Abstract
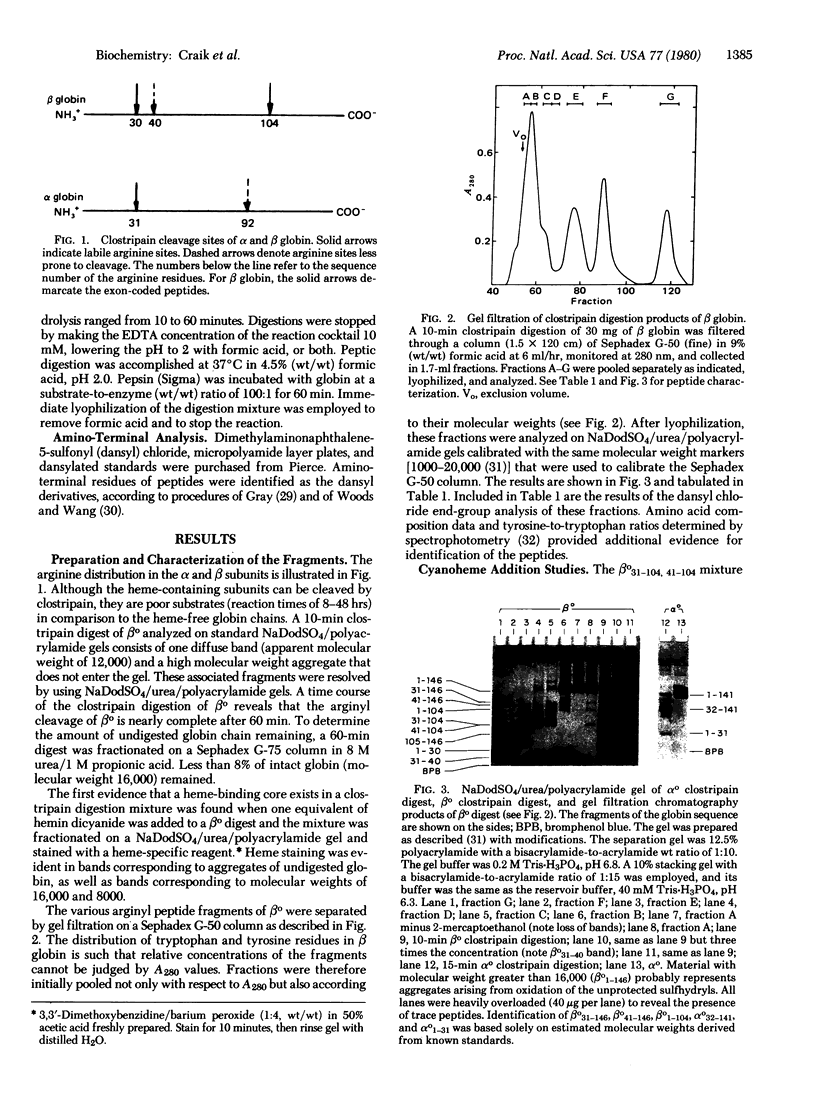
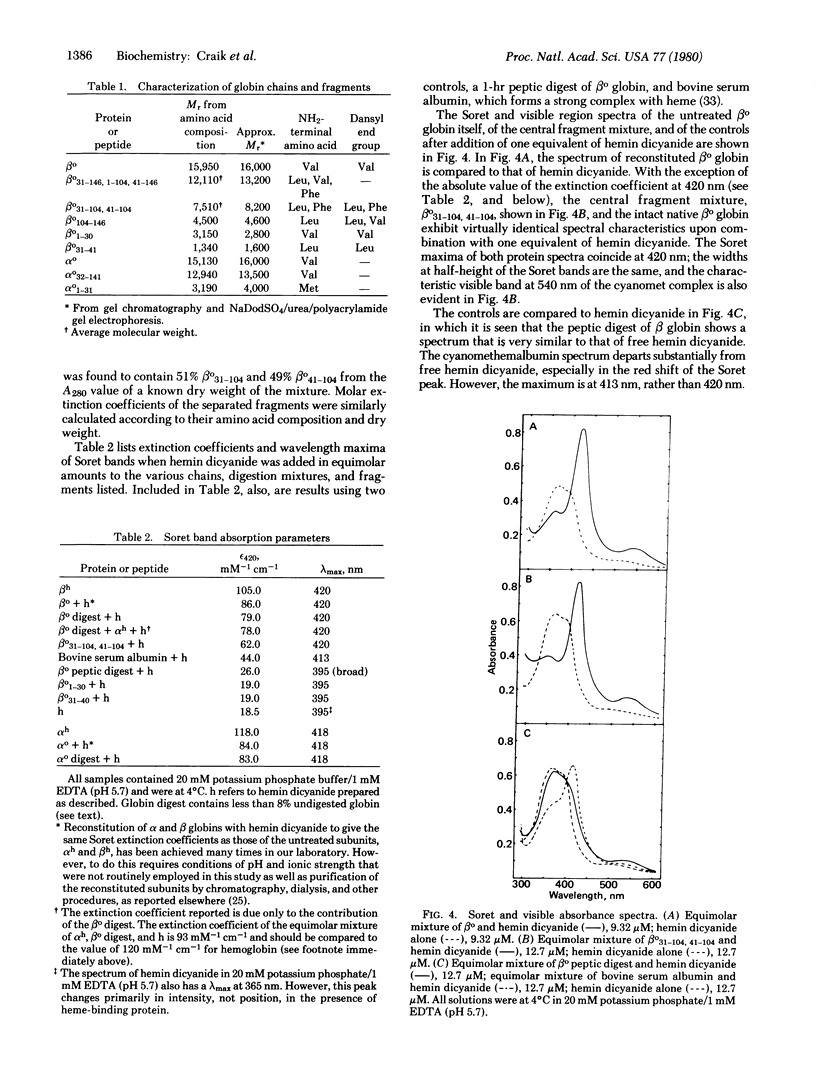
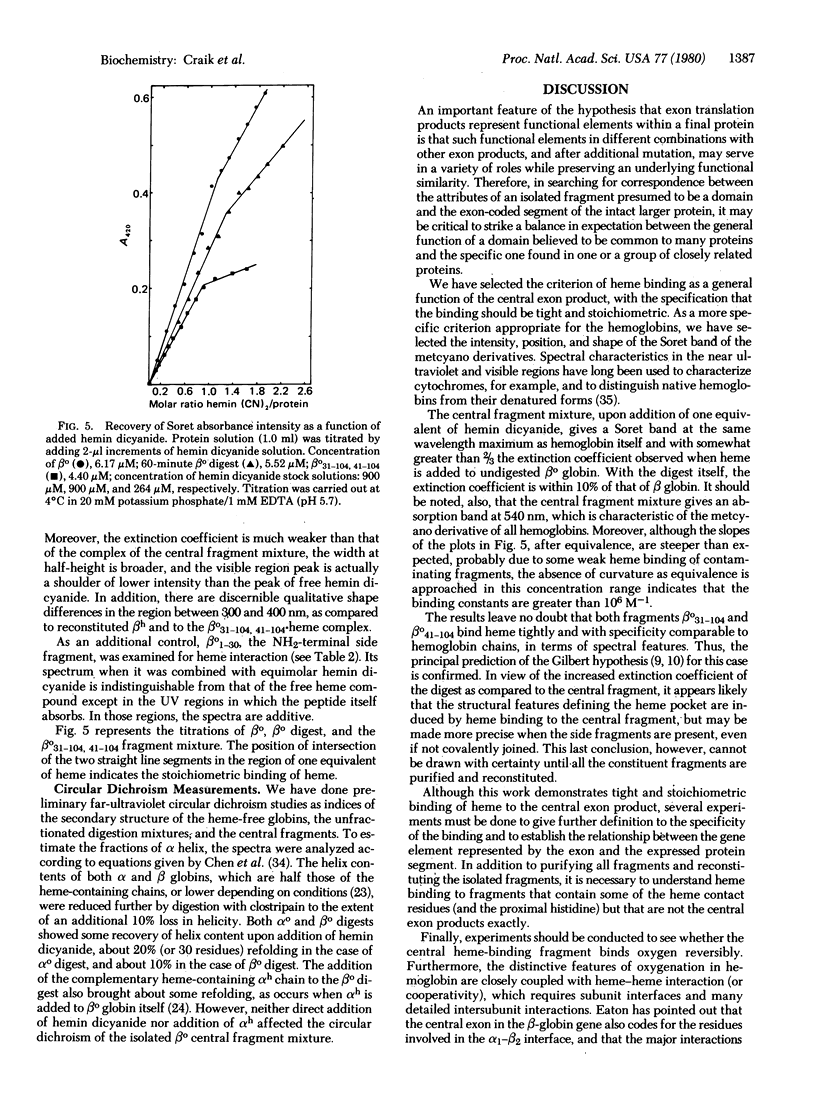
We have prepared and isolated the peptide fragments coded for by the three exons of the human β-globin gene, using the arginine-specific protease clostripain (EC 3.4.22.8). The region encoded by the central exon (amino acid residues 31-104) contains an arginine at position 40. This site was less susceptible to cleavage than the two sites that correspond to the exon-intron boundaries, and the isolated central fragment was an approximately equimolar mixture of the entire central fragment, βo31-104, and the somewhat smaller fragment contained within it, βo41-104. This central fragment mixture bound heme stoichiometrically and tightly at micromolar concentrations, generating a strong Soret absorption band as well as a characteristic absorption band in the visible spectrum. The Soret band occurred at the same wavelength and had the same shape as in hemoglobin, exhibiting an intensity greater than ⅔ that achieved when native intact β globin is reconstituted with heme. Nearly the full intensity was regained when an equivalent of heme was added to the unfractionated digest, suggesting that the noncovalently associated side fragments add precision to the fit of the heme pocket. Three controls were used in establishing the specificity of heme binding to the central fragment mixture. Similar, but preliminary, experiments have also been undertaken with α globin. A clostripain digest containing αo1-31 and αo32-141 bound heme, yielding a Soret band identical to that observed in α subunits reconstituted from the native globin chains and heme. Measurements of circular dichroism spectra as indices of secondary structure suggested a role for the side exon products in the acquisition of the native three-dimensional structure of hemoglobin. These experiments confirm a prediction of W. Gilbert that the product of the central exon of the globin gene is a complete functional domain that binds heme tightly and specifically.
Keywords: hemoglobin genes, protein folding, evolution
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Rossmann M. G. Structural comparisons of heme binding proteins. Biochemistry. 1979 Oct 30;18(22):4951–4960. doi: 10.1021/bi00589a025. [DOI] [PubMed] [Google Scholar]
- Berget S. M., Moore C., Sharp P. A. Spliced segments at the 5' terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C. Exons encode protein functional units. Nature. 1979 Feb 22;277(5698):598–598. doi: 10.1038/277598a0. [DOI] [PubMed] [Google Scholar]
- Brack C., Tonegawa S. Variable and constant parts of the immunoglobulin light chain gene of a mouse myeloma cell are 1250 nontranslated bases apart. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5652–5656. doi: 10.1073/pnas.74.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Mandel J. L., Chambon P. Ovalbumin gene is split in chicken DNA. Nature. 1977 Nov 24;270(5635):314–319. doi: 10.1038/270314a0. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Crick F. Split genes and RNA splicing. Science. 1979 Apr 20;204(4390):264–271. doi: 10.1126/science.373120. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Implications of RNA-RNA splicing in evolution of eukaryotic cells. Science. 1978 Dec 22;202(4374):1257–1260. doi: 10.1126/science.364651. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand R. M., Scheraga H. A. The influence of long-range interactions on the structure of myoglobin. Biochemistry. 1968 Aug;7(8):2864–2872. doi: 10.1021/bi00848a024. [DOI] [PubMed] [Google Scholar]
- Fronticelli C., Gold R. Conformational relevance of the beta6Glu replaced by Val mutation in the beta subunits and in the beta(1-55) and beta(1-30) peptides of hemoglobin S. J Biol Chem. 1976 Aug 25;251(16):4968–4972. [PubMed] [Google Scholar]
- GROS P., LABOUESSE B. [Clostripain, a protease from Clostridium histolyticum. 2. Specificity]. Bull Soc Chim Biol (Paris) 1960;42:559–568. [PubMed] [Google Scholar]
- Gilbert W. Why genes in pieces? Nature. 1978 Feb 9;271(5645):501–501. doi: 10.1038/271501a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Leder A., Miller H. I., Hamer D. H., Seidman J. G., Norman B., Sullivan M., Leder P. Comparison of cloned mouse alpha- and beta-globin genes: conservation of intervening sequence locations and extragenic homology. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6187–6191. doi: 10.1073/pnas.75.12.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchins J., Beychok S. Far-ultraviolet stopped-flow circular dichroism. Science. 1978 Jan 27;199(4327):425–426. doi: 10.1126/science.619462. [DOI] [PubMed] [Google Scholar]
- Mitchell W. M., Harrington W. F. Purification and properties of clostridiopeptidase B (Clostripain). J Biol Chem. 1968 Sep 25;243(18):4683–4692. [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Stratmann M., Groner B., Wurtz T., Land H., Giesecke K., Sippel A. E., Schütz G. Chicken lysozyme gene contains several intervening sequences. Proc Natl Acad Sci U S A. 1979 Jan;76(1):76–80. doi: 10.1073/pnas.76.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Seidman J. G., Peterlin B. M., Sullivan M., Maizel J. V., Leder P. Intervening sequence of DNA identified in the structural portion of a mouse beta-globin gene. Proc Natl Acad Sci U S A. 1978 Feb;75(2):725–729. doi: 10.1073/pnas.75.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waks M., Yip Y. K., Beychok S. Influence of prosthetic groups on protein folding and subunit assembly. Recombination of separated human alpha-and beta-globin chains with heme and alloplex interactions of globin chains with heme-containing subunits. J Biol Chem. 1973 Sep 25;248(18):6462–6470. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Yip Y. K., Waks M., Beychok S. Influence of prosthetic groups on protein folding and subunit assembly. I. Conformational differences between separated human alpha- and beta- globins. J Biol Chem. 1972 Nov 25;247(22):7237–7244. [PubMed] [Google Scholar]
- Yip Y. K., Waks M., Beychok S. Reconstitution of native human hemoglobin from separated globin chains and alloplex intermediates. Proc Natl Acad Sci U S A. 1977 Jan;74(1):64–68. doi: 10.1073/pnas.74.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]



