Abstract
The CACNA2D2 gene, a new subunit of the Ca2+-channel complex, was identified in the homozygous deletion region of chromosome 3p21.3 in human lung and breast cancers. Expression deficiency of the CACNA2D2 in cancer cells suggests a possible link of it to Ca2+ signaling in the pathogenesis of lung cancer and other cancers. We investigated the effects of overexpression of CACNA2D2 on intracellular Ca2+ contents, mitochondria homeostasis, cell proliferation, and apoptosis by adenoviral vector-mediated wild-type CACNA2D2 gene transfer in 3p21.3-deficient nonsmall cell lung cancer cell lines. Exogenous expression of CACNA2D2 significantly inhibited tumor cell growth compared with the controls. Overexpression of CACNA2D2 induced apoptosis in H1299 (12.5%), H358 (13.7%), H460 (22.3%), and A549 (50.1%) cell lines. Levels of intracellular free Ca2+ were elevated in AdCACNA2D2-transduced cells compared with the controls. Mitochondria membrane depolarization was observed prior to apoptosis in Ad-CACNA2D2 and Adp53-transduced H460 and A549 cells. Release of cyt c into the cytosol, caspase 3 activation, and PARP cleavage were also detected in these cells. Together, these results suggest that one of the pathways in CACNA2D2-induced apoptosis is mediated through disruption of mitochondria membrane integrity, the release of cyt c, and the activation of caspases, a process that is associated with regulation of cytosolic free Ca2+ contents.
Keywords: tumor suppressor genes, apoptosis, calcium channel proteins, human chromosome 3p21.3, lung cancer
Introduction
The novel gene CACNA2D2 has recently been identified in the homozygous deletion region of chromosome 3p21.3 in human lung and breast cancers (Gao et al., 2000, Lerman and Minna, 2000). It is characterized structurally as a new α2δ2 auxiliary subunit of the voltage-activated calcium channel (VACC) protein complex. The CACNA2D2 gene spans an ~140 kb genomic locus in the 3p21.3 region, consists of at least 40 exons, and is expressed as a 5.5–5.7 kb mRNA. The CACNA2D2 protein consists of 1146 amino acids with a predicted molecular mass of 130 kDa (Gao et al., 2000). Three splicing variants of CACNA2D2 mRNA have been detected, which result in two protein isoforms with different N-terminals (Angeloni et al., 2000). The CACNA2D2 protein shows a 56% amino-acid sequence homology to that of the α2δ1 subunit of the VACC complexes and shares a similar secondary and tertiary structure with the CACNA2D1, as suggested by the analysis of hydrophobicity, potential glycosylation sites, and bridge-forming cysteines of the primary sequence (Angeloni et al., 2000). The CACNA2D2 protein is highly expressed in normal lung tissue, but either absent or underexpressed in more than 50% of lung cancers (Gao et al., 2000). Since cancer cells are deficient in CACNA2D2, it has been suggested that CACNA2D2 could be a tumor suppressor gene linking Ca2+ signaling with the pathogenesis of lung cancer and other cancers (Gao et al., 2000).
Growing evidence has demonstrated that Ca2+ signaling regulates and controls diverse cellular processes such as cell fertilization, development, proliferation, learning and memory, contraction and secretion, and cell death (Berridge et al., 1998, 2000). The universality of calcium as an intracellular messenger depends on the enormous range of timing, spatial, and temporal signals it can create in the complicated cellular processes (Berridge et al., 1998, 2000). Alteration of the spatial and temporal balances of intracellular calcium by either environmental stimuli or calcium effectors can result in cell death by both necrosis and apoptosis (Lemasters et al., 1998; Berridge et al., 2000; Zhu et al., 2000).
Early loss of CACNA2D2 expression in the pathogenesis of lung cancer (Angeloni et al., 2000), inactivation of expression of other calcium channel-related proteins such as calcium/calmodulin-dependent death-associated protein kinase (DAPK) (Raveh and Kimchi, 2001) and CACNA1G by promoter hypermethylation in various human cancers (Toyota et al., 1999; Ueki et al., 2000; Zochbauer-Muller et al., 2001), and the growing role of Ca2+ signaling in cell regulation (Berridge et al., 2000), and especially its involvement in the mitochondria-mediated apoptotic pathway (Rutter and Rizzuto, 2000; Zhu et al., 2000), motivated us to further investigate the function of the calcium channel protein CACNA2D2 in the regulation of cell proliferation and cell death and in the pathogenesis of human cancers. This study encompasses the effect of the ectopic expression of CACNA2D2 on mitochondria homeostasis, cell proliferation, apoptosis, and intracellular Ca2+ contents by adenoviral vector-mediated wild-type (wt)-CACNA2D2 gene transfer in various 3p21.3-deficient NSCLC cell lines. We demonstrated that CACNA2D2-induced apoptosis was mediated through a cellular process involved in the regulation of the intracellular Ca2+ contents, the disruption of mitochondria membrane integrity, the release of cyt c, and the activation of downstream caspases.
Results
Exogenous expression of CACNA2D2 inhibits tumor cell growth
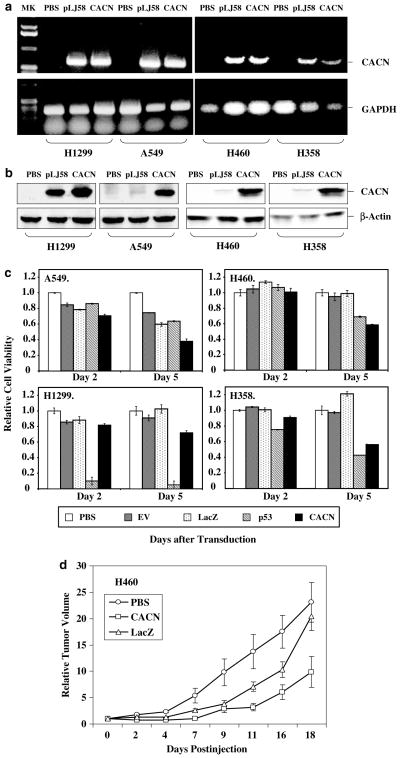
To evaluate whether the CACNA2D2 could function as a tumor suppressor or a cell death mediator by inhibition of tumor cell growth in lung cancer, we performed a series of experiments to study the effect of ectopic expression of the CACNA2D2 gene on cell proliferation in various Ad-CACNA2D2-transduced human NSCLC cell lines NCI-H1299, NCI-H460, NCI-H358, and A549, with varying status of 3p21.3 markers (Figure 1). Cells from each line were transduced in vitro by the Ad-CACNA2D2 vector administered at various MOIs, and cells treated with PBS, the empty vector Ad-EV, Ad-LacZ, or Ad-GFP were used as controls. The transduction efficiency was determined by examining the GFP-expressing cells in the Ad-GFP transduced cell population under a fluorescence microscope. The transduction efficiency of the adenoviral vectors was greater than 80% at the highest MOI applied for each cell line. Expression of CACNA2D2 was verified by RT-PCR analysis (Figure 1a) and Western blot analysis (Figure 1b), respectively, in Ad-CACNA2D2-transduced NSCLC cells. The transfection by plasmid DNA and the transduction by adenoviral vector are less efficient in A549, H460, and H358 cells than those in H1299 cells. Although the transcription of CACNA2D2 could be detected by RT-PCR (Figure 1a), the protein expression could only be detected at a trace amount by Western blot analysis (Figure 1b) in the CACNA2D2-containing plasmid DNA-transfected A459, H460, and H358 cells. A significantly elevated expression of CACNA2D2 proteins could be detected in all cell lines transduced by the Ad-CACN vector (Figure 1b).
Figure 1.
Adenoviral vector-mediated ectopic expression of CACNA2D2 gene inhibited NSCLC cell growth in vitro. (a) Expression of the CACNA2D2 gene in Ad-CACNA2D2 (CACN)-transduced NSCLC cells by RT–PCR analysis. Total RNAs were prepared from H1299, A549, H460, and H358 cells transduced by the Ad-CACNA2D2 vector for 48 h at MOIs of 1000 and 2500 vp/c, respectively, and the RNA prepared from cells transfected with a CACNA2D2-expressing plasmid DNA (pLJ58) was used as a positive control. The RNA samples were treated with DNAse prior to the RT reaction. (b) Western blot analysis of expression of CACNA2D2 protein. The crude protein lysates were prepared from NSCLC cells treated in the same way as described for RNA sample preparation in a. The rabbit anti-CACNA2D2 polyclonal antibodies were used for blotting. (c) XTT assay shows the effect of ectopic expression of CACNA2D2 on tumor cell viability. Cells from NSCLC cell lines A549, H460, H1299, and H358 were tranduced with Ad-CACNA2D2 vectors (CACN) at varied MOIs: 2500 for A549, 4000 for H460, 1000 for H1299, and 2000 vp/c for H358 cells. Untreated (PBS), Ad-EV (EV) treated, and Ad-LacZ(LacZ)-treated cells were used as negative controls and Adp53(p53)-treated cells as a positive control, at the same MOIs as CACN-treated cells for each cell line. Cell viability was calculated relative to that of untreated (PBS) controls. Differences were significant in the CACN-transduced cells compared to the untreated (PBS) control cells (P =0.021 in H1299, P<0.0001 in H358, H460, and A549 cells) and to the Ad-LacZ-transduced cells (P<0.0001 in H358, H460, and A549 cells) after 5 days of transduction. Differences between CACN-treated cells and controls were not significant in the H1299 cell line. (d) Effects of intratumoral administration of CACN on growth of human lung cancer H460 subcutaneous tumors in nu/nu mice. Results were reported as the mean±s.d. in five to 10 mice for each treatment group. Tumor volumes were normalized by the percentage increase of tumor sizes after treatment relative to those at the beginning of the treatment in each group. Mean tumor volumes±s.e. from these experiments are shown. The differences of the tumor volumes in the CACN-treated mice versus the PBS- and Ad-LacZ-treated controls were significant (P<0.0001 and 0.015, respectively)
We analysed cell proliferation by determining the viability of cells at 2 and 5 days post-transduction, respectively. Tumor cell growth was significantly inhibited in all the cell lines transduced by the Ad-CACNA2D2 vector 5 days after transduction, compared with what we observed with untreated cells (PBS) or those treated with Ad-EV and Ad-LacZ controls (Figure 1c). The A549 cell line appeared to be the most sensitive to the ectopic expression of CACNA2D2 and showed a more than 60% reduction in cell viability at day 5 (Figure 1c, A549), while moderate reduction of cell viability was observed in Ad-CACNA2D2-transduced H358 (44%), H460 (42%), and H1299 (28%) cells (Figure 1c). Adp53 was used as a positive control and was less effective than Ad-CACNA2D2 in A549 and H460, which contain wt-p53. No significant effect on cell viability was observed in controls treated with PBS, AdEV, and Ad-LacZ.
The effect of enforced expression of the wt-CAC-NA2D2 gene on tumor growth was further evaluated in vivo by direct intratumoral injection of Ad-CACNA2D2 vector, along with PBS and Ad-LacZ vector as controls, into human NSCLC H460 tumor xenografts in nu/nu mice (Figure 1d). The growth of tumors was recorded from the first injection until about 20 days after the last injection. Tumor volumes were normalized by calculating the percentage increase in tumor volume after treatment relative to volume at the beginning of treatment in each group. A significant suppression of tumor growth was observed in H460 tumors treated with Ad-CACNA2D2 vector compared with those control groups treated with PBS (P<0.0001) and with Ad-LacZ (P = 0.015) (Figure 1d). These results obtained in vivo are consistent with those observed in vitro for effects on inhibition of tumor cell growth and induction of apoptosis in the same cell line.
Induction of apoptosis by exogenous expression of CACNA2D2
One of the physiological functions associated with calcium channel proteins is their ability to induce apoptosis by regulating intracellular Ca+2 signaling and several downstream pathways (Lam et al., 1994; Walker and De Waard, 1998; Felix, 1999; Wang et al., 1999a; Zhu et al., 1999, 2000). To test whether the growth inhibition by the ectopic expression of CAC-NA2D2 was caused by induction of apoptosis, we performed FACS analysis with TUNEL reaction and PI staining to examine DNA fragmentation and cell cycle kinetics in Ad-CACNA2D2-transduced cells (Figure 2). Significant induction of apoptosis was observed in Ad-CACNA2D2-transduced A549 (50.1%) (Figure 2a), H460 (22.3%) (Figure 2b), and H358 (18.7%) (Figure 2d) cells at 5 days after transduction compared with cells treated with what was seen with the Ad-EV or Ad-LacZ (from 2 to 10%) control vectors at the same time (Figure 2). However, no significant induction of apoptosis was detected in Ad-CACNA2D2-transduced H1299 cells (Figure 2c) at the same MOIs. The magnitude of and trend toward the induction of apoptosis in these Ad-CACNA2D2-treated cells paralleled the degree and trend towards growth inhibition (Figure 1c). The correlation coefficients between the relative cell viability and the relative apoptotic cell populations in Ad-CACNA2D2-treated cells versus PBS-treated controls/PBS are significant (P<0.05) in all four NSCLC cell lines A549, H1299, H460, and H358 cells (r = −0.96198, −0.79416, −0.99436, and −0.95744, respectively), but with a less degree of correlation in H1299 cells which are less sensitive to exogenous expression of CACNA2D2, suggesting that the growth inhibition by the ectopic expression of CACNA2D2 might be mediated by the induction of apoptosis. We saw no significant alteration in cell cycle kinetics, such as G1 arrest or G2/M arrest, in these Ad-CACNA2D2-transduced cells (data not shown).
Figure 2.
Apoptosis is induced by adenoviral vector-mediated expression of CACNA2D2 in vitro. NSCLC cell lines A549 (a), H460 (b), H1299 (c), and H358 (d) were tranduced with Ad-CACNA2D2 vectors (CACN) at varied MOIs for each line as shown in Figure 1. Untreated (PBS), Ad-EV (EV)-treated, and Ad-LacZ (LacZ)-treated cells were used as negative controls and Ad-p53(p53)-treated as a positive control. The percentage of apoptosis (TUNEL-positive) in cells transduced for 2 or 5 days, respectively, was determined by FACS analysis. Induction of apoptosis was significant in CACN-treated A549, H460, and H358 cells compared to those in PBS-treated (P =0.0004, 0.0321, and 0.0003, respectively) and to those in Ad-LacZ-treated (P =0.021, 0.048, and 0.027, respectively) controls 5 days after transduction. Differences in induction of apoptosis were not significant (P>0.05) between CACN-treated cells and controls in A549, H460, and H358 cell lines at 48 h post-transduction and in H1299 at both 48 and 120 h post-transduction
Upregulation of intracellular free cytosolic Ca2+
CACNA2D2 is structurally related to the α2δ2 subunit of the VACC protein complex, which has been suggested to regulate Ca+2 trafficking through the channel and the retention of VACC at the plasma membrane without significant change in such properties as channel gating or permeation (Wang et al., 1999b; Marais et al., 2001). To examine whether the ectopic overexpression of this CACNA2D2 subunit would increase free cytosolic Ca2+ influx, we measured changes in the levels of intracellular Ca2+ in Ad-CACNA2D2-transduced cells by a sensitive FACS and fluorescence image analysis with fluorescent Fluo3-AM staining (Kao et al., 1989). Fluo3-AM dye binds specifically to free Ca2+ and shows an increase of emission fluorescence at 530 nm upon excitation at 488 nm. The fluorescence intensity depends on how much free Ca2+ is bound. We detected a significant increase of fluorescence emission in Ad-CACNA2D2-transduced H460 (P = 0.015 and 0.03) (Figure 3a (panel c) and b) and A549 (P = 0.001 and 0.002) (Figure 3a (panel g) and b) 48 h after treatment, but this was not seen in the untreated cells and the p53-transduced cells (Figures 2a (panels a and e) and 3b), but not a significant increase in Ad-CACNA2D2-transduced H1299 cells (Figure 3b). An increase in the level of fluorescence emission in Adp53-treated cells (Figure 3a (panels b and f) and b) was also detected, but exhibited a lower magnitude and a slower manifestation (the peak emission was registered 72–96 h post-treatment) than that in Ad-CACNA2D2-transduced cells (the peak emission was registered 48 h post-treatment). The increase of free cytosolic Ca2+ occurred shortly prior to apoptosis in these Ad-CACNA2D2-treated cells, suggesting a possible association of the induction of apoptosis by CACNA2D2 activity and the regulation of intracellular Ca2+ signaling, homeostasis, or both. However, this experimental setting does not allow to link mechanistically the Ca2+ increase to the apoptotic induction. The Fluo3-AM loading and staining conditions were optimized and confirmed by treating cell samples with 2 μg of ionomycin (a ionophore) (Kochegarov et al., 2001) as a positive control, which showed uniform fluorescence emission increase in all treated cells (Figure 3a, panels d and e).
Figure 3.
Ectopic expression of CACNA2D2 increased the level of the intracellular free calcium. (a) Fluorescence image analysis of free cytosolic Ca2+ in Ad-CACNA2D2 (CACN)-transduced H460 and A549 cells by Fluo 3-AM staining. Images of the CACN-treated H460 cells at an MOI of 4000 vp/c (c) and A549 cells (g) at an MOI of 2500 vp/c, untreated (PBS) (a and e) and Adp53 (p53)-treated cells (b and f), and ionomycin-treated control cells (d and h) at 48 h post-treatment are shown. (b) Free Ca2+-specific fluorescence emission was quantified by FACS analysis with Fluo 3-AM staining. Cells were treated as described in panel. (a) The differences of free intracellular Ca2+ are expressed semiquantitatively through the differences in relative fluorescence. The increase in free cytosolic Ca2+ was significant in the CACN-treated H460 (P = 0.015 and 0.032) and A549 cells (P =0.001 and 0.002), but not significant in H1299 cells (P =0.785 and 0.865) compared with the increase seen with untreated (PBS) and p53-treated controls
Interruption of mitochondria membrane potential
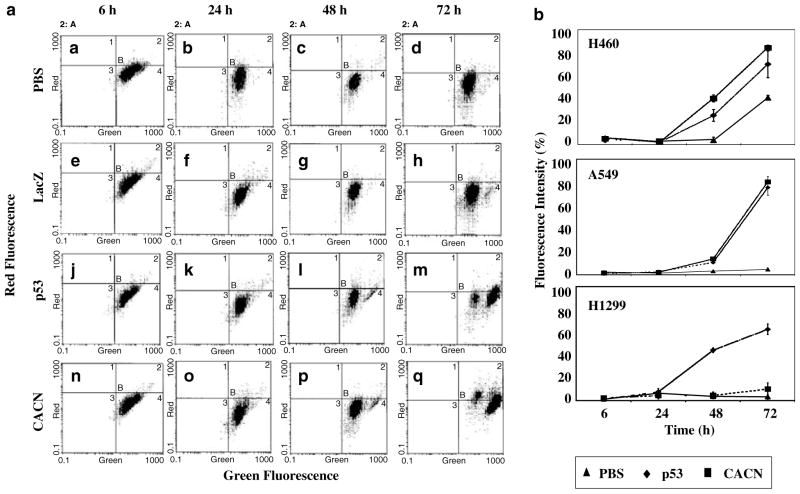
Depolarization of mitochondria and loss of mitochondria membrane potential can be a rate-limiting step in apoptosis as well as in necrotic cell death (Kroemer and Reed, 2000; Vieira et al., 2000). The emerging evidence suggests that an excessive influx of Ca2+ represents a prototypical example of a cell death stimulus where mitochondria membrane depolarization precedes cyt c release (Reed and Kroemer, 2000; Vieira et al., 2000). To investigate further the impact of the observed increase in intracellular free Ca2+ influx by ectopic expression of CACNA2D2 on mitochondria membrane integrity, we analysed the changes of mitochondria membrane potential in Ad-CACNA2D2-transduced NSCLC cells by FACS with mitochondria membrane potential-specific fluorescent JC-1 staining (Figure 4). Mitochondria depolarization, as demonstrated by a significant fluorescent shift with an increase in green (540 nm) emission (Figure 4a), was observed in Ad-CACNA2D2-transduced H460 and A549, cells between 24 and 48 h after transduction (Figure 4b), but not in untreated or Ad-LacZ-transduced cells. After 72 h of transduction by the Ad-CACNA2D2 vector, more than 84% of the A549 cells (Figure 4b, A549) and 80% of H460 (Figure 4b, H460) cells revealed mitochondria membrane depolarization; however, no significant changes were observed in the H1299 cells (Figure 4b, H1299). Adp53 induced similar changes in H460, A549 and H1299 cells (Figure 4). Mitochondria membrane depolarization preceding induction of apoptosis in cells transduced by Ad-CACNA2D2 at the same MOIs (Figure 2) suggests that the mitochondria depolarization mediated by an elevated level of Ca2+ influx is an earlier event in CACNA2D2-induced apoptosis.
Figure 4.
Ectopic expression of CACNA2D2 reduced the mitochondria membrane potential. (a) Changes in the mitochondria membrane potential in Ad-CACNA2D2-transduced A549 cells are revealed by FACS analysis with JC-1 staining. A549 cells were transduced with adenoviral vectors for 6, 24, 48, and 72 h at an MOI of 2500 vp/c. Fluorescence emission of red at 590 nm (indicating high membrane potential and aggregation of JC-1 dye) and green at 530 nm (indicating the collapse of membrane potential and the monomer of JC-1 dye) were measured by FACS. CACN (n, o, p, q)- and p53 (j, k, l, m)-transduced cells showed mitochondria membrane depolarization as evidenced by the fluorescence emission shift to the longer wavelength of green. PBS (a, b, c, d)- and LacZ (e, f, g, h)-transduced cells did not show a marked shift to the right. (b) Relative green fluorescence was higher in CACN-transduced A549, H460, and H1299 cells than in untreated (PBS) control cell. The decrease of the mitochondria membrane potential or depolarization is significant in the CACN-transduced A549 cells (P = 0.002) and H460 (P = 0.005) but not in H1299 cells after 48 h of transduction or in the untreated (PBS) control cells
Cyt c release from mitochondria
Release of cyt c from the mitochondria plays an integral role in apoptosis. To evaluate whether cyt c release might be an integral part of CACNA2D2-mediated apoptosis in a process involving regulation of Ca2+ influx and interruption of the mitochondria membrane integrity, we performed Western blot analysis of cyt c in the fractionated mitochondria and cytosolic lysates in Ad-CACNA2D2-treated A549 cells (Figure 5). The release of cyt c from mitochondria to cytosol was detected in both the Ad-CACNA2D2-transduced A549 cells at an MOI of 2500 (viral particles/cell) vp/c (Figure 5a, lanes 3 and 9) and H460 cells at an MOI of 4000 vp/c (Figure 5a, lanes 6 and 12), but no significant changes in cyt c in the cytosol fraction were detected in untreated (PBS) (Figure 5a, lanes 1, 4, 7, and 10) and Ad-LacZ-transduced (Figure 5a, lanes 2, 5, 8, and 11) cells. No significant change in levels of the cyt c in both the mitochondria and cytosol fractions was observed in Ad-CACNA2D2-transduced H1299 cells (data not shown). Cyt c release began at 48 h after transduction and increased over time as demonstrated in Ad-CACNA2D2-transduced A549 cells (Figure 5b).
Figure 5.
Western blotting was used to analyse cyt c release from mitochondria to cytosol in Ad-CACNA2D2-transduced cells. (a) Western blot of cyt c in CACN-transduced A549 and H460 cells. Cells were transduced with adenoviral vectors at an MOI of 2500 vp/c for A549 and MOI of 4000 vp/c for H460 for 48 h, and untreated (PBS) (lanes 1 and 4) and Ad-LacZ (LacZ)-transduced (lanes 2 and 5) cells were used as controls. Immunoblotting for cyt c was performed in fractionated lysates of mitochondria (lanes 1–6) and cytosol (lanes 7–12). Immunoblots of COX IV (I) were used as a mitochondria enzyme marker and β-actin as an internal loading control. (b) Time course of cyt c release in Ad-CACNA2D2-transduced A549 cells. (c) Immunofluorescence image analysis of the subcellular rearrangement of mitochondria and translocation of cyt c in Ad-CACNA2D2-transduced A549 cells. Mitochondria were probed with an FITC-labeled cyt c antibody (green) and the nucleus was counterstained with PI (red). (d) Timetable of induction of apoptosis in Ad-CACNA2D2-transduced A459 cells by FACS analysis with PI staining for DNA content. The percentage of induction of apoptosis was indicated by the increase of the SubG0-G1 cell populations (shown by a bar in each plot)
The changes in cell morphology and subcellular localization of cyt c (probed with an anti-human cyt c monoclonal antibody) were demonstrated in fluorescence images of A549 cells transduced with Ad-CACNA2D2 (Figure 5c). The characteristic pattern of the mitochondria distribution of cyt c still remained 24 h after transduction (Figure 5c, panel a) but was lost 48 h after transduction at this MOI (Figure 5c, panel b). The release of cyt c from mitochondria into the cytosol and the typical nuclear changes because of apoptosis were evident 48 h after (Figure 5c, panel b) and 72 h after (Figure 5c, panel c) transduction. The accumulation of apoptotic cell populations was also detected 48 h after transduction and increased in time as shown by FACS with PI staining (Figure 5d). The timing of the CACNA2D2-induced cyt c release was sequential and matched the timing of CACNA2D2-induced changes in intracellular Ca2+ influx (Figure 3) and mitochondria membrane potential (Figures 4 and 5d).
Activation of caspase 3 and PARP
Activation of caspases and PARP by translocation of cyt c from mitochondria to the cytosol is one of the events that establishes the mitochondrion as an important regulator of cell life and death (von Ahsen et al., 2000; Martinou and Green, 2001). Western blot analysis was performed to evaluate the activation of caspase 3 and PARP downstream of the mitochondria-mediated apoptotic pathway (Figure 6). The activation of both apoptotic executioners, caspase 3 (Figure 6a) and PARP (Figure 6b), was detected in Ad-CACNA2D2-transduced H460 and A549 cells, as demonstrated by the cleaved fragments of the procaspase3 and pro-PARP on the Western blot (Figure 6). These results provide further evidence that CACNA2D2-mediated apoptosis occurs via the mitochondrion.
Figure 6.
Downstream caspase 3 and PARP are activated by ectopic expression of the CACNA2D2 gene. (a) Western blot analysis of caspase 3. The whole cell lysate was prepared from Ad-CACNA2D2 (CACN)-transduced H460 cells (lanes 1–3) at an MOI of 4000 vp/c and A549 (lanes 5 and 6) cells at an MOI of 2500 vp/c after 72 h of transduction, and untreated (PBS) and Ad-LacZ (LacZ)- or Ad-p53 (p53)-transduced cells were used as controls. The cleaved procaspase 3 was indicated by an arrow. (b) Western blot analysis of PARP. Cells were transduced by Ad-CACNA2D2 (CACN) at the same MOI as described in a for each cell line. The cleaved PARP is indicated by the immunoblotting complexes of about 85 kDa. β-actin was used as an internal loading control
Discussion
The protein product of the recently cloned CACNA2D2 gene is structurally related to the α2δ2 auxiliary subunit of the voltage-activated calcium channel (VACC) protein complex (Angeloni et al., 2000; Gao et al., 2000). Various VACC protein subunits such as the pore-forming α1 unit and the auxiliary β, γ, and α2δ subunits have been identified and partially characterized (Singer et al., 1991; Castellano et al., 1993; Brown and Gee, 1998; Burgess et al., 1999; Felix, 1999, Hofmann et al., 1999; Varadi et al., 1999; Catterall, 2000; Lacinova et al., 2000). The α2δ2 subunit (CACNA2D2) of VACC is a regulatory subunit (Gao et al., 2000). Mutation of this gene has been found to lead to a phenotype characterized by epilepsy, ataxia, and alterations of calcium currents in cerebellar cells in mice, which is ultimately fatal (Barclay et al., 2001). Although the exact physiological function of CACNA2D2 in nonexcitable cells remains unknown, functional studies of CAC-NA2D2 have revealed that the activity of CACNA2D2 protein may alter the conductance properties of the pore-forming α1 unit as well as their membrane trafficking and, therefore dynamically regulates Ca2+ current through the VACC (Gao et al., 2000; Hobom et al., 2000; Hurley et al., 2000; Klugbauer et al., 1999). The very frequent and early loss of expression of CACNA2D2 together with a subset of genes in the 3p21.3 homozygous deletion region of human chromosome 3 in human lung and breast cancers suggest a link between the CACNA2D2 and the regulation of proliferation and cell death in lung cancer pathogenesis, possibly through the regulation of the VACC-mediated Ca2+ influx (Angeloni et al., 2000; Gao et al., 2000; Lerman and Minna, 2000). However, no direct evidence has been presented for this link. In this study, we focused on CACNA2D2-mediated apoptosis by adenoviral vector-mediated ectopic expression of the wt-CACNA2D2 gene in the CACNA2D2-deficient NSCLC cells. We presented indirect evidence to link the CACNA2D2-mediated apoptosis with the regulation of the intracellular calcium content, interruption of mitochondria membrane integrity, and activation of downstream caspases.
Inhibition of tumor cell growth by ectopic expression of CACNA2D2 is concomitant with induction of apoptosis in these Ad-CACNA2D2-transduced NSCLC cells. A significant induction of apoptosis was observed 48 h after transduction. The cell lines most sensitive to CACNA2D2-induced apoptosis were A549 and H460, which contain a wt-p53 gene and are generally resistant to either the transduction of adenoviral vectors or to wt-p53-mediated cell death. Ad-CACNA2D2-transduced H358 cells, which carry a mutated p53 gene, showed remarkable inhibition of cell growth but no significant induction of apoptosis. H1299 cells, which are p53-null, were the most resistant to CACNA2D2-induced growth inhibition and apoptosis in vitro and in vivo. These results suggest a possible association of the CAC-NA2D2-mediated apoptosis with the activities of wt-p53, which is very interesting and needs to be explored further.
Based on the evidence that the activity of CAC-NA2D2 dynamically regulates Ca2+ currents in L- and T-type calcium channels (Klugbauer et al., 1999; Gao et al., 2000; Hobom et al., 2000; Hurley et al., 2000), we expected that overexpression of CACNA2D2 might result in an increase in the level of cytosolic Ca2+ influx. A significant increase in the basal level of the intracellular free Ca2+ was indeed detected in Ad-CACNA2D2-transduced H460 and A549 cells using sensitive free-Ca2+-specific Fluo3-AM staining in a semiquantitative manner. Several factors, however, hinder the accurate determination of the kinetic events of the modulation of Ca2+ influx influenced by the adenoviral vector-mediated transient expression of CACNA2D2 protein. Gradual expression of the CAC-NA2D2 subunit after 24 h of transduction would cause the modulation of intracellular Ca2+ influx with time. Since Ca2+ is a multivalent messenger, several cytosolic Ca2+ binding proteins, such as calmodulin, can bind to the free Ca2+ to execute downstream effects on cellular processes, which would significantly reduce the availability of free Ca2+; other Ca2+ effectors or mediators, such as calbindin-D, parvalbumin, and calretinin, can buffer the cytosolic increases of Ca2+ (Berridge et al., 1998, 2000). Furthermore, Ca2+ signals have a wide range of spatial and temporal distribution and so are capable of conveying signals in a very complex way (Lemasters et al., 1998; Berridge et al., 2000; Zhu et al., 2000). Together, these factors make it difficult to detect even the global Ca2+ oscillations in our experimental setting; therefore, our data may not represent the accurate dynamic changes of intracellular Ca2+ contents and influx.
Mitochondria play a major role in apoptosis triggered by many stimuli. Disruption and permeation of the mitochondria membrane are general phenomena associated with the processes of apoptosis and necrotic cell death (Kroemer and Reed, 2000, Vieira et al., 2000). An excessive mitochondria Ca2+ influx has been suggested to be a potent cell death stimulus leading to mitochondria membrane depolarization and cyt c release (Reed and Kroemer, 2000; Vieira et al., 2000). Activation of caspases by translocation of cyt c from mitochondria to the cytosol is a downstream event through which the mitochondrion’s role as a regulator of cell life and death has become unquestioned (Chen et al., 2000; von Ahsen et al., 2000; Martinou and Green, 2001). We demonstrated that ectopic expression of CACNA2D2 was associated with the accumulation of intracellular free Ca2+ and the collapse of the mitochondria membrane potential prior to cyt c release and nuclear apoptotic changes, suggesting a physiological effect of CAC-NA2D2 activity in regulating cell survival by indirectly altering the mitochondria membrane integrity in concomitance with cytosolic Ca2+ increase. Rupture of the outer membrane results in the release of many proteins such as cyt c and some caspases (Desagher and Martinou, 2000). However, whether this is the result of a direct effect of the CACNA2D2-mediated Ca2+ oscillations on mitochondria permeability needs to be further investigated. It would also be interesting to explore the CACNA2D2-mediated Ca2+-signaling pathways involved in activation of the proapoptotic mediators such as Bad and Bax and inactivation of the antiapoptotic factors such as Bcl-2 and Bclx that convey the apoptotic signal to the mitochondrion (Gross et al., 1999; Vieira et al., 2000; von Ahsen et al., 2000).
Together, our results suggest that ectopic expression of CACNA2D2 is capable of inducing apoptosis in several NSCLC cell lines. The induction of apoptosis by CACNA2D2 activity is associated with the regulation of cytosolic Ca2+ contents and the activation of the mitochondria pathway. Further identification of the physiological functions of CACNA2D2 in unexcitable cells such as normal bronchial epithelial cells, the evaluation of the cellular modulation of endogenous and exogenous expression of CACNA2D2 in response to environmental stimuli such as DNA-damaging agents and oncogene activities in normal and tumor cells, and the characterization of the effects of CACNA2D2 activity on both L- and T-type calcium channels in the presence and absence of selective inhibitors of the various VACC subtypes will provide us insight into the molecular mechanisms in the CACNA2D2-mediated regulation of cell proliferation and cell death in the pathogenesis of lung cancers and other human cancers.
Materials and methods
Cell lines and cell culture
Four human NSCLC cell lines, A549 (homozygous for multiple 3p21.3 markers and wt-p53), NCI-H1299 (homozygous for multiple 3p21.3 markers and homozygous deletion of p53), NCI-H358 (retained heterozygosity of multiple 3p21.3 markers and homozygous deletion of p53), and NCI-H460 (homozygous for multiple 3p21.3 markers and wt-p53), with varied 3p21.3 and p53 gene status, and a normal human bronchial epithelial cell line (HBEC) or fibroblast cells were used for in vitro experiments. The multiple 3p21.3 markers located in the 630 kb region used for this analysis were described previously (Fondon et al., 1998). The A549 line was maintained in Ham’s F12 medium supplemented with 10% fetal calf serum. The H1299, H358, and H460 lines were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and 5% glutamine.
Recombinant adenoviral vectors
The recombinant Ad-CACNA2D2 was constructed using our recently developed ligation-mediated plasmid adenovirus vector construction system, named herein pAd-RAP and pAd-RAP-Shuttle. The CACNA2D2 was assembled as a mammalian gene expression cassette that is driven by a CMV promoter and tailed with a bovine growth hormone (BGH) poly (A) signal sequence. Sequences of the CAC-NA2D2 gene in the viral vectors were confirmed by automated DNA sequencing. A vector expressing the GFP (green fluorescence protein) gene (Ad-GFP) and a vector carrying the β-galactosidase gene LacZ (Ad-LacZ) were used to monitor the efficiency of transduction by the viral vectors and as nonspecific transgene expression controls. Ad-EV, an empty E1-deleted vector, was used as a negative control; Ad-p53, a vector containing the wt-p53 gene, was used as a positive control for tumor suppression. Viral titers were determined by both optical density measurement (i.e. vp/ml) and plaque assay (i.e. plaque-forming units (PFU)/ml).
Animal experiments
All animals were maintained and animal experiments were performed under NIH and institutional guidelines established for the Animal Core Facility at the University of Texas MD Anderson Cancer Center. Procedures for H460 subcutaneous tumor inoculations in nu/nu mice were described previously (Ji et al., 1999). When the average tumor size reaches about 0.5 cm in diameter, mice were injected intratumorally three times within a week with Ad-CACNA2D2 and control vectors at a dose of 3 × 1010 PFU (3 × 1012 vp)/tumor in a volume of 0.2 ml. Differences in tumor volumes between treatment groups were analysed with a mixed model ANOVA using the Statistica software (StatSoft Inc., Tulsa, OK, USA). A difference was considered to be statistically significant when P = 0.05.
Analysis of CACNA2D2 gene Expression by RT–PCR
Total RNA samples were isolated from Ad-CACNA2D2-transduced tumor cells using TRIZOL reagent (Life Technologies, Grand Island, NY, USA) as instructed by the manufacturer. The RT reaction was performed using a reverse transcription kit with the oligo-d(T)16 as a primer under the conditions recommended by the manufacturer (Perkin-Elmer Applied Biosystems, Foster City, CA, USA). The RT–PCR products amplified with human total RNA as a template and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers were used as an internal control. The primers for CACNA2D2 were 5′GACTGACCAACACCACTCTTCTC (sense, within CACNA2D2 cDNA) and 5′CTCATCGTACCTCAGCTCCTTCC (antisense, within the BGH poly (A) signaling region). The PCR was performed using an AmpliTaq PCR Kit and a 9600 PCR instrument according to the manufacturer’s instructions (Perkin-Elmer Applied Biosystems).
Cell viability assay
Inhibition of tumor cell growth by treatment with Ad-CACNA2D2 and control vectors was analysed by quantitatively determining cell viability using an improved XTT assay (Roche Molecular Biochemicals, Indianapolis, IN, USA). Briefly, cells were plated in 96-well microtiter plates at 1 × 103 cells/well in 100 μl of medium. One day after the cells were plated, a 100-μl aliquot of medium containing individual adenoviral vectors at various multiplicities of infection MOI in units of vp/cell (vp/c) was placed into each sample well, and phosphate-buffered saline (PBS), Ad-EV, Ad-LacZ, and Adp53 were added as controls. On designated sampling days after transduction, cell growth and viability were quantified by XTT assay as described previously (Nishizaki et al., 2001). The percentage of cell viability was calculated in terms of the absorbency of treated cells relative to the absorbency of untreated control cells. Experiments were repeated at least three times with quadruplicate samples for each treatment in each individual experiment.
Analysis of apoptosis and cell cycle kinetics
Induction of apoptosis in tumor cells treated with various adenoviral vectors was analysed by flow cytometry (FACS) using terminal deoxynucleotidyl transferase-mediated dUTP nickend labeling (TUNEL) reaction with fluorescein (FITC)-labeled dUTP (Roche Molecular Biochemicals, Mannheim, Germany). Briefly, cells were plated in six-well plates (1 × 106 cells/well) and treated by various Ad-CACNA2D2 vectors; PBS, Ad-EV, Ad-LacZ, and Adp53 were used as controls. At designated times after transduction, cells were harvested and washed in PBS. Cells were processed for FACS analysis to determine apoptosis and cell cycle kinetics as described previously (Ji et al., 1999).
Measurement of cytosolic free calcium
The intracellular free Ca2+ was measured by FACS and fluorescence image analysis with free-Ca2+-sensitive Fluo3-AM green fluorescent staining (Molecular Probes, Eugene, OR, USA) in Ad-CACNA2D2-transduced A549 and H460 cells. Cells were cultured in 100 mm dishes at about 5 x 106 cells/dish and transduced with adenoviral vectors at varied MOIs. After 24 and 48 h of transduction, cells were collected and washed once with 1 × HBSS supplemented with 1 mμ Ca2+, 1 mμ Mg2+, and 1% fetal bovine serum (FBS). The Fluo3-AM stock solution was prepared by first dissolving 50 μg of Fluo3-AM dye in 20 μl of DMSO containing 20% of the detergent Fluronic F-127 (Molecular Probes) and then mixing it with 117 μl of FBS. The cells were resuspended in 1 ml of HBSS containing the Fluo3-AM dye in a final concentration of 2.5–5.0 μg/ml, depending on the cell type. The anion carrier inhibitor probenecid was added at a final concentration of 4 mμ to minimize the dye leakage. The cells were incubated for 45 min at room temperature on an orbital shaker in the dark. Cells were spun down by centrifugation for 5 min at 1500 r.p.m. and washed once with HBSS. Cells were gently resuspended in HBSS containing 4 mμ of probenecid and then incubated for 20 min in the dark to allow cellular esterases to cleave the acetoxymethyl group of Fluo3-AM. Fluorescence intensity in the stained cells was measured by FACS analysis at an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Experiments were performed three times independently. To evaluate the conditions of dye loading, 2 μg of ionomycin, an ionophoric antibiotic synthesized by Streptomyces conglobatus sp. (Calbiochem, Fremont, CA, USA), was added to each of the cell samples in a separate tube, and the dynamic fluorescence emission was measured by FACS after baseline fluorescence was assessed. For fluorescence imaging analysis of Fluo3-AM stained cells, the cells were cultured in chamber slides (Falcon), and then treated and stained with Fluo3-AM with the same procedure as was described for FACS analysis. The stained cells were examined under a microscope (Nikon Labophot 2) equipped with a digital camera (Nikon DMX1200, Tokyo, Japan) and the analysis software (Nikon ACT-1 V2.0).
Analysis of mitochondria membrane potential by FACS with JC-1 staining
Changes in mitochondria membrane potential in adenoviral vector-transduced cells were measured by flow cytometry with JC-1 (5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolyl-carbocyanine iodide) staining (Molecular Probes, Eugene, OR, USA). JC-1 exists as a monomer at low concentrations or at low membrane potential and emits green fluorescence at 527 nm. However, at higher concentrations or higher membrane potentials, JC-1 forms J-aggregates and emits maximum red fluorescence at ~590 nm. The measurement of the ratio of the red to green JC-1 fluorescence in cells by flow cytometry is a sensitive and specific method for monitoring changes in mitochondria potential in living cells during induction of apoptosis by various agents (Ankarcrona et al., 1995; Cossarizza et al., 1995). Cells were cultured in six-well plates and, after reaching ~70% confluence, transduced with various adenoviral vectors at varied MOIs. Cells were collected by centrifugation for 5 min at 2000 r.p.m. at 4°C and resuspended in complete medium containing 10 mg/ml JC-1 at a density of 5 × 105 cells/ml. The cells were incubated for 10 min at room temperature in the dark, washed twice with cold PBS, resuspended in 400 ml of PBS, and analysed immediately by flow cytometry.
For in situ fluorescent staining with JC-1, cells were cultured in chamber slides. At designated time points, the medium was removed and the cells incubated in reduced serum Opti-MEM-I medium (GIBCO BRL, Grand Island, NY, USA) containing 10 μg/ml of JC-1 for 10 min in the dark. After washing and air-drying, stained cells were immediately examined by fluorescence microscopy.
Western blot analysis
Western blot analysis was performed to evaluate the expression of CACNA2D2 protein, the release of cyt c, activation of caspase 3 and PARP, and other protein expression in Ad-CACNA2D2 and control vector-transduced cells. For the preparation of crude cell lysates, cells were suspended in SDS–PAGE running buffer containing a complete set of proteinase inhibitors (Roche Molecular Biochemials, Mannheim, Germany) and lysed for 20 min at 4°C. Cell lysates were passed through a 25-gauge needle and briefly sonicated twice for 30 s. For cyt c analysis, cell fractionation was performed to separate mitochondria-enriched fractions from cytosol fractions using an Apo-Alert Cell Fractionation Kit (ClonTech, Palo Alto, CA, USA) according to the manufacturer’s instructions. Fractionated cell lysates were kept in equal volume in 2 × lysis buffer supplemented with 62.5 mμ urea. Protein concentrations were assayed using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). The crude cell lysates (about 50 μg) were used in standard SDS–PAGE and Western blot analysis.
Immunofluorescence staining
Immunofluorescence staining was performed in cells cultured in chamber slides. At designated time points, the cells were washed twice with cold PBS fixed in 4% paraformaldehyde for 15 min at 4°C and made permeable by incubation for 5 min in a solution containing 0.1% Triton X-100 and 0.1% sodium citrate. The cells were incubated with the primary monoclonal mouse anti-cyt C antibody for 60 min at 37°C, and after washing were incubated with the FITC-labeled secondary rabbit anti-mouse IgG antibodies for 60 min. After three washing steps in 0.1% Tween 20–PBS solution and air-drying, the slides were mounted with aqueous mounting medium containing 50 μg/ml of PI for nuclear staining and immediately examined under a fluorescence microscope.
Statistics
All the experiments were repeated at least two times with duplicates or triplicates of samples. The results were expressed as mean±s.d. Student’s two-sided t-test was used to compare the values of the test and control samples. A value of P<0.05 was taken as significant.
Acknowledgments
The authors would like to thank Karen Ramirez and Wendy Schober-Ditmore for their assistance in FACS analysis, and David McConkey, Leta Nutt, and Abujiang Pataer for discussions on the methodology. This work was partially supported by grants from the National Cancer Institute, the National Institutes of Health SPORE (2P50-CA70907-04); (P01 CA78778-01A1) (JAR); (CA71618) (JDM), a WM Keck Gene Therapy Career Development Grant (LJ), by a grant from the Department of the Army BESCT Lung Cancer Program (DAMD17011068902); by the Swiss National Science Foundation (GLC) and Bernische Krebsliga (GLC); by gifts to the Division of Surgery MD Anderson Cancer Center, from Tenneco and Exxon for the Core Laboratory Facility; by the M. D. Anderson Cancer Center Support Core Grant (CA16672); by a grant from the Tobacco Settlement Funds as appropriated by the Texas State Legislature (Project 8), and by a sponsored research agreement with Introgen Therapeutics, Inc. (SR93-004-1).
Abbreviation
- ADP
adenosinediphosphate
- CACNA2D2
calcium-channel alfa-2-delta-2 subunit
- COX IV (I)
cytochrome oxidase IV subunit I
- cyt C
cytochrome C
- DAPK
death-associated protein kinase
- DMSO
dimethylsulfoxide
- FBS
fetal bovine serum
- HBSS
Hanks balanced saline solution
- MOI
multiplicity of infection
- NSCLC
non-small cell lung cancer
- PARP
poly ADP-ribose polymerase
- PI
propidiumiodide
- TUNEL
terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
- VACC
voltage-activated calcium channel
- XTT
sodium 3,3′-{1-[(phenylamino)carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro)-benzene sulfonic acid hydrate
- wt
wild-type
References
- Angeloni D, Wei MH, Duh FM, Johnson BE, Lerman MI. Mol Cell Probes. 2000;14:53–54. doi: 10.1006/mcpr.1999.0277. [DOI] [PubMed] [Google Scholar]
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. J Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Brown JP, Gee NS. J Biol Chem. 1998;273:25458–25465. doi: 10.1074/jbc.273.39.25458. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Davis CF, Gefrides LA, Noebels JL. Genome Res. 1999;9:1204–1213. doi: 10.1101/gr.9.12.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano A, Wei X, Birnbaumer L, Perez-Reyes E. J Biol Chem. 1993;268:3450–3455. [PubMed] [Google Scholar]
- Catterall WA. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chen Q, Gong B, Almasan A. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossarizza A, Cooper EL, Quaglino D, Salvioli S, Kalachnikova G, Franceschi C. Biochem Biophys Res Commun. 1995;214:503–510. doi: 10.1006/bbrc.1995.2315. [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Felix R. Receptors Channels. 1999;6:351–362. [PubMed] [Google Scholar]
- Fondon JW, Mele GM, Brezinschek RI, Cummings D, Pande A, Wren J, O’Brien KM, Kupfer KC, Wei MH, Lerman M, Minna JD, Garner HR. Proc Natl Acad Sci USA. 1998;95:7514–7519. doi: 10.1073/pnas.95.13.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Sekido Y, Maximov A, Saad M, Forgacs E, Latif F, Wei MH, Lerman M, Lee JH, Perez-Reyes E, Bezprozvanny I, Minna JD. J Biol Chem. 2000;275:12237–12242. doi: 10.1074/jbc.275.16.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Hobom M, Dai S, Marais E, Lacinova L, Hofmann F, Klugbauer N. Eur J Neurosci. 2000;12:1217–1226. doi: 10.1046/j.1460-9568.2000.01009.x. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Lacinova L, Klugbauer N. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- Hurley JH, Cahill AL, Currie KP, Fox AP. Proc Natl Acad Sci USA. 2000;97:9293–9298. doi: 10.1073/pnas.160589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Fang B, Yen N, Fong K, Minna JD, Roth JA. Cancer Res. 1999;59:3333–3339. [PubMed] [Google Scholar]
- Kao JP, Harootunian AT, Tsien RY. J Biol Chem. 1989;264:8179–8184. [PubMed] [Google Scholar]
- Klugbauer N, Lacinova L, Marais E, Hobom M, Hofmann F. J Neurosci. 1999;19:684–691. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochegarov AA, Beylina SI, Matveeva NB, Leontieva GA, Zinchenko VP. Comparative Biochem Physiol. 2001;128(Part A):279–288. doi: 10.1016/s1095-6433(00)00306-8. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lacinova L, Klugbauer N, Hofmann F. Gen Physiol Biophys. 2000;19:121–136. [PubMed] [Google Scholar]
- Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Proc Natl Acad Sci USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- Lerman MI, Minna JD. Cancer Res. 2000;60:6116–6133. [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Green DR. Nat Rev Mol Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- Nishizaki M, Meyn RE, Levy LB, Atkinson EN, White RA, Roth JA, Ji L. Clin Cancer Res. 2001;7:2887–2897. [PubMed] [Google Scholar]
- Raveh T, Kimchi A. Exp Cell Res. 2001;264:185–192. doi: 10.1006/excr.2000.5134. [DOI] [PubMed] [Google Scholar]
- Reed JC, Kroemer G. Cell Death Differ. 2000;7:1145. doi: 10.1038/sj.cdd.4400777. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Rizzuto R. Trends Biochem Sci. 2000;25:215–221. doi: 10.1016/s0968-0004(00)01585-1. [DOI] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Toyota M, Ho C, Ohe-Toyota M, Baylin SB, Issa JP. Cancer Res. 1999;59:4535–4541. [PubMed] [Google Scholar]
- Ueki T, Toyota M, Sohn T, Yeo CJ, Issa JP, Hruban RH, Goggins M. Cancer Res. 2000;60:1835–1839. [PubMed] [Google Scholar]
- Varadi G, Strobeck M, Koch S, Caglioti L, Zucchi C, Palyi G. Crit Rev Biochem Mol Biol. 1999;34:181–214. doi: 10.1080/10409239991209264. [DOI] [PubMed] [Google Scholar]
- Vieira HL, Haouzi D, El Hamel C, Jacotot E, Belzacq AS, Brenner C, Kroemer G. Cell Death Differ. 2000;7:1146–1154. doi: 10.1038/sj.cdd.4400778. [DOI] [PubMed] [Google Scholar]
- von Ahsen O, Waterhouse NJ, Kuwana T, Newmeyer DD, Green DR. Cell Death Differ. 2000;7:1192–1199. doi: 10.1038/sj.cdd.4400782. [DOI] [PubMed] [Google Scholar]
- Walker D, De Waard M. Trends Neurosci. 1998;21:148–154. doi: 10.1016/s0166-2236(97)01200-9. [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Science. 1999a;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang M, Offord J, Oxender DL, Su TZ. Biochem J. 1999b;342:313–320. [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Ling S, Yu XD, Venkatesh LK, Subramanian T, Chinnadurai G, Kuo TH. J Biol Chem. 1999;274:33267–33273. doi: 10.1074/jbc.274.47.33267. [DOI] [PubMed] [Google Scholar]
- Zhu LP, Yu XD, Ling S, Brown RA, Kuo TH. Cell Calcium. 2000;28:107–117. doi: 10.1054/ceca.2000.0138. [DOI] [PubMed] [Google Scholar]
- Zochbauer-Muller S, Fong KM, Virmani AK, Geradts J, Gazdar AF, Minna JD. Cancer Res. 2001;61:249–255. [PubMed] [Google Scholar]