Abstract
In September of 2011, the National Institute of Neurological Disorders and Stroke (NINDS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the International Rett Syndrome Foundation (IRSF) and the Rett Syndrome Research Trust (RSRT) convened a workshop involving a broad cross-section of basic scientists, clinicians and representatives from the National Institutes of Health (NIH), the US Food and Drug Administration (FDA), the pharmaceutical industry and private foundations to assess the state of the art in animal studies of Rett syndrome (RTT). The aim of the workshop was to identify crucial knowledge gaps and to suggest scientific priorities and best practices for the use of animal models in preclinical evaluation of potential new RTT therapeutics. This review summarizes outcomes from the workshop and extensive follow-up discussions among participants, and includes: (1) a comprehensive summary of the physiological and behavioral phenotypes of RTT mouse models to date, and areas in which further phenotypic analyses are required to enhance the utility of these models for translational studies; (2) discussion of the impact of genetic differences among mouse models, and methodological differences among laboratories, on the expression and analysis, respectively, of phenotypic traits; and (3) definitions of the standards that the community of RTT researchers can implement for rigorous preclinical study design and transparent reporting to ensure that decisions to initiate costly clinical trials are grounded in reliable preclinical data.
Introduction
Rett syndrome (RTT) is a prototype childhood neurological disease characterized by features that are observed in many other disorders ranging from autism to Parkinson’s disease and dystonia. The disorder affects ∼1 in 10,000 females and is most often caused by mutations in the gene encoding methyl-CpG-binding protein 2 (MeCP2), a transcriptional regulatory protein. It has been shown that many of the features of RTT are reversible in mice (Gadalla et al., 2011; Guy et al., 2007), and that these features are probably due to dysfunction of neurons and supporting cells, rather than neural degeneration (Armstrong, 2002). These findings provide hope that some and perhaps most symptoms can be reversed in affected individuals if we discover effective therapies that can overcome the consequences of loss of function or dysfunction of MeCP2.
There is a crucial need to develop effective treatments for RTT. Encouragingly, several key findings suggest that RTT could be treatable in humans, and might be a promising model for developing rigorous paradigms for evaluating therapeutic interventions not only in RTT but in other postnatal childhood disorders as well. First, RTT typically manifests months after birth, arguing that key embryonic and perinatal developmental steps take place normally in affected individuals. Second, there are several excellent mouse models in which many of the somatic, behavioral and physiological changes observed in individuals with RTT are reproduced (discussed in detail below). Third, the findings that some RTT-like symptoms are reversible in mouse models following reactivation of silent Mecp2 alleles (Guy et al., 2007; Lioy et al., 2011; Robinson et al., 2012) or transgene-mediated Mecp2 replacement (Alvarez-Saavedra et al., 2007; Collins et al., 2004; Giacometti et al., 2007; Jugloff et al., 2008; Luikenhuis et al., 2004), and that most RTT-like symptoms are reproduced following loss of MeCP2 in adult animals (Cheval et al., 2012; McGraw et al., 2011; Nguyen et al., 2012), argue that most of the disease phenotypes are caused by functional disturbances of neural circuits, rather than irreversible developmental brain abnormalities. Thus, the challenge for the field now is to build on these exciting findings by identifying therapeutic opportunities, testing them rigorously in RTT models to determine their effectiveness in improving clinically relevant outcome measures, and establishing their safety with chronic use.
Given the emergence of new therapeutic leads in the field (cf. Abdala et al., 2010; De Filippis et al., 2012; Deogracias et al., 2012; Kron et al., 2012; McCauley et al., 2011; Nag and Berger-Sweeney, 2007; Ogier et al., 2007; Roux et al., 2007; Schmid et al., 2012; Tropea et al., 2009; Zanella et al., 2008), RTT models hold great promise for translational research, particularly for prioritizing and validating potential treatment strategies prior to launching costly clinical trials. Unfortunately, as discussed later in this review, there is a long history of failure in translating promising findings from preclinical animal models to clinical success, especially for neurological disorders. Therefore, it is crucial that the RTT research community develops best practices and standards for performing preclinical trials, and identifies the best path forward for justifying the advancement of preclinical discoveries to clinical trials. These steps are essential to avoid involving individuals with RTT in unnecessary clinical trials, wasting precious research money, and raising unwarranted expectations for the patients and their families.
With these goals in mind, the National Institute of Neurological Disorders and Stroke (NINDS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and private United States organizations supporting RTT research [the International Rett Syndrome Foundation (IRSF) and the Rett Syndrome Research Trust (RSRT)] held a workshop entitled ‘Setting Priorities for Therapy Development in Rett Syndrome’ at the Hyatt Regency Bethesda (Bethesda, MD) on 25–27 September 2011 to discuss how to optimize the predictive value of animal models in RTT preclinical research and avoid the pitfalls that often lead to failure on clinical translation. In addition to these funding agencies and foundations, workshop participants included members of the RTT research community and the pharmaceutical industry, clinicians, and representatives from the US Food and Drug Administration (FDA). The workshop agenda and participant list are available in supplementary material Tables S1 and S2, respectively.
A key component of the workshop was the creation of working groups that were responsible for reviewing the state of the art in phenotypic analyses of mouse models of RTT. Four groups were created, each focusing on different phenotypic domains: (1) general health, locomotion and lifespan (led by Dr James Eubanks, University of Toronto); (2) respiratory and autonomic control (led by Drs David Katz, Case Western Reserve University School of Medicine, and Jeffrey Neul, Baylor College of Medicine); (3) cognitive, social and anxiety phenotypes (led by Dr Joanne Berger-Sweeney, Tufts University); and (4) cellular and synaptic phenotypes (led by Dr Lucas Pozzo-Miller, The University of Alabama at Birmingham). Reporting on key outcomes from the workshop, as well as subsequent discussions among the working groups, this paper reviews the phenotypic characteristics of currently available mouse models of RTT, highlights current knowledge gaps, and identifies criteria and best practices for preclinical study design that we hope will optimize the ability of the RTT research community to translate basic findings into new therapeutic approaches. We begin with a brief overview of the principal clinical features of RTT, which is a necessary foundation for evaluating the degree to which existing mouse models reproduce phenotypes of the human disease, and for identifying sensitive and relevant outcome measures for preclinical and clinical trials.
Genetic and clinical-pathological features of RTT
Loss-of-function mutations in MECP2, an X-linked gene, account for the vast majority (∼95%) of typical RTT cases (Amir et al., 1999). Most mutations arise spontaneously (de novo) in the paternal germ line; thus, individuals with RTT are typically females who, owing to X-chromosome inactivation, are somatic mosaics for normal and mutant MECP2. Boys with mutations that cause RTT in females typically die before or soon after birth with a severe encephalopathy (discussed in more detail below).
RTT is distinguished by its unique time course and phenotypic complexity. Affected individuals present with postnatal neurological regression, usually starting between 1.5 and 3 years of age (but sometimes as early as 6 months of age), with loss of acquired hand skills and spoken language and, in some cases, social withdrawal or extreme irritability that can resemble autism (Hagberg, 2002; Neul et al., 2010). After regression, there is a stabilization of skills, rather than a relentless progression, a feature that differentiates RTT from neurodegenerative conditions such as Batten disease or Huntington’s disease. During this pseudo-stationary or plateau stage, characteristic features of RTT such as repetitive hand movements (stereotypies), which can be present before or during regression, become more prominent. Later in life, many affected individuals enter a stage of motor decline in which ambulation can be lost, and Parkinsonian features such as rigidity and hypomimia become prominent (FitzGerald et al., 1990a; FitzGerald et al., 1990b). Mutations in other genes such as cyclin-dependent kinase like 5 (CDKL5) and forkhead box G1 (FOXG1) can cause phenotypes overlapping with those seen in RTT (Archer et al., 2006; Ariani et al., 2008); however, several features, such as congenital onset and infantile spasms in CDKL5-mutant patients, and congenital onset and hypoplasia of the corpus callosum in FOXG1-mutant patients, distinguish these disorders from typical RTT (Kortüm et al., 2011).
During the regression stage, some individuals with RTT develop autistic features that include social withdrawal, avoidance of eye contact and indifference to visual or auditory stimuli (Mount et al., 2002a; Mount et al., 2003). After regression, some of these autistic features decrease, and most affected individuals develop intense eye gaze that they use for communication (Coenraads, 2007; Kaufmann et al., 2012). Recent work has shown that features such as stereotypies and lack of language skills persist throughout the life of affected individuals, although hand stereotypies can change from rapid movements to midline hand clasping with age. Additional behavioral problems include anxiety in response to novel situations (Mount et al., 2002b), increased behavioral rigidity and increased pain tolerance (Downs et al., 2010). Individuals with RTT are considered to have severe intellectual disability; however, because affected individuals have severe impairments in their ability to communicate, it is difficult to make accurate assessments of their intellectual ability (Baptista et al., 2006; Neul et al., 2010).
Movement abnormalities are a major issue in RTT (FitzGerald et al., 1990a; FitzGerald et al., 1990b), with the most obvious being the repetitive hand stereotypies, which seem to interfere with volitional hand use. Gait is almost always disrupted, with evidence of ataxia and apraxia. Dystonia is common, seen first in the ankles and eventually progressing to many joints. Axial hypotonia is present early in the disease course but, as children become young adults, increased tone with features of rigidity becomes more prominent. Additional movement abnormalities include tremor, myoclonus, chorea, facial grimacing and severe teeth grinding. Most individuals with RTT have scoliosis, and some require surgical intervention (Percy et al., 2010).
Nutrition and gastrointestinal function are also major clinical issues in RTT, and there is marked growth failure in most affected individuals (Tarquinio et al., 2012). It has long been recognized that head growth is impaired, resulting in acquired microcephaly (Hagberg et al., 1983), and height and weight are usually markedly diminished (Schultz et al., 1993). However, a subset of individuals with RTT are overweight or obese (Renieri et al., 2009), a feature that is often associated with higher functioning and possibly improved oromotor skills (Motil et al., 1999). Many individuals with RTT have various gastrointestinal problems, including significant chewing and swallowing difficulties, gastroesophageal reflux, gastrointestinal dysmotility and severe constipation, which severely decrease the quality of life for patients and their families (Motil et al., 2012).
Dysregulation of breathing and autonomic homeostasis are very common in RTT. Respiratory abnormalities, which include periods of forceful breathing (hyperventilation), severe pauses in breathing (including breath holds) that can cause cyanosis and even loss of consciousness, and abnormal cardiorespiratory coupling, are more severe during wakefulness than during sleep (Elian and Rudolf, 1991; Julu et al., 2001; Julu and Witt Engerström, 2005; Marcus et al., 1994; Weese-Mayer et al., 2008; Weese-Mayer et al., 2006) and can be exaggerated during periods of excitement or stress. Autonomic abnormalities include periods of vasomotor disturbance (usually associated with cold hands and feet), abnormal sweating, decreased heart rate variability, evidence of sympathetic-parasympathetic imbalance and prolongation of corrected QT interval (an indication of abnormal cardiac electrical activity) in a subset of individuals (Guideri et al., 2004; McCauley et al., 2011; Sekul et al., 1994). One quarter of deaths in RTT are sudden and unexpected (Kerr et al., 1997), and might result from complications of cardiorespiratory dysfunction.
Brain electrical activity is not typical in individuals with RTT, as shown by the markedly disrupted pattern observed on electroencephalograms (EEGs) (Glaze et al., 1998) and the high probability of seizures (Glaze et al., 2010). Seizures, ranging from complex partial to generalized tonic-clonic, are most commonly seen after other symptoms appear (usually after age two) and correlate with the severity of the phenotype. In addition to true epileptic events, individuals with RTT also have non-epileptic paroxysmal events, and video EEG is needed to differentiate between them (Glaze et al., 1998).
Despite the severity and phenotypic complexity of RTT, the brains of individuals with RTT do not show gross neuropathological changes, nor evidence of neuronal or glial atrophy, degeneration, gliosis, or demyelination, indicating that RTT is not a neurodegenerative disorder (Jellinger et al., 1988; Reiss et al., 1993). Smaller total brain volume and smaller neurons (but with a higher cell density) have been observed in several brain regions, including the cerebral cortex, hypothalamus and the hippocampal formation (Bauman et al., 1995a; Bauman et al., 1995b). The size and complexity of dendritic trees are reduced in cortical pyramidal cells (Armstrong et al., 1995; Armstrong et al., 1998), and levels of microtubule-associated protein-2 (MAP-2), a protein involved in microtubule stabilization, are lower throughout the neocortex of RTT autopsy material (Kaufmann et al., 2000; Kaufmann et al., 1995). In addition, the density of dendritic spines is lower in pyramidal neurons of the frontal cortex (Belichenko et al., 1994; Jellinger et al., 1988) and in the CA1 region of the hippocampus (Chapleau et al., 2009).
Mouse models of RTT
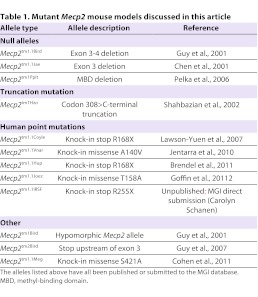
Identification of MECP2 as the disease-causing gene led rapidly to the development of mouse models of RTT (Table 1) that recapitulate, to varying degrees, the underlying molecular and genetic defects and symptoms of the human disease (Table 2). Established models include mice carrying either global alleles (null, hypomorphic, large deletions and point mutations) or conditional null alleles (reviewed in Calfa et al., 2011b). As discussed below, a major focus at the workshop concerned the utility of the various models for translational studies. One point of consensus was that conditional alleles (i.e. cell-specific deletions created by cross-breeding of floxed Mecp2 mice with Credeleter lines), although useful for elucidating mechanisms of neurological dysfunction, are unlikely to be of value in translational studies because, by design, they do not recapitulate the global loss of MeCP2 that is characteristic of RTT.
Table 1.
Mutant Mecp2 mouse models discussed in this article
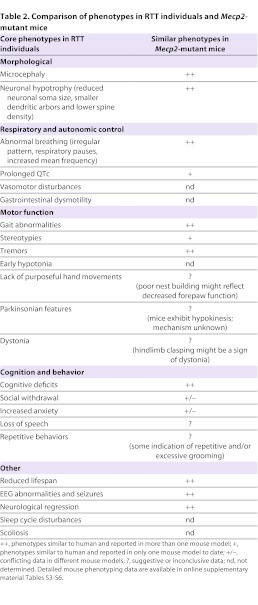
Table 2.
Comparison of phenotypes in RTT individuals and Mecp2-mutant mice
Global Mecp2 alleles generally fall into three categories: (1) null mutations (Mecp2tm1.1Bird, Mecp2tm1.1Jae or Mecp2tm1Pplt) (Chen et al., 2001; Guy et al., 2001; Pelka et al., 2006), which are similar to the large deletions found in ∼10% of affected people; (2) truncations and single-nucleotide mutations that approximate or reproduce mutations found in individuals with RTT (codon 308-C-terminal truncation: Mecp2tm1Hzo; R168X: Mecp2tm1.1Coyle and Mecp2tm1Hup; T158A: Mecp2tm1.1Joez; and R255X: Mecp2tm1.1Irsf) (Brendel et al., 2011; Goffin et al., 2012; Jentarra et al., 2010; Lawson-Yuen et al., 2007; Shahbazian et al., 2002); and (3) point mutations that either model related neurodevelopmental disorders [A140V: Mecp2tm1.1Vnar (Jentarra et al., 2010)] or provide mechanistic insight into MeCP2 function [S421A: Mecp2tm1.1Meg (Cohen et al., 2011)]. Mutant mice that are hypomorphic for Mecp2 (Mecp2tm1Bird) (Kerr et al., 2008; Samaco et al., 2008) have also been created; these animals express MeCP2 at ∼50% of wild-type levels due to retention of a selectable marker cassette in the floxed Mecp2 allele and, as expected, exhibit more modest behavioral impairments compared with the complete nulls. Phenotypic details of the various RTT mouse models are summarized in supplementary material Tables S3–S7.
During the workshop, discussion of mouse models focused on three key concepts used to evaluate the utility of a model for translational studies: (1) construct validity (similarities in underlying molecular mechanisms in humans and mice); (2) reproducibility (findings replicated in more than one laboratory) and robustness (effect size, and whether or not findings are generalizable to more than one model and/or experimental condition); and (3) face validity (similarities in anatomical, physiological and/or behavioral phenotypes in humans and mice).
Construct validity
To some degree, all global null or hypomorphic alleles have construct validity, given that most disease-causing mutations in human RTT cause loss of MeCP2 function (e.g. Kudo et al., 2001). However, as noted above, only 10% of mutations in individuals with RTT are deletions that are large enough to approximate a true null condition. Moreover, although male mice have, until recently, been used most frequently in preclinical studies of RTT, male nulls and hypomorphs do not exhibit the genetic mosaicism that is characteristic of the vast majority of individuals with RTT, who are female. Furthermore, it is not known whether complete loss of MeCP2 in cells (either in hemizygous males or heterozygous females) recapitulates the effects of a point mutation or truncated protein, including the possibility of toxic gain of function with some of the human mutations. Thus, translational studies in mice carrying null alleles might only be relevant to a relatively small subset of individuals with RTT. However, many MECP2 point mutations, in addition to large deletions, are believed to eliminate gene function; in particular, some mutations in the methyl-binding and transcriptional repression domains cause the most severe RTT phenotypes and might be relevant to the null allele. Therefore, current attempts to establish the construct validity of various RTT mouse models (e.g. null versus point mutations) would benefit from detailed comparisons of how the corresponding alleles, when studied in the same genetic background, alter target gene expression, cell physiology and neurological function. It would also be helpful to know whether the two most commonly used null alleles, Mecp2tm1.1Bird and Mecp2tm1.1Jae, are functionally equivalent, given that they were generated using targeting strategies directed against slightly different sequences in the coding region of Mecp2. Ultimately, choosing mouse models for translational studies might require compromises between construct validity and practical concerns – i.e. to optimize phenotypes that are robust and that develop within a timeframe that is cost effective for large-scale preclinical studies.
What is notable in RTT mouse models is evidence of construct validity beyond the genetic mutations themselves, including neurochemical abnormalities found in individuals with RTT. For example, diverse mouse models reproduce the reduced levels of biogenic amines and brain-derived neurotrophic factor (BDNF) that are found in the brains of RTT patients (Wang et al., 2006; Deng et al., 2007; Li et al., 2012; Chang et al., 2006). Thus, removing MeCP2 function from mice reproduces at least some of the same cell signaling deficits as MECP2 mutations in humans.
Reproducibility and robustness
In addition to the allelic diversity of current RTT models, RTT research is further confounded by the fact that different laboratories study different alleles on different genetic backgrounds and at different developmental stages, making it difficult to discern the effects of a specific allele from genetic, environmental and maturational influences. Expression of the same Mecp2 allele on different genetic backgrounds can confer significant differences in phenotypic effects, including but not limited to time of onset and symptom severity. Similarly, the degree to which animal husbandry practices (including food, water, lighting, noise, handling and bedding), maternal age and quality of maternal care influence the phenotypic effects of Mecp2 alleles has not been studied. Furthermore, disease progression across the lifespan has not been carefully evaluated in most models, especially in heterozygous females, as discussed below. For translational studies, therefore, the variations in phenotype that are influenced by genetic background and animal husbandry practices highlight the importance of validating phenotypes and therapeutic efficacy in multiple models and in multiple laboratories. Such validation will hopefully mitigate the possibility that a given study is treating idiosyncratic gene-gene or gene-environment interactions that might not generalize or be relevant to the human disease.
Fortunately, and as would be expected based on the high penetrance of MECP2 mutations in humans, at least some phenotypes that mimic symptoms of human RTT are common to multiple Mecp2-mutant mouse models (see below). However, relatively little is known about whether RTT mouse models recapitulate the unique features of progression, regression and stabilization that define the human disease. This might reflect the overall bias of the scientific community, which has performed most phenotypic analyses in male hemizygous mice. These animals might best model the small number of human male patients who are hemizygous for mutant MECP2 and present, not with clinically defined RTT, but with a severe encephalopathy accompanied by a failure to acquire skills, rather than regression. An increasing number of laboratories are shifting to phenotypic analysis of heterozygous female mice, which might yield models that more closely phenocopy human RTT, including temporal features such as disease regression.
Face validity: RTT phenotypes recapitulated in mouse models
The following sections provide an overview of the key pathophysiological findings that have been reported in RTT mouse models carrying global (rather than cell-specific) Mecp2 mutant alleles. Most models exhibit a broad spectrum of phenotypes that are similar to those seen in RTT individuals, including shortened lifespan, motor and sensory impairments, breathing abnormalities, cognitive and behavioral dysfunction, and cellular and synaptic defects (reviewed in Calfa et al., 2011b) (summarized in Table 2). However, care must be taken not to assume, on the basis of face validity alone, that similar behavioral phenotypes in mice and humans necessarily arise from the same underlying pathophysiological mechanisms. Emphasis here is given to those findings that are most robust and reproducible across different models and/or genetic backgrounds and in different laboratories, with the goal of identifying the models and phenotypes that will be most useful for translational studies. This overview is by no means exhaustive; further details and additional references for each model are provided in the accompanying online supplemental material (supplementary material Tables S3–S7). Male mice that are hemizygous for Mecp2 null alleles are referred to as ‘Nulls’, and females that are heterozygous for Mecp2 null alleles are referred to as ‘Hets’.
General health, locomotion and lifespan
Gross motor dysfunction and shortened lifespan are common features of diverse RTT mouse models on different genetic backgrounds and broadly recapitulate the clinical presentation in individuals with RTT (Table 2; supplementary material Table S3). Mecp2-mutant mice exhibit robust and reproducible abnormalities in motor behaviors, including hypoactivity (Chen et al., 2001; Guy et al., 2001; Jugloff et al., 2008; Stearns et al., 2007), impaired balance and coordination (Alvarez-Saavedra et al., 2007; Jugloff et al., 2008; Kondo et al., 2008; Shahbazian et al., 2002; Stearns et al., 2007), spontaneous tremors (Alvarez-Saavedra et al., 2007; Brendel et al., 2011; Chen et al., 2001; Goffin et al., 2012; Guy et al., 2001; Lawson-Yuen et al., 2007; Pelka et al., 2006; Shahbazian et al., 2002; Stearns et al., 2007), hindlimb clasping (Brendel et al., 2011; Chen et al., 2001; Goffin et al., 2012; Guy et al., 2007; Guy et al., 2001; Kondo et al., 2008; Lawson-Yuen et al., 2007; Pelka et al., 2006; Stearns et al., 2007), and impaired limb and postural reflexes (Picker et al., 2006; Santos et al., 2007). In male Nulls, these deficits occur early (within 6 weeks of birth) and are generally more pronounced than in female Hets; female Hets typically develop milder motor impairments (usually not seen before 10 weeks of age) and exhibit greater phenotypic variability (Stearns et al., 2007). In addition, excessive and repetitive grooming has been observed in male Mecp2tm1.1Jae Nulls and might represent a form of motor stereotypy (Stearns et al., 2007). To a first approximation, many of these phenotypes seem to mimic motor deficits exhibited by individuals with RTT. For example, hindlimb clasping, although not specific to RTT mice, might represent a form of motor dysfunction. However, further work is required to determine the degree to which these human and mouse phenotypes truly share common underlying neurological mechanisms.
Lifespan is severely shortened in male Nulls (including both the commonly employed Mecp2tm1.1Bird and Mecp2tm1.1Jae models), which rarely live longer than 3 months. Prior to death, male Nulls often exhibit severe hypoactivity, kyphosis and disheveled fur, and typically undergo severe weight loss. In female Hets carrying null alleles, there is a higher than normal rate of sudden and unexpected death (unpublished observations from multiple groups), although kyphosis and disheveled fur are less reproducibly observed. Lifespan is also reduced in most of the male hemizygous human (targeted) mutation models (Goffin et al., 2012; Lawson-Yuen et al., 2007; Shahbazian et al., 2002), but the degree varies with the specific Mecp2 allele and/or genetic background. An exception is the Mecp2tm1.1Vnar (A140V) mutant line, which has an apparently normal lifespan and, other than cellular abnormalities, does not exhibit many obvious phenotypic defects (Jentarra et al., 2010); these findings are consistent with clinical data showing that missense A140V mutations are usually associated with milder phenotypes in males and have not been reported to cause classical RTT in females.
In contrast to the relatively robust phenotypes described above, genetic background seems to have a significant impact on body weight. Mecp2 null alleles on a C57BL/6 background tend to have reduced body weight (Guy et al., 2001; Pelka et al., 2006; Stearns et al., 2007; Ward et al., 2011), whereas alleles on the 129 background often exhibit increased body weight (Chen et al., 2001; Shahbazian et al., 2002). Lonetti and colleagues used a mixed genetic background (B6.129SF1) and found no evidence of increased body weight (Lonetti et al., 2010).
Respiratory and autonomic phenotypes
The most robust breathing phenotype in RTT mouse models that has been reproducibly observed by different laboratories and in different mouse strains is abnormal variation in respiratory cycle length in room air, including respiratory pauses and periods of tachypnea associated with decreased expiratory time and increased mean breathing frequency (Table 2; supplementary material Table S4). These breathing abnormalities closely phenocopy respiratory dysfunction observed in individuals with RTT. Respiratory pauses occur in male Nulls, and female Hets and hypomorphs, with an earlier onset in Nulls (by ∼5 weeks) compared with Hets (∼10 weeks). Null males and Het females also exhibit exaggerated respiratory reflexes, including enhanced vagally induced respiratory pauses and increased hypoxic ventilatory responses (Bissonnette and Knopp, 2006; Johnson et al., 2012; Roux et al., 2008; Stettner et al., 2007; Voituron et al., 2009).
Although cardiac phenotypes have been less well characterized, recent work indicates that male Nulls and female Hets exhibit prolongation of the corrected QT interval and an increased susceptibility to induced cardiac arrhythmia and cardiac death. This seems to be a progressive phenotype as female Hets exhibit these phenotypes at 11 but not 4 months of age (McCauley et al., 2011).
Cognitive, social and anxiety phenotypes
As noted above, the severity of communication deficits in individuals with RTT has hampered systematic evaluation of their cognitive abilities. Thus, although cognitive deficits have been identified in RTT mouse models (Table 2; supplementary material Table S5), the face validity of these findings has been difficult to establish. One of the most reliable and robust cognitive phenotypes in RTT mice – i.e. replicated by different laboratories, in different RTT mouse models with diverse Mecp2 alleles and/or genetic backgrounds – is impairment in contextual fear conditioning, which is a test of associative learning and memory. This phenotype is apparent in male Nulls by about 6 weeks of age (Stearns et al., 2007); similarly, the human mutation models tested to date – male Mecp2tm1.1Joez and Mecp2tm1Hzo mice – show impairments in contextual fear conditioning by 10 and 20 weeks of age, respectively (Shahbazian et al., 2002; Moretti et al., 2006; Goffin et al., 2012). Object recognition testing has also revealed learning deficits in Mecp2tm1.1Jae male Nulls and female Hets (Schaevitz et al., 2010; Stearns et al., 2007) and Mecp2tm1.1Meg knock-in mice (Cohen et al., 2011), the models tested thus far. Likewise, motor-cerebellar learning is impaired in all Mecp2 models examined to date (Goffin et al., 2012; Lonetti et al., 2010; Pelka et al., 2006). In one study, female Mecp2tm1.1Jae Hets were found to have milder, more variable cognitive impairments as adults (Stearns et al., 2007), and more recent evidence indicates that behavioral phenotypes, including cognitive impairments, are detectable in young Mecp2tm1.1Bird Hets (Samaco et al., 2012).
In contrast to the robust cognitive phenotypes, social behavior phenotypes in RTT mouse models vary widely, depending on the Mecp2 allele and genetic background. Comparison among models is further complicated by methodological differences in social testing paradigms used in different studies. Nonetheless, all models tested thus far show a preference for spending time with another mouse rather than an inanimate object, unlike some models of other autism spectrum disorders that show no preference, such as BTBR mice (McFarlane et al., 2008). However, the extent and even nature of sociability differs across models, depending on the allele and/or genetic background. For example, male Mecp2tm1.1Jae and Mecp2tm1.1Bird mice show evidence of increased sociability, including increased time at partitions and more time exploring unfamiliar mice (Kerr et al., 2008; Schaevitz et al., 2010). Similarly, male Mecp2tm1Hzo mice on a C57BL/6J background exhibit enhanced pro-social behavior compared with wild-type mice (Pearson et al., 2012). In contrast, the same allele (male Mecp2tm1Hzo) on a 129/SvEv or mixed 129SvEv:B6 background exhibits reduced social interactions as demonstrated in male resident-intruder and partition tests (Moretti et al., 2005; Moretti et al., 2006; Shahbazian et al., 2002). Thus, the impact of reduced MeCP2 function per se on social behavior remains very unclear, as is the relevance of the mouse phenotypes identified thus far to the social withdrawal and autistic features seen in some individuals with RTT.
Likewise, measures of anxiety vary widely depending on allele, genetic background and testing paradigm. Using the elevated plus and zero mazes, male Nulls and Mecp2tm1.1Joez mutants seem less anxious than wild types (i.e. they spend more time in light spaces of the maze) (Goffin et al., 2012; Kerr et al., 2012; Pelka et al., 2006; Stearns et al., 2007). The findings are the opposite in male Mecp2tm1Hzo mutants, which are more anxious in the same tasks (De Filippis et al., 2010; McGill et al., 2006). Using the open field, where mice ambulate between the center of the circular field (less anxious behavior) and the periphery of the field (more anxious behavior), male Mecp2tm1Hzo mutants and female Mecp2tm1.1Jae Hets seem more anxious than wild types (De Filippis et al., 2010; Lonetti et al., 2010).
A few laboratories have demonstrated that RTT mice exhibit abnormal ultrasonic vocalizations when pups are separated from their mothers during early postnatal development. Specifically, beginning at postnatal days 4–6, neonatal ultrasonic vocalizations are altered, although in opposite directions in the two Mecp2 models tested to date: vocalizations are decreased in male Mecp2tm1Hzo mice and increased in both male Null and female Het Mecp2tm1.1Jae mice compared with wild-type littermates (De Filippis et al., 2010; Picker et al., 2006). Whether these apparently conflicting results reflect true strain differences remains to be determined. Furthermore, although many individuals with RTT lack speech, most are very vocal. A better understanding of the underlying neural circuit dysfunction might help to differentiate between the preservation of vocalization and the loss of speech in RTT. Clearly, this is an important area for further investigation, because loss of acquired speech is a hallmark of classical RTT, and identifying a robust mouse model for this feature would be a valuable tool for understanding the underlying pathophysiology and for future translational studies.
Importantly, many behavioral tasks, including all of the anxiety measures, require an animal to ambulate. Therefore, testing of behavioral outcomes is often confounded by the severe motor impairments that are characteristic of most RTT mouse models. Furthermore, anxiety measures are highly sensitive (more so than for most behavioral tasks) to prior handling, the order of testing and the laboratory environment. Thus, for behavioral tasks in general, and anxiety measures in particular, it is important to control carefully for these variables.
Cellular, synaptic and circuit phenotypes
To some degree, the reproducibility and robustness of cellular and synaptic phenotypes in different Mecp2-mutant mice is difficult to evaluate owing to the methodological differences between studies published from different laboratories, and because not all available mouse models have been systematically analyzed. However, the prototypical features of human RTT neuropathology – such as smaller neurons, higher neuronal packing density, reduced dendritic arbors and abnormal dendritic spines – have all been found in models analyzed thus far (Table 2; supplementary material Table S6). In addition, studies of different strains of MeCP2-deficient mice have revealed consistent impairments in one or more physiological properties at the cellular and synaptic level in all brain regions studied so far (supplementary material Table S6). These impairments include network hyperexcitability in the brainstem and hippocampus (Calfa et al., 2011a; Kline et al., 2010; Kron et al., 2012; Medrihan et al., 2008; Zhang et al., 2008), network hypoexcitability and synaptic hypoconnectivity in the cerebral cortex (Dani et al., 2005; Dani and Nelson, 2009), altered intrinsic neuronal electrical properties in the locus ceruleus and substantia nigra (Gantz et al., 2011; Taneja et al., 2009), and dysregulation of transmitter release in cultured hippocampal neurons and chromaffin cells (Nelson et al., 2006; Wang et al., 2006). However, these physiological impairments exhibit significant regional specificity: decreased excitatory synaptic drive is found in cortical circuits, whereas increased neuronal or synaptic excitability is found in the hippocampus and multiple brainstem regions involved in autonomic control (reviewed in Shepherd and Katz, 2011) (see also Kron et al., 2012). Modest impairments in individual elements (e.g. GABAergic synapses) might lead to different outcomes depending on the spatiotemporal construction and connectivity of the specific neuronal network. In addition, because most physiological studies at the cellular and synaptic level have been carried out in male Nulls, it has rarely been feasible to distinguish primary cell-autonomous deficits from the secondary or compensatory changes that occur in females with a mosaic pattern of neuronal and glial Mecp2 deficiency [see, however, Taneja et al. (Taneja et al., 2009)].
Several laboratories have documented impairments in long-term potentiation (LTP) and depression (LTD), which are two forms of synaptic plasticity associated with learning and memory, in brain slice preparations from Mecp2 mutants, including Mecp2tm1.1Jae, Mecp2tm1.1Bird and Mecp2tm1Hzo Null mice (reviewed in Boggio et al., 2010). Reduced LTP has been found at excitatory CA3-to-CA1 synapses in the hippocampus (Asaka et al., 2006) and in layer II/III of the primary somatosensory cortex (Lonetti et al., 2010). Reduced LTD has been demonstrated in area CA1 (Asaka et al., 2006; Moretti et al., 2006). So far, most studies of synaptic plasticity have analyzed populations of neurons and synapses using extracellular electrodes. Importantly, analysis of monosynaptic connections between pyramidal neurons in layer V of the primary somatosensory cortex of Mecp2tm1.1Jae Null mice using intracellular electrodes demonstrated that LTP was intact at these synapses, provided that sufficient postsynaptic depolarization was achieved by step depolarization or by evoked action potentials during the induction of spike-timing-dependent plasticity (Dani and Nelson, 2009). Thus, it is not yet clear whether LTP phenotypes that are identified using extracellular electrodes actually reflect weak and sparse connections rather than specific deficits in classical mechanisms of LTP generation.
The presentation of seizure-like behaviors and atypical EEGs in RTT individuals [i.e. focal, multifocal and generalized epileptiform abnormalities, with rhythmic slow theta activity in the frontal-central regions (Glaze, 2005)] is recapitulated in Mecp2-mutant mice as a constellation of EEG abnormalities, with or without associated seizure-like behaviors. Indeed, abnormal EEGs have been described in male Null and female Het Mecp2tm1.1Bird mice (D’Cruz et al., 2010; Goffin et al., 2012; Liao et al., 2012; Wither et al., 2012), as well as in Mecp2tm1Hzo Null mice (Shahbazian et al., 2002) and Mecp2tm1.1Joez mutants (Goffin et al., 2012).
Improving the quality and rigor of preclinical research in RTT: a necessary step to achieving translational success
In addition to the importance of establishing well-characterized and validated animal models, the workshop participants discussed the need to improve the quality and rigor of preclinical studies as an important step in enhancing the predictive value of RTT translational research. Barriers to translational success, regardless of the disease in question, often include a lack of rigorous standards and transparency in reporting preclinical studies, as well as publication bias caused by the under-reporting of negative results in the scientific literature. At the workshop, Dr Shai Silberberg (NINDS) reviewed recently published meta-analyses showing that unintended biases in animal studies tend to substantially inflate effect sizes, or lead to Type 1 errors (false positives) and overestimations of potential therapeutic efficacy. These unintended biases include the lack of allocation concealment [i.e. the investigators have no knowledge of the experimental group to which an animal belongs (Fisher et al., 2009)], blinded assessment of outcome and random allocation of subjects to experimental groups (see Bebarta et al., 2003; Macleod et al., 2005; Ransohoff and Gourlay, 2010; Sena et al., 2007; Landis et al., 2012). Furthermore, in recent efforts to reproduce original findings of potential drug targets in animal models of amyotrophic lateral sclerosis (ALS) (Scott et al., 2008), women’s health and cardiovascular disease (Prinz et al., 2011), cancer (Begley and Ellis, 2012; Prinz et al., 2011) or spinal cord injury (Steward et al., 2012), the authors of follow-up studies were unable to replicate most data published by others, including those published in high-profile journals. This failure to replicate has been attributed to a number of factors, including chance observations due to small sample size, concerns about study quality and transparency in reporting, lack of robustness or generalization of the original findings, and publication bias [see Brunner et al. for a discussion of statistical ramifications associated with publication bias (Brunner et al., 2012)]. These and other alarming data have prompted a number of disease-focused groups, including the NINDS, to publish guidelines and policy statements for improving the quality and transparency of preclinical animal research (see Box 1).
Box 1. A call for increased rigor and transparent reporting in preclinical research.
Given the remarkably low success rate in translating preclinical research into clinical success, a number of disease-focused groups, including the NINDS, are recognizing the urgent need to raise the standards of preclinical studies to encompass the rigor and transparency that is already expected of human clinical trials [see CONSORT 2010 statement (Schulz et al., 2010)]. Recently published guidelines and policy statements from these groups outline some of the principles and standards of good study design and reporting when conducting preclinical trials of candidate therapeutics – e.g. allocation concealment, blinded assessment of outcome, random allocation of subjects to experimental groups and other methods designed to minimize bias and Type 1 (‘false positive’) errors [ARRIVE Guidelines, 2010 (http://www.nc3rs.org.uk/page.asp?id=1357); STAIR preclinical recommendations (Fisher et al., 2009); NINDS Notice NOT-NS-11-023 (http://www.ninds.nih.gov/funding/transparency_in_reporting_guidance.pdf); Alzheimer’s disease preclinical research guidelines (Shineman et al., 2011)]. In addition to publishing guidance (NINDS Notice NOT-NS-11-023), the NINDS has incorporated these guidelines into enhanced review considerations for NINDS-supported translational (e.g. http://grants.nih.gov/grants/guide/pa-files/PAR-11-294.html) and clinical (e.g. http://grants.nih.gov/grants/guide/pa-files/PAR-11-343.html) research programs. Moreover, the NINDS convened a 2-day workshop (20–21 June 2012 in Washington, DC) entitled ‘Optimizing the Predictive Value of Preclinical Research’ to identify the key causes of deficiencies in preclinical studies and provide recommendations for addressing them; the outcomes of this workshop have been reported (Landis et al., 2012) and the workshop agenda can be accessed online (http://www.ninds.nih.gov/funding/areas/channels_synapses_and_circuits/rigor_and_transparency/index.htm). By raising standards and awareness, these initiatives strive to increase the reliability, reproducibility and predictive value of preclinical research, and ultimately to improve the likelihood of success on clinical translation.
Moreover, the NINDS considers independent replication to be an asset in evaluating the readiness of a candidate therapy for NINDS-supported translational programs or clinical trials (NINDS Notice NOT-NS-023; http://www.ninds.nih.gov/funding/transparency_in_reporting_guidance.pdf). Ideally, replication should be conducted by an independent laboratory (or by a contract research organization) with no financial, scientific or other vested interest in the original study. Steward et al. discuss some of the challenges in carrying out independent replication studies (Steward et al., 2012). For example, exact replication of original findings can be difficult or impossible owing to subtle differences in experimental procedures between laboratories (such as different animal suppliers, genetic drift, animal housing and handling, different lots of reagents, variations in surgical or experimental procedures, etc.). In some cases, exact replication might be necessary to validate the original findings and evaluate their statistical significance. However, models that minimize variability might not accurately reflect the heterogeneity of patients in the clinical setting. Instead, replications that introduce some variation in the procedures (e.g. testing in more than one model or strain, as discussed below) will also test the robustness and generalization of the outcomes and might better serve the goal of validating candidate therapeutics (Steward et al., 2012).
Despite the challenges and many disappointing attempts to translate positive results from animal models into effective human therapies, there are also some notable successes. Among these is the recent finding that arbaclofen (STX209) might reduce behavioral dysfunction in individuals with Fragile X syndrome (Berry-Kravis et al., 2012). This randomized, controlled Phase 2 clinical trial was initiated on the basis of evidence that the drug reverses disease pathologies in Fmr1 mutant mice, a model of Fragile X syndrome (Henderson et al., 2012), as well as findings from previous work in a Drosophila model of the disease (Chang et al., 2008). As with RTT, mouse models of Fragile X syndrome have good construct validity, and exhibit several robust and reproducible phenotypes that can serve as a foundation for preclinical testing. Similarly, the therapeutic efficacy of several drugs now used for the treatment of epilepsy, including lacosamide and retigabine, was first established in animal studies organized under the auspices of the Anticonvulsant Screening Program at NINDS (Choi et al., 1996; Rostock et al., 1996; Stöhr et al., 2007). This program incorporates a battery of standardized, rigorously implemented in vivo rodent assays to screen candidate anticonvulsant compounds for epilepsy. Importantly, these examples provide clear evidence that preclinical models have the potential to predict clinical efficacy in humans, provided that the models are robust, studies are well designed and that preclinical outcome measures are relevant to the desired clinical end points.
Summary of knowledge gaps and workshop recommendations
Despite considerable progress in describing the pathophysiological consequences of Mecp2 mutations in mice, crucial gaps remain that must be addressed in order to establish optimal models for preclinical evaluation of potential RTT therapeutics. The workshop participants concluded that addressing the following research goals in RTT would substantially improve the potential for translating promising preclinical findings into clinical success; these include the need to:
obtain a thorough understanding of the effects of genetic background on the phenotypic consequences of different Mecp2 alleles
generate and characterize Mecp2 alleles that model all of the most common human RTT mutations
develop a detailed characterization of female Hets carrying different Mecp2 alleles (cf. Samaco et al., 2012), including how phenotypes appear and evolve across the lifespan; such assessments are crucial given the progressive nature of RTT and the fact that symptoms develop (and change) over the span of years or decades in humans
better characterize behavioral phenotypes in Mecp2 mutants, particularly those that are relevant to RTT clinical features (e.g. measures of cognitive function, social interactions, communication, anxiety); develop behavioral assays that are not confounded by motor defects
characterize in more detail the phenotypes that represent significant challenges for medical management of individuals with RTT, including seizures, and gastrointestinal, cardiorespiratory, sensory and sleep-wake problems
develop more sensitive, reliable and relevant preclinical outcome measures that align with clinical end points and can better predict success in RTT clinical trials; in some cases, this will require a better understanding of whether RTT mouse phenotypes, despite having apparent face validity, share common underlying circuits and neuropathological mechanisms with respective clinical phenotypes
develop in vivo measures of target engagement (e.g. molecular biomarkers of desired ligand-receptor activity or downstream signaling responses), and biomarkers of disease progression and amelioration that, ideally, are non-invasive, to facilitate translation to clinical studies
obtain a better understanding of the therapeutic window in RTT – i.e. the optimal timing and duration of treatment in RTT models.
There was agreement at the workshop that outcome measures for preclinical testing in RTT models should involve phenotypes that are robust and reproducible, and ideally possess good construct and face validity with corresponding RTT disease phenotypes in humans. Although mouse phenotypes have been identified that meet these criteria (Table 2; supplementary material Tables S3–S7), some phenotypic domains, particularly those involving complex behaviors (e.g. social behaviors and anxiety), still lack consistent, reliable readouts in current RTT models. This prompts an important question: will amelioration of less complex behavioral phenotypes, or even cellular and molecular defects, in preclinical trials be sufficient to predict success in clinical trials involving more complex behavioral end points? This is a possibility, as supported by the recent Phase 2 clinical trial mentioned above involving Fragile X syndrome patients (Berry-Kravis et al., 2012). This study reported improvements in social functioning following treatment with arbaclofen, even though the preclinical data supporting this trial consisted largely of positive drug effects on synaptic and neuronal phenotypes in Fmr1 mutant mice (Henderson et al., 2012). Hopefully, as the RTT field moves into clinical testing of therapeutic leads, we can validate and refine the predictive potential of the models by comparing outcomes in mice with results obtained from human clinical trials.
To assess the reproducibility and robustness of promising preclinical interventions, the workshop participants recommended independent replication of the original findings and validation in more than one RTT mouse model or strain prior to launching later-stage translational or clinical projects. In addition, if the original studies were performed in male Nulls, it will be vital to replicate the findings in female Hets whenever feasible, because these models have better construct and face validity for RTT, as discussed above. Adopting standardized assays, tests and outcome measures in animal studies has also been debated in the field (Paylor, 2009; van der Staay and Steckler, 2002). At the workshop, there was agreement that standardized testing procedures can be valuable, especially for phenotypic characterizations (to allow head-to-head comparisons of a phenotype across mouse models or strains, for example); however, overly rigid adherence to ‘accepted’ standards could ultimately inhibit the development or adoption of more sensitive and reliable measures. The workshop participants recommended adopting standardized testing procedures and data analysis protocols whenever possible so that results can be meaningfully compared across different interventions and among different laboratories; modifications and/or improvements to standardized protocols should be justified.
The workshop participants recognized that research consortia, perhaps organized around specific themes (e.g. cognitive function, respiratory control, etc.) could be a valuable tool for generating standardized testing protocols, developing or improving preclinical outcome measures and cross-validating preclinical trial results using best practices for study design (described above). Support for such activities could potentially fall within the scope of the current NIH funding initiative PAR-11-038 (http://grants.nih.gov/grants/guide/pa-files/PAR-11-038.html). The participants also felt that consideration should be given to evaluating the potential value and cost effectiveness of centralized core facilities for preclinical trials, and for validating results from other laboratories.
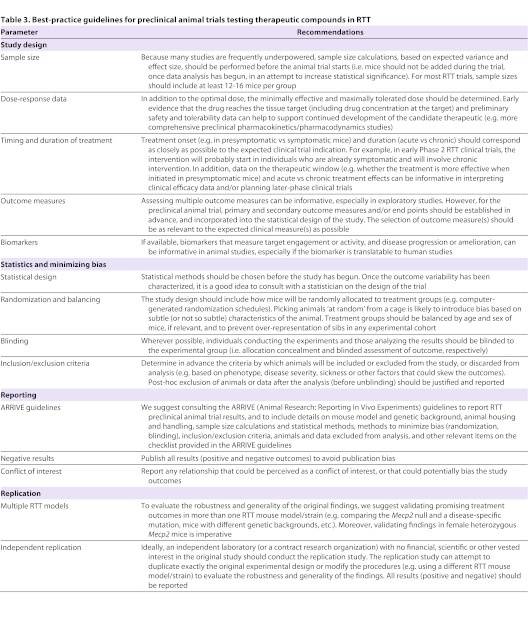
Finally, we need to bring the rigor and quality of study design that is expected of human clinical trials [see CONSORT 2010 statement (Schulz et al., 2010)] to animal trials that test candidate therapeutics. This will require a commitment among RTT researchers to fully disclose all aspects of study design in their publications, including (but not limited to) allocation concealment, blinded assessment of outcome, random allocation of subjects to experimental groups, experimental group sizes, breeding strategies and statistical methods. Table 3 outlines best-practice guidelines for animal trials in RTT that were derived from the workshop recommendations, and adapted from the STAIR (Fisher et al., 2009), ARRIVE (http://www.nc3rs.org.uk/page.asp?id=1357) and NINDS [NINDS Notice NOT-NS-11-023 (http://www.ninds.nih.gov/funding/transparency_in_reporting_guidance.pdf)] guidelines.
Table 3.
Best-practice guidelines for preclinical animal trials testing therapeutic compounds in RTT
Conclusions
The compelling need for effective treatments for RTT, coupled with the availability of good mouse models, is fuelling interest in translational studies aimed at identifying potential new therapeutics. However, given the substantial financial costs and high expectations of clinical trials, it is incumbent upon the RTT research community to rigorously validate models, outcome measures and study designs (see Table 3) that will generate robust and reproducible preclinical findings with clear relevance to the human disease. The NIH, as well as private Rett syndrome funding organizations (IRSF and RSRT), recognize the added demands that rigorous high-quality animal trials place on investigators, especially in requesting independent replication of promising therapeutic leads before moving forward. Current NIH research funding initiatives target these priorities for both animal (http://grants.nih.gov/grants/guide/pa-files/PAR-11-038.html) and clinical (http://grants.nih.gov/grants/guide/pa-files/PAR-11-045.html) research in RTT.
It is also imperative that the complexity of RTT pathophysiology is taken fully into account in the design of preclinical studies. This includes the recognition that loss of MeCP2 function can have direct effects on neuronal function (e.g. synaptic strength) and development (e.g. neuronal cell growth), as well as indirect and cumulative effects on long-term maturation, leading to distinct phenotypic consequences and possibly requiring different treatment strategies. Similarly, it will be important to determine in RTT individuals which specific features might be reversible with pharmacological treatment alone (and at what developmental age) and which might require more complex interventions (e.g. pharmacotherapy combined with behavioral, cognitive and/or speech therapies). Understanding this complexity will be essential in selecting therapeutic end points and to understanding how best to reach them.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge contributions from Monica Coenraads (RSRT), Janice Ascano (IRSF), Stephen Bajardi (IRSF), John McCall (PharMac LLC), Melisa Parisi (NICHD) and MaryLou Oster-Granite (NICHD) in organizing the workshop. We thank all workshop speakers and participants (see supplementary material Table S2) for productive discussions, many of which are represented in this paper. We also thank Shai Silberberg (NINDS) and Naomi Kleitman (NINDS) for helpful comments on the manuscript, Nino Ramirez for input on respiratory phenotypes in RTT, and Elsa Perez for compiling the bibliography. Special thanks go to the editorial staff at Disease Models & Mechanisms for attending the workshop and providing invaluable support for this article. This article represents the views of the authors and not the NIH.
Footnotes
COMPETING INTERESTS
The authors declare that they do not have any financial or competing interests.
FUNDING
Support for the September 2011 workshop on which this paper reports (‘Setting Priorities for Therapy Development in Rett Syndrome’) was provided by the National Institute of Neurological Disorders and Stroke (NINDS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the International Rett Syndrome Research Foundation (IRSF) and the Rett Syndrome Research Trust (RSRT). Grant support includes NINDS (NS-057398 to D.M.K. and NS-065027 to L.P.-M.), Rett Syndrome Research Trust (to M.J.J.), the International Rett Syndrome Foundation (ANGEL award 2608 to M.J.J. and ANGEL award 2583 to D.M.K.) and Canadian Institutes of Health (MOP-106481 to J.H.E.).
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.011007/-/DC1
REFERENCES
- Abdala A. P., Dutschmann M., Bissonnette J. M., Paton J. F. (2010). Correction of respiratory disorders in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 107, 18208–18213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Saavedra M., Sáez M. A., Kang D., Zoghbi H. Y., Young J. I. (2007). Cell-specific expression of wild-type MeCP2 in mouse models of Rett syndrome yields insight about pathogenesis. Hum. Mol. Genet. 16, 2315–2325 [DOI] [PubMed] [Google Scholar]
- Amir R. E., Van den Veyver I. B., Wan M., Tran C. Q., Francke U., Zoghbi H. Y. (1999). Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 23, 185–188 [DOI] [PubMed] [Google Scholar]
- Archer H. L., Evans J., Edwards S., Colley J., Newbury-Ecob R., O’Callaghan F., Huyton M., O’Regan M., Tolmie J., Sampson J., et al. (2006). CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J. Med. Genet. 43, 729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariani F., Hayek G., Rondinella D., Artuso R., Mencarelli M. A., Spanhol-Rosseto A., Pollazzon M., Buoni S., Spiga O., Ricciardi S., et al. (2008). FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 83, 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D., Dunn J. K., Antalffy B., Trivedi R. (1995). Selective dendritic alterations in the cortex of Rett syndrome. J. Neuropathol. Exp. Neurol. 54, 195–201 [DOI] [PubMed] [Google Scholar]
- Armstrong D. D. (2002). Neuropathology of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 8, 72–76 [DOI] [PubMed] [Google Scholar]
- Armstrong D. D., Dunn K., Antalffy B. (1998). Decreased dendritic branching in frontal, motor and limbic cortex in Rett syndrome compared with trisomy 21. J. Neuropathol. Exp. Neurol. 57, 1013–1017 [DOI] [PubMed] [Google Scholar]
- Asaka Y., Jugloff D. G., Zhang L., Eubanks J. H., Fitzsimonds R. M. (2006). Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol. Dis. 21, 217–227 [DOI] [PubMed] [Google Scholar]
- Baptista P. M., Mercadante M. T., Macedo E. C., Schwartzman J. S. (2006). Cognitive performance in Rett syndrome girls: a pilot study using eyetracking technology. J. Intellect. Disabil. Res. 50, 662–666 [DOI] [PubMed] [Google Scholar]
- Bauman M. L., Kemper T. L., Arin D. M. (1995a). Microscopic observations of the brain in Rett syndrome. Neuropediatrics 26, 105–108 [DOI] [PubMed] [Google Scholar]
- Bauman M. L., Kemper T. L., Arin D. M. (1995b). Pervasive neuroanatomic abnormalities of the brain in three cases of Rett’s syndrome. Neurology 45, 1581–1586 [DOI] [PubMed] [Google Scholar]
- Bebarta V., Luyten D., Heard K. (2003). Emergency medicine animal research: does use of randomization and blinding affect the results? Acad. Emerg. Med. 10, 684–687 [DOI] [PubMed] [Google Scholar]
- Begley C. G., Ellis L. M. (2012). Drug development: Raise standards for preclinical cancer research. Nature 483, 531–533 [DOI] [PubMed] [Google Scholar]
- Belichenko P. V., Oldfors A., Hagberg B., Dahlström A. (1994). Rett syndrome: 3-D confocal microscopy of cortical pyramidal dendrites and afferents. Neuroreport 5, 1509–1513 [PubMed] [Google Scholar]
- Berry-Kravis E. M., Hessl D., Rathmell B., Zarevics P., Cherubini M., Walton-Bowen K., Mu Y., Nguyen D. V., Gonzalez-Heydrich J., Wang P. P., et al. (2012). Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci. Transl. Med. 4, 152ra127. [DOI] [PubMed] [Google Scholar]
- Bissonnette J. M., Knopp S. J. (2006). Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr. Res. 59, 513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio E. M., Lonetti G., Pizzorusso T., Giustetto M. (2010). Synaptic determinants of rett syndrome. Front. Synaptic Neurosci. 2, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendel C., Belakhov V., Werner H., Wegener E., Gärtner J., Nudelman I., Baasov T., Huppke P. (2011). Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J. Mol. Med. (Berl.) 89, 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D., Balci F., Ludvig E. A. (2012). Comparative psychology and the grand challenge of drug discovery in psychiatry and neurodegeneration. Behav. Processes 89, 187–195 [DOI] [PubMed] [Google Scholar]
- Calfa G., Hablitz J. J., Pozzo-Miller L. (2011a). Network hyperexcitability in hippocampal slices from Mecp2 mutant mice revealed by voltage-sensitive dye imaging. J. Neurophysiol. 105, 1768–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfa G., Percy A. K., Pozzo-Miller L. (2011b). Experimental models of Rett syndrome based on Mecp2 dysfunction. Exp. Biol. Med. (Maywood) 236, 3–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Bray S. M., Li Z., Zarnescu D. C., He C., Jin P., Warren S. T. (2008). Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat. Chem. Biol. 4, 256–263 [DOI] [PubMed] [Google Scholar]
- Chang Q., Khare G., Dani V., Nelson S., Jaenisch R. (2006). The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 2, 341–348 [DOI] [PubMed] [Google Scholar]
- Chapleau C. A., Calfa G. D., Lane M. C., Albertson A. J., Larimore J. L., Kudo S., Armstrong D. L., Percy A. K., Pozzo-Miller L. (2009). Dendritic spine pathologies in hippocampal pyramidal neurons from Rett syndrome brain and after expression of Rett-associated MECP2 mutations. Neurobiol. Dis. 35, 219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. Z., Akbarian S., Tudor M., Jaenisch R. (2001). Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat. Genet. 27, 327–331 [DOI] [PubMed] [Google Scholar]
- Cheval H., Guy J., Merusi C., De Sousa D., Selfridge J., Bird A. (2012). Postnatal inactivation reveals enhanced requirement for MeCP2 at distinct age windows. Hum. Mol. Genet. 21, 3806–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D., Stables J. P., Kohn H. (1996). Synthesis and anticonvulsant activities of N-Benzyl-2-acetamidopropionamide derivatives. J. Med. Chem. 39, 1907–1916 [DOI] [PubMed] [Google Scholar]
- Coenraads M. (2007). Face to face with Rett syndrome. Epigenetics 2, 2–4 [DOI] [PubMed] [Google Scholar]
- Cohen S., Gabel H. W., Hemberg M., Hutchinson A. N., Sadacca L. A., Ebert D. H., Harmin D. A., Greenberg R. S., Verdine V. K., Zhou Z., et al. (2011). Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron 72, 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. L., Levenson J. M., Vilaythong A. P., Richman R., Armstrong D. L., Noebels J. L., David Sweatt J., Zoghbi H. Y. (2004). Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum. Mol. Genet. 13, 2679–2689 [DOI] [PubMed] [Google Scholar]
- D’Cruz J. A., Wu C., Zahid T., El-Hayek Y., Zhang L., Eubanks J. H. (2010). Alterations of cortical and hippocampal EEG activity in MeCP2-deficient mice. Neurobiol. Dis. 38, 8–16 [DOI] [PubMed] [Google Scholar]
- Dani V. S., Nelson S. B. (2009). Intact long-term potentiation but reduced connectivity between neocortical layer 5 pyramidal neurons in a mouse model of Rett syndrome. J. Neurosci. 29, 11263–11270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani V. S., Chang Q., Maffei A., Turrigiano G. G., Jaenisch R., Nelson S. B. (2005). Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Pro c. Natl. Acad. Sci. USA 102, 12560–12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis B., Ricceri L., Laviola G. (2010). Early postnatal behavioral changes in the Mecp2-308 truncation mouse model of Rett syndrome. Genes Brain Behav. 9, 213–223 [DOI] [PubMed] [Google Scholar]
- De Filippis B., Fabbri A., Simone D., Canese R., Ricceri L., Malchiodi-Albedi F., Laviola G., Fiorentini C. (2012). Modulation of RhoGTPases improves the behavioral phenotype and reverses astrocytic deficits in a mouse model of Rett syndrome. Neuropsychopharm. 37,1152–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng V., Matagne V., Banine F., Frerking M., Ohliger P., Budden S., Pevsner J., Dissen G. A., Sherman L. S., Ojeda S. R. (2007). FXYD1 is an MeCP2 target gene overexpressed in the brains of Rett syndrome patients and Mecp2-null mice. Hum. Mol. Genet. 16, 640–650 [DOI] [PubMed] [Google Scholar]
- Deogracias R., Yazdani M., Dekkers M. P., Guy J., Ionescu M. C., Vogt K. E., Barde Y. A. (2012). Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl. Acad Sci. USA 109, 14230–14235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs J., Géranton S. M., Bebbington A., Jacoby P., Bahi-Buisson N., Ravine D., Leonard H. (2010). Linking MECP2 and pain sensitivity: the example of Rett syndrome. Am. J. Med. Genet. 152A, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elian M., Rudolf N. D. (1991). EEG and respiration in Rett syndrome. Acta Neurol. Scand. 83, 123–128 [DOI] [PubMed] [Google Scholar]
- Fisher M., Feuerstein G., Howells D. W., Hurn P. D., Kent T. A., Savitz S. I., Lo E. H. (2009). Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald P. M., Jankovic J., Glaze D. G., Schultz R., Percy A. K. (1990a). Extrapyramidal involvement in Rett’s syndrome. Neurology 40, 293–295 [DOI] [PubMed] [Google Scholar]
- FitzGerald P. M., Jankovic J., Percy A. K. (1990b). Rett syndrome and associated movement disorders. Mov. Disord. 5, 195–202 [DOI] [PubMed] [Google Scholar]
- Gadalla K. K., Bailey M. E., Cobb S. R. (2011). MeCP2 and Rett syndrome: reversibility and potential avenues for therapy. Biochem. J. 439, 1–14 [DOI] [PubMed] [Google Scholar]
- Gantz S. C., Ford C. P., Neve K. A., Williams J. T. (2011). Loss of Mecp2 in substantia nigra dopamine neurons compromises the nigrostriatal pathway. J. Neurosci. 31, 12629–12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti E., Luikenhuis S., Beard C., Jaenisch R. (2007). Partial rescue of MeCP2 deficiency by postnatal activation of MeCP2. Proc. Natl. Acad. Sci. USA 104, 1931–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze D. G. (2005). Neurophysiology of Rett syndrome. J. Child Neurol. 20, 740–746 [DOI] [PubMed] [Google Scholar]
- Glaze D. G., Schultz R. J., Frost J. D. (1998). Rett syndrome: characterization of seizures versus non-seizures. Electroencephalogr. Clin. Neurophysiol. 106, 79–83 [DOI] [PubMed] [Google Scholar]
- Glaze D. G., Percy A. K., Skinner S., Motil K. J., Neul J. L., Barrish J. O., Lane J. B., Geerts S. P., Annese F., Graham J., et al. (2010). Epilepsy and the natural history of Rett syndrome. Neurology 74, 909–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin D., Allen M., Zhang L., Amorim M., Wang I. T., Reyes A. R., Mercado-Berton A., Ong C., Cohen S., Hu L., et al. (2012). Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci. 15, 274–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guideri F., Acampa M., Calamandrei G., Aloe L., Zappella M., Hayek Y. (2004). Nerve growth factor plasma levels and ventricular repolarization in Rett syndrome. Pediatr. Cardiol. 25, 394–396 [DOI] [PubMed] [Google Scholar]
- Guy J., Hendrich B., Holmes M., Martin J. E., Bird A. (2001). A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat. Genet. 27, 322–326 [DOI] [PubMed] [Google Scholar]
- Guy J., Gan J., Selfridge J., Cobb S., Bird A. (2007). Reversal of neurological defects in a mouse model of Rett syndrome. Science 315, 1143–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B. (2002). Clinical manifestations and stages of Rett syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 8, 61–65 [DOI] [PubMed] [Google Scholar]
- Hagberg B., Aicardi J., Dias K., Ramos O. (1983). A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann. Neurol. 14, 471–479 [DOI] [PubMed] [Google Scholar]
- Henderson C., Wijetunge L., Kinoshita M. N., Shumway M., Hammond R. S., Postma F. R., Brynczka C., Rush R., Thomas A., Paylor R., et al. (2012). Reversal of disease-related pathologies in the fragile X mouse model by selective activation of GABAB receptors with arbaclofen. Sci. Transl. Med. 4, 152ra128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K., Armstrong D., Zoghbi H. Y., Percy A. K. (1988). Neuropathology of Rett syndrome. Acta Neuropathol. 76, 142–158 [DOI] [PubMed] [Google Scholar]
- Jentarra G. M., Olfers S. L., Rice S. G., Srivastava N., Homanics G. E., Blue M., Naidu S., Narayanan V. (2010). Abnormalities of cell packing density and dendritic complexity in the MeCP2 A140V mouse model of Rett syndrome/X-linked mental retardation. BMC Neurosci. 11, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. A., Lam M., Punzo A. M., Li H., Lin B. R., Ye K., Mitchell G. S., Chang Q. (2012). 7,8-dihydroxyflavone exhibits therapeutic efficacy in a mouse model of Rett syndrome. J. Appl. Physiol. 112, 704–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugloff D. G., Vandamme K., Logan R., Visanji N. P., Brotchie J. M., Eubanks J. H. (2008). Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum. Mol. Genet. 17, 1386–1396 [DOI] [PubMed] [Google Scholar]
- Julu P. O., Witt Engerström I. (2005). Assessment of the maturity-related brainstem functions reveals the heterogeneous phenotypes and facilitates clinical management of Rett syndrome. Brain Dev. 27 Suppl. 1, S43–S53 [DOI] [PubMed] [Google Scholar]
- Julu P. O., Kerr A. M., Apartopoulos F., Al-Rawas S., Engerström I. W., Engerström L., Jamal G. A., Hansen S. (2001). Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch. Dis. Child. 85, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann W. E., Naidu S., Budden S. (1995). Abnormal expression of microtubule-associated protein 2 (MAP-2) in neocortex in Rett syndrome. Neuropediatrics 26, 109–113 [DOI] [PubMed] [Google Scholar]
- Kaufmann W. E., MacDonald S. M., Altamura C. R. (2000). Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett syndrome. Cereb. Cortex 10, 992–1004 [DOI] [PubMed] [Google Scholar]
- Kaufmann W. E., Tierney E., Rohde C. A., Suarez-Pedraza M. C., Clarke M. A., Salorio C. F., Bibat G., Bukelis I., Naram D., Lanham D. C., et al. (2012). Social impairments in Rett syndrome: characteristics and relationship with clinical severity. J. Intellect. Disabil. Res. 56, 233–247 [DOI] [PubMed] [Google Scholar]
- Kerr A. M., Armstrong D. D., Prescott R. J., Doyle D., Kearney D. L. (1997). Rett syndrome: analysis of deaths in the British survey. Eur. Child Adolesc. Psychiatry 6 Suppl. 1, 71–74 [PubMed] [Google Scholar]
- Kerr B., Alvarez-Saavedra M., Sáez M. A., Saona A., Young J. I. (2008). Defective body-weight regulation, motor control and abnormal social interactions in Mecp2 hypomorphic mice. Hum. Mol. Genet. 17, 1707–1717 [DOI] [PubMed] [Google Scholar]
- Kerr B., Soto C. J., Saez M., Abrams A., Walz K., Young J. I. (2012). Transgenic complementation of MeCP2 deficiency: phenotypic rescue of Mecp2-null mice by isoform-specific transgenes. Eur. J. Hum. Genet. 20, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline D. D., Ogier M., Kunze D. L., Katz D. M. (2010). Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J. Neurosci. 30, 5303–5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Gray L. J., Pelka G. J., Christodoulou J., Tam P. P., Hannan A. J. (2008). Environmental enrichment ameliorates a motor coordination deficit in a mouse model of Rett syndrome – Mecp2 gene dosage effects and BDNF expression. Eur. J. Neurosci. 27, 3342–3350 [DOI] [PubMed] [Google Scholar]
- Kortüm F., Das S., Flindt M., Morris-Rosendahl D. J., Stefanova I., Goldstein A., Horn D., Klopocki E., Kluger G., Martin P., et al. (2011). The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis. J. Med. Genet. 48, 396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron M., Howell C. J., Adams I. T., Ransbottom M., Ogier M., Katz D. M. (2012). Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J. Neurosci. 32, 13860–13872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo S., Nomura Y., Segawa M., Fujita N., Nakao M., Dragich J., Schanen C., Tamura M. (2001). Functional analyses of MeCP2 mutations associated with Rett syndrome using transient expression systems. Brain Dev. 23 Suppl. 1, S165–S173 [DOI] [PubMed] [Google Scholar]
- Landis S. C., Amara S. G., Asadullah K., Austin C. P., Blumenstein R., Bradley E. W., Crystal R. G., Darnell R. B., Ferrante R. J., H. Fillit H., et al. (2012). A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490, 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson-Yuen A., Liu D., Han L., Jiang Z. I., Tsai G. E., Basu A. C., Picker J., Feng J., Coyle J. T. (2007). Ube3a mRNA and protein expression are not decreased in Mecp2R168X mutant mice. Brain Res. 1180, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Calfa G., Larimore J., Pozzo-Miller L. (2012). Activity-dependent BDNF release and TRPC signaling is impaired in hippocampal neurons of Mecp2 mutant mice. Proc. Natl. Acad. Sci. USA 109, 17087–17092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Gandal M. J., Ehrlichman R. S., Siegel S. J., Carlson G. C. (2012). MeCP2+/− mouse model of RTT reproduces auditory phenotypes associated with Rett syndrome and replicate select EEG endophenotypes of autism spectrum disorder. Neurobiol. Dis. 46, 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy D. T., Garg S. K., Monaghan C. E., Raber J., Foust K. D., Kaspar B. K., Hirrlinger P. G., Kirchhoff F., Bissonnette J. M., Ballas N., et al. (2011). A role for glia in the progression of Rett’s syndrome. Nature 475, 497–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonetti G., Angelucci A., Morando L., Boggio E. M., Giustetto M., Pizzorusso T. (2010). Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol. Psychiatry 67, 657–665 [DOI] [PubMed] [Google Scholar]
- Luikenhuis S., Giacometti E., Beard C. F., Jaenisch R. (2004). Expression of MeCP2 in postmitotic neurons rescues Rett syndrome in mice. Proc. Natl. Acad. Sci. USA 101, 6033–6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod M. R., O’Collins T., Horky L. L., Howells D. W., Donnan G. A. (2005). Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J. Cereb. Blood Flow M etab. 25, 713–721 [DOI] [PubMed] [Google Scholar]
- Marcus C. L., Carroll J. L., McColley S. A., Loughlin G. M., Curtis S., Pyzik P., Naidu S. (1994). Polysomnographic characteristics of patients with Rett syndrome. J. Pediatr. 125, 218–224 [DOI] [PubMed] [Google Scholar]
- McCauley M. D., Wang T., Mike E., Herrera J., Beavers D. L., Huang T. W., Ward C. S., Skinner S., Percy A. K., Glaze D. G., et al. (2011). Pathogenesis of lethal cardiac arrhythmias in Mecp2 mutant mice: implication for therapy in Rett syndrome. Sci. Transl. Med. 3, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane H. G., Kusek G. K., Yang M., Phoenix J. L., Bolivar V. J., Crawley J. N. (2008). Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 7, 152–163 [DOI] [PubMed] [Google Scholar]
- McGill B. E., Bundle S. F., Yaylaoglu M. B., Carson J. P., Thaller C., Zoghbi H. Y. (2006). Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA 103, 18267–18272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw C. M., Samaco R. C., Zoghbi H. Y. (2011). Adult neural function requires MeCP2. Science 333, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrihan L., Tantalaki E., Aramuni G., Sargsyan V., Dudanova I., Missler M., Zhang W. (2008). Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J. Neurophysiol. 99, 112–121 [DOI] [PubMed] [Google Scholar]
- Moretti P., Bouwknecht J. A., Teague R., Paylor R., Zoghbi H. Y. (2005). Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum. Mol. Genet. 14, 205–220 [DOI] [PubMed] [Google Scholar]
- Moretti P., Levenson J. M., Battaglia F., Atkinson R., Teague R., Antalffy B., Armstrong D., Arancio O., Sweatt J. D., Zoghbi H. Y. (2006). Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J. Neurosci. 26, 319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motil K. J., Schultz R. J., Browning K., Trautwein L., Glaze D. G. (1999). Oropharyngeal dysfunction and gastroesophageal dysmotility are present in girls and women with Rett syndrome. J. Pediatr. Gastroenterol. Nutr. 29, 31–37 [DOI] [PubMed] [Google Scholar]
- Motil K. J., Caeg E., Barrish J. O., Geerts S., Lane J. B., Percy A. K., Annese F., McNair L., Skinner S. A., Lee H. S., et al. (2012). Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with rett syndrome. J. Pediatr. Gastroenterol. Nutr. 55, 292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount R. H., Charman T., Hastings R. P., Reilly S., Cass H. (2002a). The Rett Syndrome Behaviour Questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. J. Child Psychol. Psychiatry 43, 1099–1110 [DOI] [PubMed] [Google Scholar]
- Mount R. H., Hastings R. P., Reilly S., Cass H., Charman T. (2002b). Behaviour problems in adult women with Rett syndrome. J. Intellect. Disabil. Res. 46, 619–624 [DOI] [PubMed] [Google Scholar]
- Mount R. H., Charman T., Hastings R. P., Reilly S., Cass H. (2003). Features of autism in Rett syndrome and severe mental retardation. J. Autism Dev. Disord. 33, 435–442 [DOI] [PubMed] [Google Scholar]
- Nag N., Berger-Sweeney J. E. (2007). Postnatal dietary choline supplementation alters behavior in a mouse model of Rett syndrome. Neurobiol. Dis. 26, 473–480 [DOI] [PubMed] [Google Scholar]
- Nelson E. D., Kavalali E. T., Monteggia L. M. (2006). MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr. Biol. 16, 710–716 [DOI] [PubMed] [Google Scholar]
- Neul J. L., Kaufmann W. E., Glaze D. G., Christodoulou J., Clarke A. J., Bahi-Buisson N., Leonard H., Bailey M. E., Schanen N. C., Zappella M., et al. (2010). Rett syndrome: revised diagnostic criteria and nomenclature. Ann. Neurol. 68, 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M. V., Du F., Felice C. A., Shan X., Nigam A., Mandel G., Robinson J. K., Ballas N. (2012). MeCP2 is critical for maintaining mature neuronal networks and global brain anatomy during late stages of postnatal brain development and in the mature adult brain. J. Neurosci. 32, 10021–10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier M., Wang H., Hong E., Wang Q., Greenberg M. E., Katz D. M. (2007). Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J. Neurosci. 27, 10912–10917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor R. (2009). Questioning standardization in science. Nat. Methods 6, 253–254 [DOI] [PubMed] [Google Scholar]
- Pearson B. L., Defensor E. B., Pobbe R. L., Yamamoto L. H., Bolivar V. J., Blanchard D. C., Blanchard R. J. (2012). Mecp2 truncation in male mice promotes affiliative social behavior. Behav. Genet. 42, 299–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelka G. J., Watson C. M., Radziewic T., Hayward M., Lahooti H., Christodoulou J., Tam P. P. (2006). Mecp2 deficiency is associated with learning and cognitive deficits and altered gene activity in the hippocampal region of mice. Brain 129, 887–898 [DOI] [PubMed] [Google Scholar]
- Percy A. K., Lee H. S., Neul J. L., Lane J. B., Skinner S. A., Geerts S. P., Annese F., Graham J., McNair L., Motil K. J., et al. (2010). Profiling scoliosis in Rett syndrome. Pediatr. Res. 67, 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker J. D., Yang R., Ricceri L., Berger-Sweeney J. (2006). An altered neonatal behavioral phenotype in Mecp2 mutant mice. Neuroreport 17, 541–544 [DOI] [PubMed] [Google Scholar]
- Prinz F., Schlange T., Asadullah K. (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nat. Rev. Drug Discov. 10, 712. [DOI] [PubMed] [Google Scholar]
- Ransohoff D. F., Gourlay M. L. (2010). Sources of bias in specimens for research about molecular markers for cancer. J. Clin. Oncol. 28, 698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A. L., Faruque F., Naidu S., Abrams M., Beaty T., Bryan R. N., Moser H. (1993). Neuroanatomy of Rett syndrome: a volumetric imaging study. Ann. Neurol. 34, 227–234 [DOI] [PubMed] [Google Scholar]
- Renieri A., Mari F., Mencarelli M. A., Scala E., Ariani F., Longo I., Meloni I., Cevenini G., Pini G., Hayek G., et al. (2009). Diagnostic criteria for the Zappella variant of Rett syndrome (the preserved speech variant). Brain Dev. 31, 208–216 [DOI] [PubMed] [Google Scholar]
- Robinson L., Guy J., McKay L., Brockett E., Spike R. C., Selfridge J., De Sousa D., Merusi C., Riedel G., Bird A., et al. (2012). Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain 135, 2699–2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostock A., Tober C., Rundfeldt C., Bartsch R., Engel J., Polymeropoulos E. E., Kutscher B., Löscher W., Hönack D., White H. S., et al. (1996). D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 23, 211–223 [DOI] [PubMed] [Google Scholar]
- Roux J. C., Dura E., Moncla A., Mancini J., Villard L. (2007). Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur. J. Neurosci. 25, 1915–1922 [DOI] [PubMed] [Google Scholar]
- Roux J. C., Dura E., Villard L. (2008). Tyrosine hydroxylase deficit in the chemoafferent and the sympathoadrenergic pathways of the Mecp2 deficient mouse. Neurosci. L ett. 447, 82–86 [DOI] [PubMed] [Google Scholar]
- Samaco R. C., Fryer J. D., Ren J., Fyffe S., Chao H. T., Sun Y., Greer J. J., Zoghbi H. Y., Neul J. L. (2008). A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum. Mol. Genet. 17, 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco R. C., McGraw C. M., Ward C. S., Sun Y., Neul J. L., Zoghbi H. Y. (2012). Female Mecp2+/− mice display robust behavioral deficits on two different genetic backgrounds providing a framework for pre-clinical studies. Hum. Mol. Genet. [Epub ahead of print] doi: 10.1093/hmg/dds406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M., Silva-Fernandes A., Oliveira P., Sousa N., Maciel P. (2007). Evidence for abnormal early development in a mouse model of Rett syndrome. Genes Brain Behav. 6, 277–286 [DOI] [PubMed] [Google Scholar]
- Schaevitz L. R., Moriuchi J. M., Nag N., Mellot T. J., Berger-Sweeney J. (2010). Cognitive and social functions and growth factors in a mouse model of Rett syndrome. Physiol. Behav. 100, 255–263 [DOI] [PubMed] [Google Scholar]
- Schmid D. A., Yang T., Ogier M., Adams I., Mirakhur Y., Wang Q., Massa S. M., Longo F. M., Katz D. M. (2012). A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J. Neurosci. 32, 1803–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. J., Glaze D. G., Motil K. J., Armstrong D. D., del Junco D. J., Hubbard C. R., Percy A. K. (1993). The pattern of growth failure in Rett syndrome. Am. J. Dis. Child. 147, 633–637 [DOI] [PubMed] [Google Scholar]
- Schulz K. F., Altman D. G., Moher D. (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 7, e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S., Kranz J. E., Cole J., Lincecum J. M., Thompson K., Kelly N., Bostrom A., Theodoss J., Al-Nakhala B. M., Vieira F. G., et al. (2008). Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 9, 4–15 [DOI] [PubMed] [Google Scholar]
- Sekul E. A., Moak J. P., Schultz R. J., Glaze D. G., Dunn J. K., Percy A. K. (1994). Electrocardiographic findings in Rett syndrome: an explanation for sudden death? J. Pediatr. 125, 80–82 [DOI] [PubMed] [Google Scholar]
- Sena E., van der Worp H. B., Howells D., Macleod M. (2007). How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 30, 433–439 [DOI] [PubMed] [Google Scholar]
- Shahbazian M., Young J., Yuva-Paylor L., Spencer C., Antalffy B., Noebels J., Armstrong D., Paylor R., Zoghbi H. (2002). Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron 35, 243–254 [DOI] [PubMed] [Google Scholar]
- Shepherd G. M., Katz D. M. (2011). Synaptic microcircuit dysfunction in genetic models of neurodevelopmental disorders: focus on Mecp2 and Met. Curr. Opin. Neurobiol. 21, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shineman D. W., Basi G. S., Bizon J. L., Colton C. A., Greenberg B. D., Hollister B. A., Lincecum J., Leblanc G. G., Lee L. B., Luo F., et al. (2011). Accelerating drug discovery for Alzheimer’s disease: best practices for preclinical animal studies. Alzheimers Res. Ther. 3, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns N. A., Schaevitz L. R., Bowling H., Nag N., Berger U. V., Berger-Sweeney J. (2007). Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience 146, 907–921 [DOI] [PubMed] [Google Scholar]
- Stettner G. M., Huppke P., Brendel C., Richter D. W., Gärtner J., Dutschmann M. (2007). Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J. Physiol. 579, 863–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Popovich P. G., Dietrich W. D., Kleitman N. (2012). Replication and reproducibility in spinal cord injury research. Exp. Neurol. 233, 597–605 [DOI] [PubMed] [Google Scholar]
- Stöhr T., Kupferberg H. J., Stables J. P., Choi D., Harris R. H., Kohn H., Walton N., White H. S. (2007). Lacosamide, a novel anti-convulsant drug, shows efficacy with a wide safety margin in rodent models for epilepsy. Epilepsy Res. 74, 147–154 [DOI] [PubMed] [Google Scholar]
- Taneja P., Ogier M., Brooks-Harris G., Schmid D. A., Katz D. M., Nelson S. B. (2009). Pathophysiology of locus ceruleus neurons in a mouse model of Rett syndrome. J. Neurosci. 29, 12187–12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquinio D., Motil K., Hou W., Lee H., Glaze D. G., Skinner S. A., Neul J. L., Annese F., McNair L., Barrish J. O., et al. (2012). Growth failure and outcome in Rett syndrome: Specific growth references. Neurology 79, 1653–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropea D., Giacometti E., Wilson N. R., Beard C., McCurry C., Fu D. D., Flannery R., Jaenisch R., Sur M. (2009). Partial reversal of Rett Syndrome-like symptoms in MeCP2 mutant mice. Proc. Natl. Acad. Sci. USA 106, 2029–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Staay F. J., Steckler T. (2002). The fallacy of behavioral phenotyping without standardisation. Genes Brain Behav. 1, 9–13 [DOI] [PubMed] [Google Scholar]
- Voituron N., Zanella S., Menuet C., Dutschmann M., Hilaire G. (2009). Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir. Physiol. Neurobiol. 168, 109–118 [DOI] [PubMed] [Google Scholar]
- Wang H., Chan S. A., Ogier M., Hellard D., Wang Q., Smith C., Katz D. M. (2006). Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J. Neurosci. 26, 10911–10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. S., Arvide E. M., Huang T. W., Yoo J., Noebels J. L., Neul J. L. (2011). MeCP2 is critical within HoxB1-derived tissues of mice for normal lifespan. J. Neurosci. 31, 10359–10370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Lieske S. P., Boothby C. M., Kenny A. S., Bennett H. L., Silvestri J. M., Ramirez J. M. (2006). Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr. Res. 60, 443–449 [DOI] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Lieske S. P., Boothby C. M., Kenny A. S., Bennett H. L., Ramirez J. M. (2008). Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr. Pulmonol. 43, 1045–1060 [DOI] [PubMed] [Google Scholar]
- Wither R. G., Colic S., Wu C., Bardakjian B. L., Zhang L., Eubanks J. H. (2012). Daily rhythmic behaviors and thermoregulatory patterns are disrupted in adult female MeCP2-deficient mice. PLoS ONE 7, e35396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella S., Mebarek S., Lajard A. M., Picard N., Dutschmann M., Hilaire G. (2008). Oral treatment with desipramine improves breathing and life span in Rett syndrome mouse model. Respir. Physiol. Neurobiol. 160, 116–121 [DOI] [PubMed] [Google Scholar]
- Zhang L., He J., Jugloff D. G., Eubanks J. H. (2008). The MeCP2-null mouse hippocampus displays altered basal inhibitory rhythms and is prone to hyperexcitability. Hippocampus 18, 294–309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.