Abstract
The saxitoxin-binding component (SBC) of the excitable membrane sodium channel has been solubilized and purified from rat skeletal muscle sarcolemma. Phospholipid was required in mixed micelles with detergent for stability of the mammalian SBC. Even at optimal detergent-to-phospholipid ratio, the solubilized SBC showed significant temperature-dependent loss of specific toxin binding with time, necessitating maintenance of low temperatures during purification. Characteristics of saxitoxin binding to the solubilized material closely resembled those seen in intact membranes. A weak anion-exchange column was synthesized; it provided rapid 10- to 20-fold purification of the solubilized SBC. Additional necessary purification was obtained by chromatography on immobilized wheat germ agglutinin. Specific saxitoxin-binding activity of the purified material averaged approximately 1500 pmol of saxitoxin bound per mg of protein. Three bands were present in this material on sodium dodecyl sulfate/polyacrylamide gel electrophoresis. The purified material sedimented on a sucrose gradient with an apparent s20,w of 9.9 S.
Full text
PDF
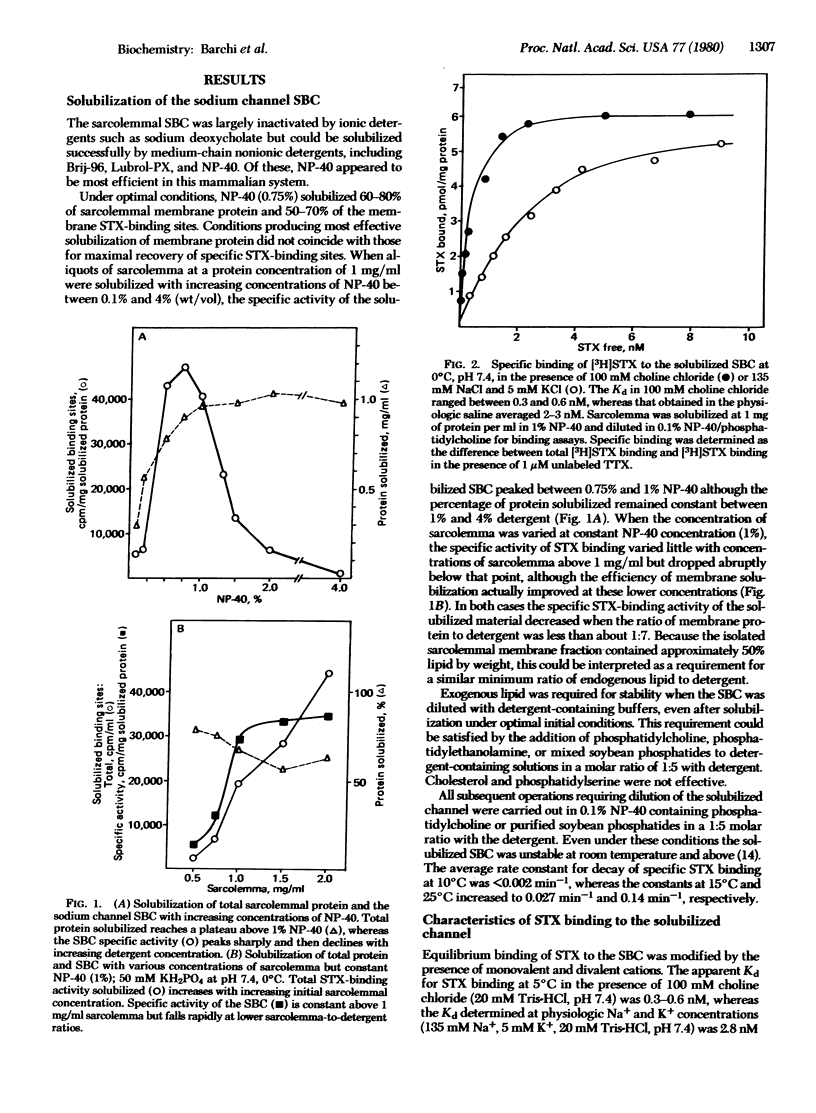
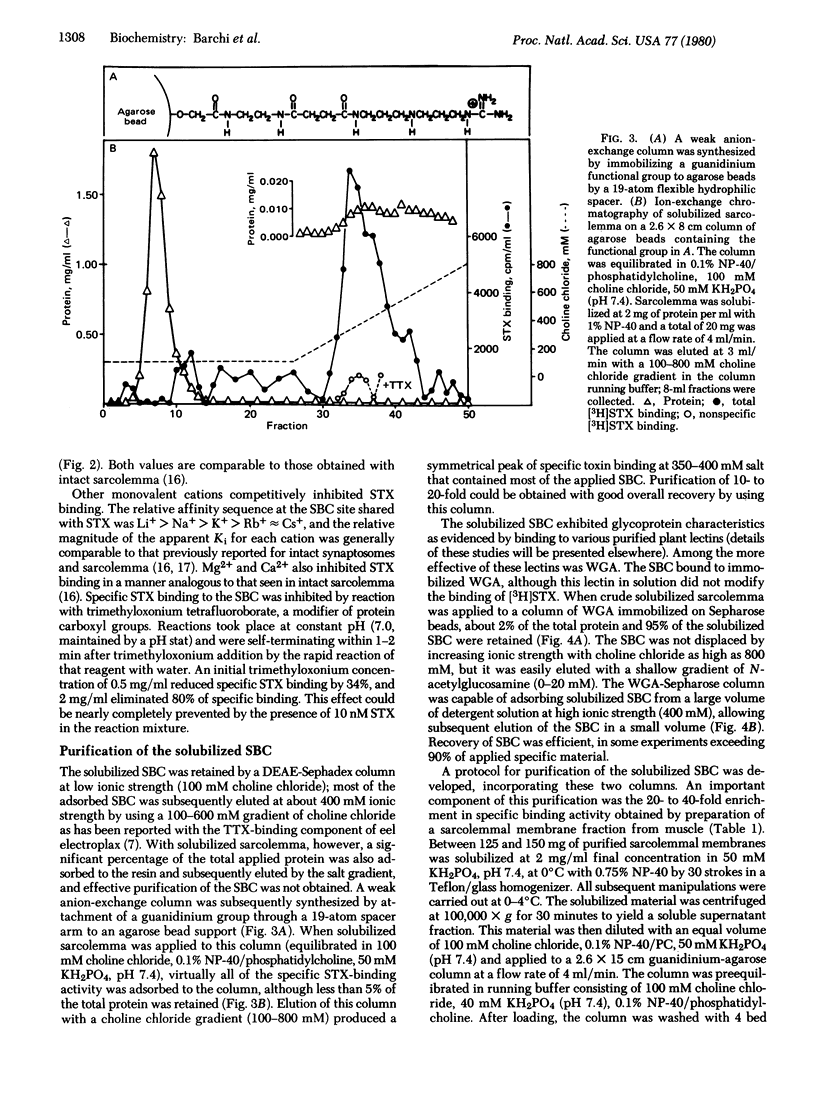
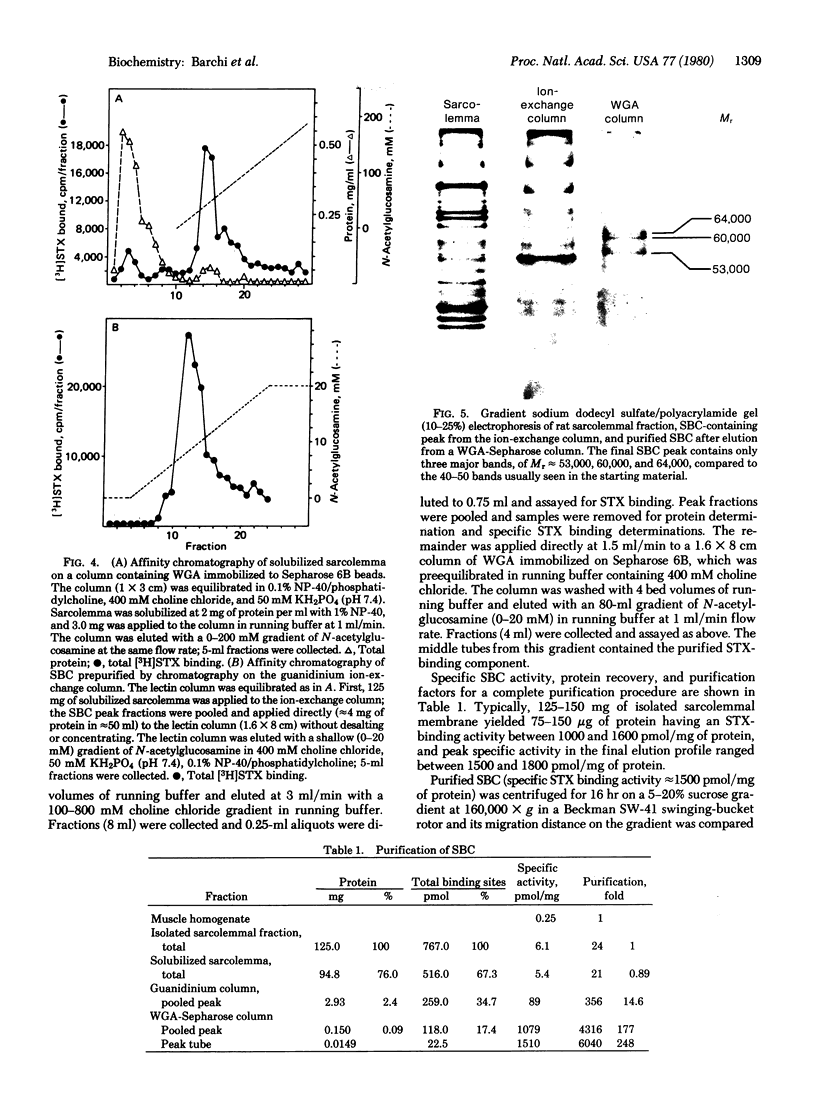

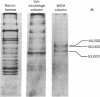
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew W. S., Raftery M. A. Solubilized tetrodotoxin binding component from the electroplax of Electrophorus electricus. Stability as a function of mixed lipid-detergent micelle composition. Biochemistry. 1979 May 15;18(10):1912–1919. doi: 10.1021/bi00577a010. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M. Ionic pores, gates, and gating currents. Q Rev Biophys. 1974 May;7(2):179–210. doi: 10.1017/s0033583500001402. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B., Chalikian D. M., Murphy L. E. Muscle surface membranes: preparative methods affect apparent chemical properties and neurotoxin binding. Biochim Biophys Acta. 1979 Jan 5;550(1):59–76. doi: 10.1016/0005-2736(79)90115-9. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Weigele J. B. Characteristics of saxitoxin binding to the sodium channel of sarcolemma isolated from rat skeletal muscle. J Physiol. 1979 Oct;295:383–396. doi: 10.1113/jphysiol.1979.sp012975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Engel W. K. In vitro analysis of the general properties and junctional receptor characteristics of skeletal muscle membranes. Isolation, purification, and partial characterization of sarcolemmal fragments. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2435–2439. doi: 10.1073/pnas.71.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Wang J. H. Solubilization of a specific tetrodotoxin-binding component from garfish olfactory nerve membrane. Biochemistry. 1972 Nov 21;11(24):4565–4569. doi: 10.1021/bi00774a022. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman J. K. Covalent linkage of functional groups, ligands, and proteins to polyacrylamide beads. Methods Enzymol. 1974;34:30–58. doi: 10.1016/s0076-6879(74)34006-2. [DOI] [PubMed] [Google Scholar]
- KLEE W. A., RICHARDS F. M. The reaction of O-methylisourea with bovine pancreatic ribonuclease. J Biol Chem. 1957 Nov;229(1):489–504. [PubMed] [Google Scholar]
- Lefkowitz R. J., Haber E., O'Hara D. Identification of the cardiac beta-adrenergic receptor protein: solubilization and purification by affinity chromatography. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2828–2832. doi: 10.1073/pnas.69.10.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson S. R., Ellory J. C. Molecular size of the tetrodotoxin binding site estimated by irradiation inactivation. Nat New Biol. 1973 Sep 26;245(143):122–123. doi: 10.1038/newbio245122a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Analysis of saxitoxin binding in isolated rat synaptosomes using a rapid filtration assay. FEBS Lett. 1978 Jul 15;91(2):310–314. doi: 10.1016/0014-5793(78)81199-5. [DOI] [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Saxitoxin binding to the mammalian sodium channel. Competition by monovalent and divalent cations. FEBS Lett. 1978 Nov 1;95(1):49–53. doi: 10.1016/0014-5793(78)80049-0. [DOI] [PubMed] [Google Scholar]