Abstract
D-Glucosamine is toxic to several malignant cell lines and in vivo tumors at concentrations that have little effect upon normal host tissues. Evidence is presented to support the hypothesis that cellular membranes may be the primary targets of glucosamine's tumoricidal activity. Treatment of rat C6 glioma cells with a cytotoxic concentration of glucosamine (20 mM) caused fragmentation of rough endoplasmic reticulum, proliferation of Golgi complexes, evagination of outer nuclear and mitochondrial membranes, and the accumulation of membranous vacuoles and lipid droplets in the cytoplasm. These changes were detected within the first 3 hr after treatment of cultures with glucosamine and became increasingly severe until cell lysis occurred between 24 and 48 hr of treatment. The cytotoxicity of glucosamine was potentiated by the local anesthetic lidocaine, and by other membrane-active drugs, at concentrations that were growth inhibitory but nonlytic. Most of these drugs possessed local anesthetic activity and inhibited glioma sterol synthesis. Within the same period of time required for ultrastructural changes in cellular membranes, glucosamine inhibited the incorporation of [2-14C]acetate into sterols and into an unidentified 400-dalton lipid that migrated close to sterols on thin-layer chromatograms. This inhibition was potentiated by lidocaine and increased over the same range of D-glucosamine concentrations that led to increased cell toxicity after a 48-hr treatment. These findings suggest that the effects of glucosamine upon cellular membranes may be central to its tumoricidal activity and that glucosamine, in combination with membrane-active drugs, may be useful in the treatment of certain types of tumors, particularly those of the central nervous system.
Keywords: lidocaine, anesthetics, sterols, glial tumor cells
Full text
PDF
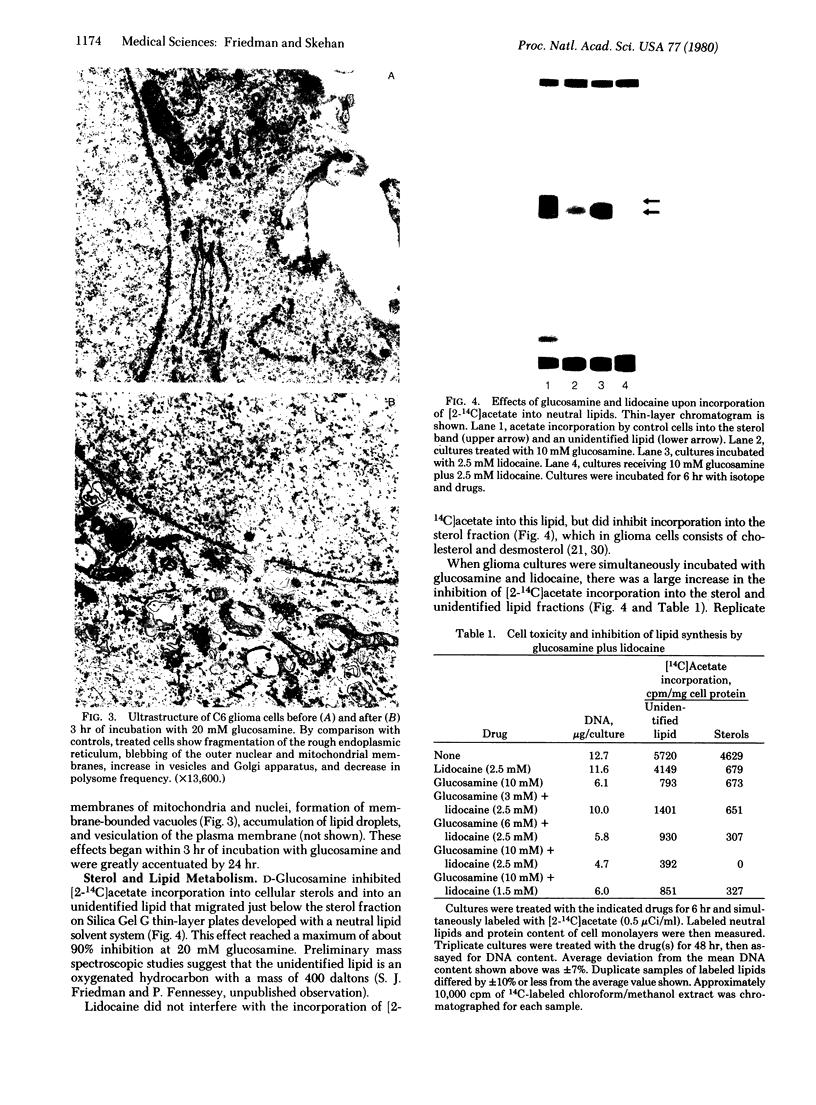


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALL H. A., WICK A. N., SANDERS C. Influence of glucose antimetabolites on the Walker tumor. Cancer Res. 1957 Apr;17(3):235–239. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekesi J. G., Bekesi E., Winzler R. J. Inhibitory effect of D-glucosamine and other sugars on the biosynthesis of protein, ribonucleic acid, and deoxyribonucleic acid in normal and neoplastic tissues. J Biol Chem. 1969 Jul 25;244(14):3766–3772. [PubMed] [Google Scholar]
- Bekesi J. G., Molnar Z., Winzler R. J. Inhibitory effect of d-glucosamine and other sugar analogs on the viability and transplantability of ascites tumor cells. Cancer Res. 1969 Feb;29(2):353–359. [PubMed] [Google Scholar]
- Bekesi J. G., Winzler R. J. Inhibitory effects of D-glucosamine on the growth of Walker 256 carcinosarcoma and on protein, RNA, and DNA synthesis. Cancer Res. 1970 Dec;30(12):2905–2912. [PubMed] [Google Scholar]
- Bekesi J. G., Winzler R. J. The effect of D-glucosamine on the adenine and uridine nucleotides of sarcoma 180 ascites tumor cells. J Biol Chem. 1969 Oct 25;244(20):5663–5668. [PubMed] [Google Scholar]
- Bernacki R., Porter C., Korytnyk W., Mihich E. Plasma membrane as a site for chemotherapeutic intervention. Adv Enzyme Regul. 1977 Oct 3;16:217–237. doi: 10.1016/0065-2571(78)90075-4. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Inhibition of protein, glycoprotein, ribonucleic acid and deoxyribonucleic acid synthesis by D-glucosamine and other sugars in mouse leukemic cells L5178Y and selective inhibition in SV-3T3 compared with 3T3 cells. Biochim Biophys Acta. 1971 Jun 17;240(1):74–93. doi: 10.1016/0005-2787(71)90515-6. [DOI] [PubMed] [Google Scholar]
- COPIUS-PEEREBOOM J. W., BEEKES H. W. THE ANALYSIS OF MIXTURES OF ANIMAL AND VEGETABLE FATS. V. SEPARATION OF STEROL ACETATES BY THIN-LAYER CHROMATOGRAPHY IN REVERSED-PHASE SYSTEMS AND ON SILICA GEL G-SILVER NITRATE LAYERS. J Chromatogr. 1965 Jan;17:99–113. doi: 10.1016/s0021-9673(00)99839-x. [DOI] [PubMed] [Google Scholar]
- Chou T. C., Talaly P. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J Biol Chem. 1977 Sep 25;252(18):6438–6442. [PubMed] [Google Scholar]
- Decker K., Keppler D., Rudigier J., Domschke W. Cell damage by trapping of biosynthetic intermediates. The role of uracil nucleotides in experimental hepatitis. Hoppe Seylers Z Physiol Chem. 1971 Mar;352(3):412–418. doi: 10.1515/bchm2.1971.352.1.412. [DOI] [PubMed] [Google Scholar]
- El-Mofty S. K., Scrutton M. C., Serroni A., Nicolini C., Farber J. L. Early, reversible plasma membrane injury in galactosamine-induced liver cell death. Am J Pathol. 1975 Jun;79(3):579–596. [PMC free article] [PubMed] [Google Scholar]
- FJELDE A., SORKIN E., RHODES J. M. The effect of glucosamine on human epidermoid carcinoma cells in tissue culture. Exp Cell Res. 1956 Feb;10(1):88–98. doi: 10.1016/0014-4827(56)90075-1. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Friedman S. J., Kimball T., Trotter C. D., Skehan P. J. The inhibition of thymidine kinase in glial tumor cells by an amino sugar, D-glucosamine. Cancer Res. 1977 Apr;37(4):1068–1074. [PubMed] [Google Scholar]
- Friedman S. J., Skehan P. The inhibition of sterol synthesis by anesthetics. FEBS Lett. 1979 Jun 15;102(2):235–240. doi: 10.1016/0014-5793(79)80008-3. [DOI] [PubMed] [Google Scholar]
- Friedman S. J., Trotter C. D., Kimball T., Skehan P. J. The inhibition of thymidine metabolism in tumor cells treated with D-glucosamine. Cancer Res. 1977 Apr;37(4):1141–1146. [PubMed] [Google Scholar]
- GARBER B. Inhibition by glucosamine of aggregation of dissociated embryonic cells. Dev Biol. 1963 Mar;6:630–641. doi: 10.1016/0012-1606(63)90147-7. [DOI] [PubMed] [Google Scholar]
- HARPUR R. P., QUASTEL J. H. Phosphorylation of d-glucosamine by brain extracts. Nature. 1949 Oct 22;164(4173):693–693. doi: 10.1038/164693a0. [DOI] [PubMed] [Google Scholar]
- Kaluza G., Scholtissek C., Rott R. Inhibition of the multiplication of enveloped RNA-viruses by glucosamine and 2-deoxy-D-glucose. J Gen Virol. 1972 Mar;14(3):251–259. doi: 10.1099/0022-1317-14-3-251. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Ginsburg V. The metabolism of glucosamine by tissue culture cells. Exp Cell Res. 1966 Mar;41(3):592–600. doi: 10.1016/s0014-4827(66)80109-x. [DOI] [PubMed] [Google Scholar]
- Kuroda Y. Effects of hexosamines and their acetyl derivatives on aggregation of rat hepatoma cells in rotation culture. Cancer Res. 1974 Feb;34(2):403–409. [PubMed] [Google Scholar]
- LINDNER J., von SCHWEINITZH, BECKER K. [On the tumor-inhibiting effect of ground substance components. Part 2. Metabolism studies]. Klin Wochenschr. 1960 Aug 1;38:763–768. doi: 10.1007/BF01487279. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Molnar Z., Bekesi J. G. Cytotoxic effects of D-glucosamine on the ultrastructures of normal and neoplastic tissues in vivo. Cancer Res. 1972 Apr;32(4):756–765. [PubMed] [Google Scholar]
- Molnar Z., Bekesi J. G. Effects of D-glucosamine, D-mannosamine, and 2-deoxy-D-glucose on the ultrastructure of ascites tumor cells in vitro. Cancer Res. 1972 Feb;32(2):380–389. [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Transport and metabolism of glucosamine by cultured Novikoff rat hepatoma cells and effects on nucleotide pools. Cancer Res. 1973 Mar;33(3):482–492. [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. Transport and metabolism of glucosamine by cultured Novikoff rat hepatoma cells and effects on nucleotide pools. Cancer Res. 1973 Mar;33(3):482–492. [PubMed] [Google Scholar]
- QUASTEL J. H., CANTERO A. Inhibition of tumour growth by D-glucosamine. Nature. 1953 Feb 7;171(4345):252–254. doi: 10.1038/171252a0. [DOI] [PubMed] [Google Scholar]
- RUBIN A., SPRINGER G. F., HOGUE M. J. The effect of D-glucosamine hydrochloride and related compounds on tissue cultures of the solid form of mouse sarcoma 37. Cancer Res. 1954 Jul;14(6):456–458. [PubMed] [Google Scholar]
- Scholtissek C. Influence of glucosamine on the uptake of nucleosides by chick fibroblasts and on the incorporation into RNA. Biochim Biophys Acta. 1972 Sep 14;277(3):459–465. doi: 10.1016/0005-2787(72)90088-3. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Rott R., Klenk H. D. Two different mechanisms of the inhibition of the multiplication of enveloped viruses by glucosamine. Virology. 1975 Jan;63(1):191–200. doi: 10.1016/0042-6822(75)90384-0. [DOI] [PubMed] [Google Scholar]
- Sukeno T., Kikuchi H., Saeki H., Tsuiki S. Transformation of glucosamine to glycogen and lactate by ascites tumor cells. Biochim Biophys Acta. 1971 Jul 20;244(1):19–29. doi: 10.1016/0304-4165(71)90116-4. [DOI] [PubMed] [Google Scholar]
- Tesoriere G., Vento R., Calvaruso G. Inhibitory effect of D-glucosamine on glycolysis in bovine retina. Biochim Biophys Acta. 1975 Mar 14;385(1):58–67. doi: 10.1016/0304-4165(75)90074-4. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Hennessy S. W. Cholesterol biosynthesis and 3-hydroxy-3-methyl-glutaryl coenzyme A reductase in cultured glial and neuronal cells. Regulation by lipoprotein and by certain free sterols. Biochim Biophys Acta. 1977 Mar 25;486(3):408–420. doi: 10.1016/0005-2760(77)90090-x. [DOI] [PubMed] [Google Scholar]