Abstract
The mouse alpha-fetoprotein mRNA is the product of a single-copy gene whose mRNA coding sequences are represented discontinuously in the genome. Several EcoRI genomic fragments which contain portions of the alpha-fetoprotein gene have been cloned using the EK2 vector lambda gt WES . lambda B. In addition, a mouse genomic library has been screened to obtain a 15.75-kilobase segment of DNA that includes more than 85% of the alpha-fetoprotein coding sequence. Analyses by restriction endonuclease mapping and electron microscopy showed that the mRNA sequence is interrupted by at least 11 intervening sequences which occupy 90% of the cloned DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABELEV G. I., PEROVA S. D., KHRAMKOVA N. I., POSTNIKOVA Z. A., IRLIN I. S. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963 Apr;1:174–180. doi: 10.1097/00007890-196301020-00004. [DOI] [PubMed] [Google Scholar]
- Abelev G. I. Alpha-fetoprotein as a marker of embryo-specific differentiations in normal and tumor tissues. Transplant Rev. 1974;20(0):3–37. doi: 10.1111/j.1600-065x.1974.tb00139.x. [DOI] [PubMed] [Google Scholar]
- Abelev G. I., Assecritova I. V., Kraevsky N. A., Perova S. D., Perevodchikova N. I. Embryonal serum alpha-globulin in cancer patients: diagnostic value. Int J Cancer. 1967 Sep 15;2(5):551–558. doi: 10.1002/ijc.2910020517. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Gitlin D., Boesman M. Serum alpha-fetoprotein, albumin, and gamma-G-globulin in the human conceptus. J Clin Invest. 1966 Nov;45(11):1826–1838. doi: 10.1172/JCI105486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Miller D. L. Quantitation of rat alpha-fetoprotein messenger RNA with a complementary DNA probe. J Biol Chem. 1977 Dec 10;252(23):8469–8475. [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Polsky F., Edgell M. H., Seidman J. G., Leder P. High capacity gel preparative electrophoresis for purification of fragments of genomic DNA. Anal Biochem. 1978 Jul 1;87(2):397–410. doi: 10.1016/0003-2697(78)90689-9. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Immunological crossreaction between alpha-fetoprotein and albumin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4641–4644. doi: 10.1073/pnas.73.12.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pihko H., Seppälä M. Alpha-fetoprotein: immunochemical purification and chemical properties. Expression in normal state and in malignant and non-malignant liver disease. Transplant Rev. 1974;20(0):38–60. doi: 10.1111/j.1600-065x.1974.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Seppälä M. alpha-Fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/s0065-230x(08)60849-0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Terry W. D. alpha foetoprotein and serum albumin show sequence homology. Nature. 1976 Apr 29;260(5554):804–805. doi: 10.1038/260804a0. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Thomas K., Michalson M., Salatrepat J., Bonner J. Control of albumin and alpha-fetoprotein expression in rat liver and in some transplantable hepatocellular carcinomas. Biochim Biophys Acta. 1979 Aug 29;564(1):173–178. doi: 10.1016/0005-2787(79)90198-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki T., Thomas K., Schindler I. Cell-free studies of developmental changes in synthesis of alpha-foetoprotein and albumin in the mouse liver. Nature. 1974 May 17;249(454):269–271. doi: 10.1038/249269b0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Cameron J. R., Davis R. W. Viable molecular hybrids of bacteriophage lambda and eukaryotic DNA. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4579–4583. doi: 10.1073/pnas.71.11.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Leder P. Purification and cloning of a mouse ribosomal gene fragment in coliphage lambda. Gene. 1977;2(3-4):173–191. doi: 10.1016/0378-1119(77)90016-6. [DOI] [PubMed] [Google Scholar]
- Tiemeier D. C., Tilghman S. M., Polsky F. I., Seidman J. G., Leder A., Edgell M. H., Leder P. A comparison of two cloned mouse beta-globin genes and their surrounding and intervening sequences. Cell. 1978 Jun;14(2):237–245. doi: 10.1016/0092-8674(78)90110-1. [DOI] [PubMed] [Google Scholar]
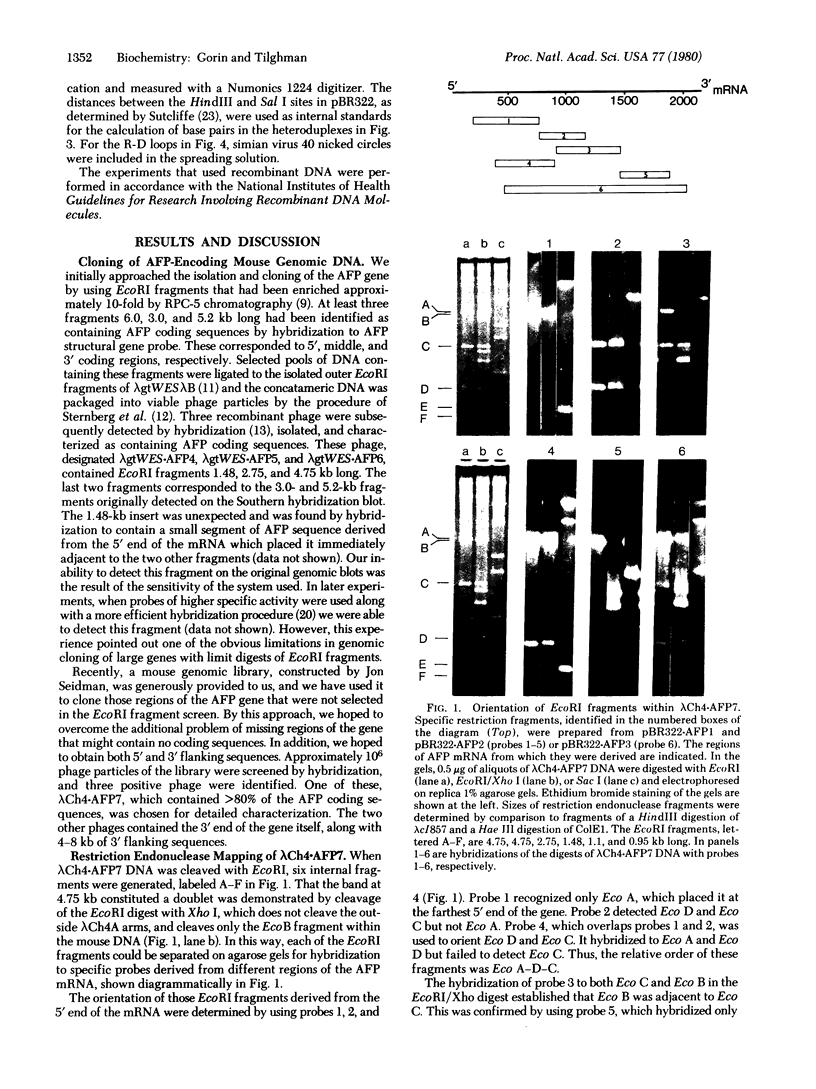
- Tilghman S. M., Kioussis D., Gorin M. B., Ruiz J. P., Ingram R. S. The presence of intervening sequences in the alpha-fetoprotein gene of the mouse. J Biol Chem. 1979 Aug 10;254(15):7393–7399. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]