M. Statins alter smooth muscle cell accumulation and collagen content in established atheroma of watanabe heritable hyperlipidemic rabbits. Circulation. 2001;103:993–999. doi: 10.1161/01.cir.103.7.993. [DOI] [PubMed] [Google Scholar]
- 38.Shiomi M, Ito T, Hirouchi Y, Enomoto M. Fibromuscular cap composition is important for the stability of established atherosclerotic plaques in mature WHHL rabbits treated with statins. Atherosclerosis. 2001;157:75–84. doi: 10.1016/s0021-9150(00)00708-5. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa M, Magata Y, Kato T, Hatano K, Ishino S, Mukai T, Shiomi M, Ito K, Saji H. Application of 18F-FDG PET for monitoring the therapeutic effect of antiinflammatory drugs on stabilization of vulnerable atherosclerotic plaques. J Nucl Med. 2006;47:1845–1850. [PubMed] [Google Scholar]
- 40.Tahara N, Kai H, Yamagishi S, Mizoguchi M, Nakaura H, Ishibashi M, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49:1533–1539. doi: 10.1016/j.jacc.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 41.Wu YW, Kao HL, Chen MF, Lee BC, Tseng WY, Jeng JS, Tzen KY, Yen RF, Huang PJ, Yang WS. Characterization of plaques using 18F-FDG PET/CT in patients with carotid atherosclerosis and correlation with matrix metalloproteinase-1. J Nucl Med. 2007;48:227–233. [PubMed] [Google Scholar]
- 42.Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, Hayabuchi N, Imaizumi T. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–1831. doi: 10.1016/j.jacc.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 43.Lee SJ, On YK, Lee EJ, Choi JY, Kim BT, Lee KH. Reversal of vascular 18F-FDG uptake with plasma high-density lipoprotein elevation by atherogenic risk reduction. J Nucl Med. 2008;49:1277–1282. doi: 10.2967/jnumed.108.052233. [DOI] [PubMed] [Google Scholar]
- 44.Matter CM, Wyss MT, Meier P, Spath N, von Lukowicz T, Lohmann C, Weber B, Ramirez de Molina A, Lacal JC, Ametamey SM, von Schulthess GK, Luscher TF, Kaufmann PA, Buck A. 18F-choline images murine atherosclerotic plaques ex vivo. Arterioscl Throm Vas. 2006;26:584–589. doi: 10.1161/01.ATV.0000200106.34016.18. [DOI] [PubMed] [Google Scholar]
- 45.Laitinen IE, Luoto P, Nagren K, Marjamaki PM, Silvola JM, Hellberg S, Laine VJ, Yla-Herttuala S, Knuuti J, Roivainen A. Uptake of 11C-choline in mouse atherosclerotic plaques. J Nucl Med. 2010;51:798–802. doi: 10.2967/jnumed.109.071704. [DOI] [PubMed] [Google Scholar]
- 46.Kato K, Schober O, Ikeda M, Schafers M, Ishigaki T, Kies P, Naganawa S, Stegger L. Evaluation and comparison of 11C-choline uptake and calcification in aortic and common carotid arterial walls with combined PET/CT. Eur J Nucl Med Mol Imaging. 2009;36:1622–1628. doi: 10.1007/s00259-009-1152-7. [DOI] [PubMed] [Google Scholar]
- 47.Semenkovich CF. Regulation of fatty acid synthase (FAS) Prog Lipid Res. 1997;36:43–53. doi: 10.1016/s0163-7827(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 48.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–89. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derlin T, Habermann CR, Lengyel Z, Busch JD, Wisotzki C, Mester J, Pavics L. Feasibility of 11C-acetate PET/CT for imaging of fatty acid synthesis in the atherosclerotic vessel wall. J Nucl Med. 2011;52:1848–1854. doi: 10.2967/jnumed.111.095869. [DOI] [PubMed] [Google Scholar]
- 50.Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 51.Kume N, Kita T. Apoptosis of vascular cells by oxidized LDL: involvement of caspases and LOX-1 and its implication in atherosclerotic plaque rupture. Circ Res. 2004;94:269–270. doi: 10.1161/01.RES.0000119804.92239.97. [DOI] [PubMed] [Google Scholar]
- 52.Smirnova IV, Kajstura M, Sawamura T, Goligorsky MS. Asymmetric dimethylarginine up-regulates LOX-1 in activated macrophages: role in foam cell formation. Am J Physiol Heart Circ Physiol. 2004;287:H782–790. doi: 10.1152/ajpheart.00822.2003. [DOI] [PubMed] [Google Scholar]
- 53.Moriwaki H, Kume N, Kataoka H, Murase T, Nishi E, Sawamura T, Masaki T, Kita T. Expression of lectin-like oxidized low density lipoprotein receptor-1 in human and murine macrophages: upregulated expression by TNF-alpha. FEBS Lett. 1998;440:29–32. doi: 10.1016/s0014-5793(98)01414-8. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida H, Kondratenko N, Green S, Steinberg D, Quehenberger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J. 1998;334:9–13. doi: 10.1042/bj3340009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D, Liu L, Chen H, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized lowdensity lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–617. doi: 10.1161/01.cir.0000047276.52039.fb. [DOI] [PubMed] [Google Scholar]
- 56.Kataoka H, Kume N, Miyamoto S, Minami M, Morimoto M, Hayashida K, Hashimoto N, Kita T. Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arterioscl Throm Vas. 2001;21:955–960. doi: 10.1161/01.atv.21.6.955. [DOI] [PubMed] [Google Scholar]
- 57.Kume N, Kita T. Roles of lectin-like oxidized LDL receptor-1 and its soluble forms in atherogenesis. Curr Opin Lipidol. 2001;12:419–423. doi: 10.1097/00041433-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 58.Ishino S, Mukai T, Kume N, Asano D, Ogawa M, Kuge Y, Minami M, Kita T, Shiomi M, Saji H. Lectin-like oxidized LDL receptor-1 (LOX-1) expression is associated with atherosclerotic plaque instability-analysis in hypercholesterolemic rabbits. Atherosclerosis. 2007;195:48–56. doi: 10.1016/j.atherosclerosis.2006.11.031. [DOI] [PubMed] [Google Scholar]
- 59.Ishino S, Mukai T, Kuge Y, Kume N, Ogawa M, Takai N, Kamihashi J, Shiomi M, Minami M, Kita T, Saji H. Targeting of lectinlike oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med. 2008;49:1677–1685. doi: 10.2967/jnumed.107.049536. [DOI] [PubMed] [Google Scholar]
- 60.Li D, Patel AR, Klibanov AL, Kramer CM, Ruiz M, Kang BY, Mehta JL, Beller GA, Glover DK, Meyer CH. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ Cardiovasc Imaging. 2010;3:464–472. doi: 10.1161/CIRCIMAGING.109.896654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bjorkerud S, Bjorkerud B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am J Pathol. 1996;149:367–380. [PMC free article] [PubMed] [Google Scholar]
- 62.Geng YJ, Henderson LE, Levesque EB, Muszynski M, Libby P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscl Throm Vas. 1997;17:2200–2208. doi: 10.1161/01.atv.17.10.2200. [DOI] [PubMed] [Google Scholar]
- 63.Blankenberg FG, Katsikis PD, Tait JF, Davis RE, Naumovski L, Ohtsuki K, Kopiwoda S, Abrams MJ, Darkes M, Robbins RC, Maecker HT, Strauss HW. In vivo detection and imaging of phosphatidylserine expression during programmed cell death. Proc Natl Acad Sci USA. 1998;95:6349–6354. doi: 10.1073/pnas.95.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mochizuki T, Kuge Y, Zhao S, Tsukamoto E, Hosokawa M, Strauss HW, Blankenberg FG, Tait JF, Tamaki N. Detection of apoptotic tumor response in vivo after a single dose of chemotherapy with 99mTc-annexin V. J Nucl Med. 2003;44:92–97. [PubMed] [Google Scholar]
- 65.Takei T, Kuge Y, Zhao S, Sato M, Strauss HW, Blankenberg FG, Tait JF, Tamaki N. Time course of apoptotic tumor response after a single dose of chemotherapy: comparison with 99mTc-annexin V uptake and histologic findings in an experimental model. J Nucl Med. 2004;45:2083–2087. [PubMed] [Google Scholar]
- 66.Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem. 1990;265:17420–17423. [PubMed] [Google Scholar]
- 67.Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, Narula J. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004;350:1472–1473. doi: 10.1056/NEJM200404013501425. [DOI] [PubMed] [Google Scholar]
- 68.Kolodgie FD, Petrov A, Virmani R, Narula N, Verjans JW, Weber DK, Hartung D, Steinmetz N, Vanderheyden JL, Vannan MA, Gold HK, Reutelingsperger CP, Hofstra L, Narula J. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation. 2003;108:3134–3139. doi: 10.1161/01.CIR.0000105761.00573.50. [DOI] [PubMed] [Google Scholar]
- 69.Laufer EM, Winkens MH, Narula J, Hofstra L. Molecular imaging of macrophage cell death for the assessment of plaque vulnerability. Arterioscl Throm Vas. 2009;29:1031–1038. doi: 10.1161/ATVBAHA.108.165522. [DOI] [PubMed] [Google Scholar]
- 70.Sarai M, Hartung D, Petrov A, Zhou J, Narula N, Hofstra L, Kolodgie F, Isobe S, Fujimoto S, Vanderheyden JL, Virmani R, Reutelingsperger C, Wong ND, Gupta S, Narula J. Broad and specific caspase inhibitor-induced acute repression of apoptosis in atherosclerotic lesions evaluated by radiolabeled annexin A5 imaging. J Am Coll Cardiol. 2007;50:2305–2312. doi: 10.1016/j.jacc.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 71.Ishino S, Kuge Y, Takai N, Tamaki N, Strauss HW, Blankenberg FG, Shiomi M, Saji H. 99mTc-Annexin A5 for noninvasive characterization of atherosclerotic lesions: imaging and histological studies in myocardial infarctionprone Watanabe heritable hyperlipidemic rabbits. Eur J Nucl Med Mol Imaging. 2007;34:889–899. doi: 10.1007/s00259-006-0289-x. [DOI] [PubMed] [Google Scholar]
- 72.Nguyen QD, Smith G, Glaser M, Perumal M, Arstad E, Aboagye EO. Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-3/7 specific [18F] -labeled isatin sulfonamide. Proc Natl Acad Sci USA. 2009;106:16375–16380. doi: 10.1073/pnas.0901310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou D, Chu W, Chen DL, Wang Q, Reichert DE, Rothfuss J, D’Avignon A, Welch MJ, Mach RH. [18F] - and [11C] -labeled N-benzyl-isatin sulfonamide analogues as PET tracers for apoptosis: synthesis, radiolabeling mechanism, and in vivo imaging study of apoptosis in Fas-treated mice using [11C] WC-98. Org Biomol Chem. 2009;7:1337–1348. doi: 10.1039/b819024k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 76.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–823. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 77.Knauper V, Bailey L, Worley JR, Soloway P, Patterson ML, Murphy G. Cellular activation of proMMP-13 by MT1-MMP depends on the Cterminal domain of MMP-13. FEBS Lett. 2002;532:127–130. doi: 10.1016/s0014-5793(02)03654-2. [DOI] [PubMed] [Google Scholar]
- 78.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 79.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 80.Brown DL, Hibbs MS, Kearney M, Loushin C, Isner JM. Identification of 92-kD gelatinase in human coronary atherosclerotic lesions. Association of active enzyme synthesis with unstable angina. Circulation. 1995;91:2125–2131. doi: 10.1161/01.cir.91.8.2125. [DOI] [PubMed] [Google Scholar]
- 81.Li Z, Li L, Zielke HR, Cheng L, Xiao R, Crow MT, Stetler-Stevenson WG, Froehlich J, Lakatta EG. Increased expression of 72-kd type IV collagenase (MMP-2) in human aortic atherosclerotic lesions. Am J Pathol. 1996;148:121–128. [PMC free article] [PubMed] [Google Scholar]
- 82.Kuge Y, Takai N, Ishino S, Temma T, Shiomi M, Saji H. Distribution profiles of membrane Type-1 matrix metalloproteinase (MT1-MMP), matrix metalloproteinase-2 (MMP-2) and cyclooxygenase-2 (COX-2) in rabbit atherosclerosis: comparison with plaque instability analysis. Biol Pharm Bull. 2007;30:1634–1640. doi: 10.1248/bpb.30.1634. [DOI] [PubMed] [Google Scholar]
- 83.Stawowy P, Meyborg H, Stibenz D, Borges Pereira Stawowy N, Roser M, Thanabalasingam U, Veinot JP, Chretien M, Seidah NG, Fleck E, Graf K. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation. 2005;111:2820–2827. doi: 10.1161/CIRCULATIONAHA.104.502617. [DOI] [PubMed] [Google Scholar]
- 84.Rajavashisth TB, Xu XP, Jovinge S, Meisel S, Xu XO, Chai NN, Fishbein MC, Kaul S, Cercek B, Sharifi B, Shah PK. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999;99:3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 85.Hartung D, Schafers M, Fujimoto S, Levkau B, Narula N, Kopka K, Virmani R, Reutelingsperger C, Hofstra L, Kolodgie FD, Petrov A, Narula J. Targeting of matrix metalloproteinase activation for noninvasive detection of vulnerable atherosclerotic lesions. Eur J Nucl Med Mol Imaging. 2007;34(Suppl 1):S1–8. doi: 10.1007/s00259-007-0435-0. [DOI] [PubMed] [Google Scholar]
- 86.Davies JR, Rudd JH, Weissberg PL, Narula J. Radionuclide imaging for the detection of inflammation in vulnerable plaques. J Am Coll Cardiol. 2006;47:C57–68. doi: 10.1016/j.jacc.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 87.Schafers M, Schober O, Hermann S. Matrix-metalloproteinases as imaging targets for inflammatory activity in atherosclerotic plaques. J Nucl Med. 2010;51:663–666. doi: 10.2967/jnumed.109.065698. [DOI] [PubMed] [Google Scholar]
- 88.Bremer C, Tung CH, Weissleder R. In vivo molecular target assessment of matrix metalloproteinase inhibition. Nat Med. 2001;7:743–748. doi: 10.1038/89126. [DOI] [PubMed] [Google Scholar]
- 89.Watkins GA, Jones EF, Scott Shell M, VanBrocklin HF, Pan MH, Hanrahan SM, Feng JJ, He J, Sounni NE, Dill KA, Contag CH, Coussens LM, Franc BL. Development of an optimized activatable MMP-14 targeted SPECT imaging probe. Bioorg Med Chem. 2009;17:653–659. doi: 10.1016/j.bmc.2008.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohshima S, Petrov A, Fujimoto S, Zhou J, Azure M, Edwards DS, Murohara T, Narula N, Tsimikas S, Narula J. Molecular imaging of matrix metalloproteinase expression in atherosclerotic plaques of mice deficient in apolipoprotein e or low-density-lipoprotein receptor. J Nucl Med. 2009;50:612–617. doi: 10.2967/jnumed.108.055889. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J, Nie L, Razavian M, Ahmed M, Dobrucki LW, Asadi A, Edwards DS, Azure M, Sinusas AJ, Sadeghi MM. Molecular imaging of activated matrix metalloproteinases in vascular remodeling. Circulation. 2008;118:1953–1960. doi: 10.1161/CIRCULATIONAHA.108.789743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fujimoto S, Hartung D, Ohshima S, Edwards DS, Zhou J, Yalamanchili P, Azure M, Fujimoto A, Isobe S, Matsumoto Y, Boersma H, Wong N, Yamazaki J, Narula N, Petrov A, Narula J. Molecular imaging of matrix metalloproteinase in atherosclerotic lesions: resolution with dietary modification and statin therapy. J Am Coll Cardiol. 2008;52:1847–1857. doi: 10.1016/j.jacc.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 93.Tekabe Y, Li Q, Luma J, Weisenberger D, Sedlar M, Harja E, Narula J, Johnson LL. Noninvasive monitoring the biology of atherosclerotic plaque development with radiolabeled annexin V and matrix metalloproteinase inhibitor in spontaneous atherosclerotic mice. J Nucl Cardiol. 2010;17:1073–1081. doi: 10.1007/s12350-010-9276-5. [DOI] [PubMed] [Google Scholar]
- 94.Kuge Y, Takai N, Ogawa Y, Temma T, Zhao Y, Nishigori K, Ishino S, Kamihashi J, Kiyono Y, Shiomi M, Saji H. Imaging with radiolabelled anti-membrane type 1 matrix metalloproteinase (MT1-MMP) antibody: potentials for characterizing atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2010;37:2093–2104. doi: 10.1007/s00259-010-1521-2. [DOI] [PubMed] [Google Scholar]
- 95.Johnson JL, Devel L, Czarny B, George SJ, Jackson CL, Rogakos V, Beau F, Yiotakis A, Newby AC, Dive V. A selective matrix metalloproteinase-12 inhibitor retards atherosclerotic plaque development in apolipoprotein E-knockout mice. Arterioscl Throm Vas. 2011;31:528–535. doi: 10.1161/ATVBAHA.110.219147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci USA. 2005;102:15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Watanabe S, Fan J. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- 98.Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M, Watanabe T, Yasumoto K, Sasaguri Y. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008;172:1419–1429. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HG, Wickline SA, Parks WC. Matrilysin is expressed by lipidladen macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci USA. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas AC, Sala-Newby GB, Ismail Y, Johnson JL, Pasterkamp G, Newby AC. Genomics of foam cells and nonfoamy macrophages from rabbits identifies arginase-I as a differential regulator of nitric oxide production. Arterioscl Throm Vas. 2007;27:571–577. doi: 10.1161/01.ATV.0000256470.23842.94. [DOI] [PubMed] [Google Scholar]
- 101.Devel L, Rogakos V, David A, Makaritis A, Beau F, Cuniasse P, Yiotakis A, Dive V. Development of selective inhibitors and substrate of matrix metalloproteinase-12. J Biol Chem. 2006;281:11152–11160. doi: 10.1074/jbc.M600222200. [DOI] [PubMed] [Google Scholar]
- 102.Moons AH, Levi M, Peters RJ. Tissue factor and coronary artery disease. Cardiovasc Res. 2002;53:313–325. doi: 10.1016/s0008-6363(01)00452-7. [DOI] [PubMed] [Google Scholar]
- 103.Jeanpierre E, Le Tourneau T, Six I, Zawadzki C, Van Belle E, Ezekowitz MD, Bordet R, Susen S, Jude B, Corseaux D. Dietary lipid lowering modifies plaque phenotype in rabbit atheroma after angioplasty: a potential role of tissue factor. Circulation. 2003;108:1740–1745. doi: 10.1161/01.CIR.0000089370.84709.51. [DOI] [PubMed] [Google Scholar]
- 104.Kuge Y, Kume N, Ishino S, Takai N, Ogawa Y, Mukai T, Minami M, Shiomi M, Saji H. Prominent lectin-like oxidized low density lipoprotein (LDL) receptor-1 (LOX-1) expression in atherosclerotic lesions is associated with tissue factor expression and apoptosis in hypercholesterolemic rabbits. Biol Pharm Bull. 2008;31:1475–1482. doi: 10.1248/bpb.31.1475. [DOI] [PubMed] [Google Scholar]
- 105.Temma T, Ogawa Y, Kuge Y, Ishino S, Takai N, Nishigori K, Shiomi M, Ono M, Saji H. Tissue factor detection for selectively discriminating unstable plaques in an atherosclerotic rabbit model. J Nucl Med. 2010;51:1979–1986. doi: 10.2967/jnumed.110.081216. [DOI] [PubMed] [Google Scholar]
- 106.Shaw SY. Molecular imaging in cardiovascular disease: targets and opportunities. Nat Rev Cardiol. 2009;6:569–579. doi: 10.1038/nrcardio.2009.119. [DOI] [PubMed] [Google Scholar]
- 107.Katoh M, Haage P, Wiethoff AJ, Gunther RW, Bucker A, Tacke J, Spuentrup E. Molecular magnetic resonance imaging of deep vein thrombosis using a fibrin-targeted contrast agent: a feasibility study. Invest Radiol. 2009;44:146–150. doi: 10.1097/RLI.0b013e318195886d. [DOI] [PubMed] [Google Scholar]
- 108.Spuentrup E, Botnar RM, Wiethoff AJ, Ibrahim T, Kelle S, Katoh M, Ozgun M, Nagel E, Vymazal J, Graham PB, Gunther RW, Maintz D. MR imaging of thrombi using EP-2104R, a fibrinspecific contrast agent: initial results in patients. Eur Radiol. 2008;18:1995–2005. doi: 10.1007/s00330-008-0965-2. [DOI] [PubMed] [Google Scholar]
- 109.Vymazal J, Spuentrup E, Cardenas-Molina G, Wiethoff AJ, Hartmann MG, Caravan P, Parsons EC Jr. Thrombus imaging with fibrin-specific gadolinium-based MR contrast agent EP-2104R: results of a phase II clinical study of feasibility. Invest Radiol. 2009;44:697–704. doi: 10.1097/RLI.0b013e3181b092a7. [DOI] [PubMed] [Google Scholar]
- 110.Jaffer FA, Tung CH, Wykrzykowska JJ, Ho NH, Houng AK, Reed GL, Weissleder R. Molecular imaging of factor XIIIa activity in thrombosis using a novel, near-infrared fluorescent contrast agent that covalently links to thrombi. Circulation. 2004;110:170–176. doi: 10.1161/01.CIR.0000134484.11052.44. [DOI] [PubMed] [Google Scholar]
- 111.Tung CH, Ho NH, Zeng Q, Tang Y, Jaffer FA, Reed GL, Weissleder R. Novel factor XIII probes for blood coagulation imaging. Chembiochem. 2003;4:897–899. doi: 10.1002/cbic.200300602. [DOI] [PubMed] [Google Scholar]
- 112.Elmaleh DR, Fischman AJ, Tawakol A, Zhu A, Shoup TM, Hoffmann U, Brownell AL, Zamecnik PC. Detection of inflamed atherosclerotic lesions with diadenosine-5’, 5’’’-P1, P4-tetraphosphate (Ap4A) and positron-emission tomography. Proc Natl Acad Sci USA. 2006;103:15992–15996. doi: 10.1073/pnas.0607246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscl Throm Vas. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 114.Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S126–138. doi: 10.1007/s00259-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 115.Beer AJ, Haubner R, Wolf I, Goebel M, Luderschmidt S, Niemeyer M, Grosu AL, Martinez MJ, Wester HJ, Weber WA, Schwaiger M. PET-based human dosimetry of 18F-galacto-RGD, a new radiotracer for imaging alpha v beta 3 expression. J Nucl Med. 2006;47:763–769. [PubMed] [Google Scholar]
- 116.Laitinen I, Saraste A, Weidl E, Poethko T, Weber AW, Nekolla SG, Leppanen P, Yla-Herttuala S, Holzlwimmer G, Walch A, Esposito I, Wester HJ, Knuuti J, Schwaiger M. Evaluation of alpha v beta 3 integrin-targeted positron emission tomography tracer 18F-galacto-RGD for imaging of vascular inflammation in atherosclerotic mice. Circ Cardiovasc Imaging. 2009;2:331–338. doi: 10.1161/CIRCIMAGING.108.846865. [DOI] [PubMed] [Google Scholar]
- 117.van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J, Arbo B, Rogstad A, Cuthbertson A, Chettibi S, Reutelingsperger C, Blankesteijn WM, Smits JF, Daemen MJ, Zannad F, Vannan MA, Narula N, Pitt B, Hofstra L, Narula J. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52:2017–2028. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 118.Liu Y, Pressly ED, Abendschein DR, Hawker CJ, Woodard GE, Woodard PK, Welch MJ. Targeting angiogenesis using a C-type atrial natriuretic factor-conjugated nanoprobe and PET. J Nucl Med. 2011;52:1956–1963. doi: 10.2967/jnumed.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu Y, Abendschein D, Woodard GE, Rossin R, McCommis K, Zheng J, Welch MJ, Woodard PK. Molecular imaging of atherosclerotic plaque with 64Cu-labeled natriuretic peptide and PET. J Nucl Med. 2010;51:85–91. doi: 10.2967/jnumed.109.066977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 121.Veenman L, Gavish M. The peripheral-type benzodiazepine receptor and the cardiovascular system. Implications for drug development. Pharmacol Therapeut. 2006;110:503–524. doi: 10.1016/j.pharmthera.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 122.Bird JL, Izquierdo-Garcia D, Davies JR, Rudd JH, Probst KC, Figg N, Clark JC, Weissberg PL, Davenport AP, Warburton EA. Evaluation of translocator protein quantification as a tool for characterising macrophage burden in human carotid atherosclerosis. Atherosclerosis. 2010;210:388–391. doi: 10.1016/j.atherosclerosis.2009.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lamare F, Hinz R, Gaemperli O, Pugliese F, Mason JC, Spinks T, Camici PG, Rimoldi OE. Detection and quantification of large-vessel inflammation with 11C-(R)-PK11195 PET/CT. J Nucl Med. 2011;52:33–39. doi: 10.2967/jnumed.110.079038. [DOI] [PubMed] [Google Scholar]
- 124.Gaemperli O, Shalhoub J, Owen DR, Lamare F, Johansson S, Fouladi N, Davies AH, Rimoldi OE, Camici PG. Imaging intraplaque inflammation in carotid atherosclerosis with 11C-PK11195 positron emission tomography/computed tomography. Eur Heart J. 2011;33:1902–10. doi: 10.1093/eurheartj/ehr367. [DOI] [PubMed] [Google Scholar]
- 125.Silvola JM, Laitinen I, Sipila HJ, Laine VJ, Leppanen P, Yla-Herttuala S, Knuuti J, Roivainen A. Uptake of 68gallium in atherosclerotic plaques in LDLR-/-ApoB100/100 mice. EJNMMI research. 2011;1:14. doi: 10.1186/2191-219X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lees AM, Lees RS, Schoen FJ, Isaacsohn JL, Fischman AJ, McKusick KA, Strauss HW. Imaging human atherosclerosis with 99mTc-labeled low density lipoproteins. Arteriosclerosis. 1988;8:461–470. doi: 10.1161/01.atv.8.5.461. [DOI] [PubMed] [Google Scholar]
- 127.Rosen JM, Butler SP, Meinken GE, Wang TS, Ramakrishnan R, Srivastava SC, Alderson PO, Ginsberg HN. Indium-111-labeled LDL: a potential agent for imaging atherosclerotic disease and lipoprotein biodistribution. J Nucl Med. 1990;31:343–350. [PubMed] [Google Scholar]
- 128.Vallabhajosula S, Paidi M, Badimon JJ, Le NA, Goldsmith SJ, Fuster V, Ginsberg HN. Radiotracers for low density lipoprotein biodistribution studies in vivo: technetium-99m low density lipoprotein versus radioiodinated low density lipoprotein preparations. J Nucl Med. 1988;29:1237–1245. [PubMed] [Google Scholar]
- 129.Pietzsch J, Bergmann R, Wuest F, Pawelke B, Hultsch C, van den Hoff J. Catabolism of native and oxidized low density lipoproteins: in vivo insights from small animal positron emission tomography studies. Amino Acids. 2005;29:389–404. doi: 10.1007/s00726-005-0203-z. [DOI] [PubMed] [Google Scholar]
- 130.Kircher MF, Grimm J, Swirski FK, Libby P, Gerszten RE, Allport JR, Weissleder R. Noninvasive in vivo imaging of monocyte trafficking to atherosclerotic lesions. Circulation. 2008;117:388–395. doi: 10.1161/CIRCULATIONAHA.107.719765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nahrendorf M, Keliher E, Panizzi P, Zhang H, Hembrador S, Figueiredo JL, Aikawa E, Kelly K, Libby P, Weissleder R. 18F-4V for PET-CT imaging of VCAM-1 expression in atherosclerosis. JACC Cardiovasc Imaging. 2009;2:1213–1222. doi: 10.1016/j.jcmg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Annovazzi A, Bonanno E, Arca M, D’Alessandria C, Marcoccia A, Spagnoli LG, Violi F, Scopinaro F, De Toma G, Signore A. 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2006;33:117–126. doi: 10.1007/s00259-005-1899-4. [DOI] [PubMed] [Google Scholar]
- 133.Hartung D, Petrov A, Haider N, Fujimoto S, Blankenberg F, Fujimoto A, Virmani R, Kolodgie FD, Strauss HW, Narula J. Radiolabeled Monocyte Chemotactic Protein 1 for the detection of inflammation in experimental atherosclerosis. J Nucl Med. 2007;48:1816–1821. doi: 10.2967/jnumed.107.043463. [DOI] [PubMed] [Google Scholar]
- 134.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]