Abstract
Background
Phosphorus (P) is essential for plant growth and development. Phosphate (Pi) transporter genes in the Pht1 family play important roles in Pi uptake and translocation in plants. Although Pht1 family genes have been well studied in model plants, little is known about their functions in soybean, an important legume crop worldwide.
Principal Findings
We identified and isolated a complete set of 14 Pi transporter genes (GmPT1-14) in the soybean genome and categorized them into two subfamilies based on phylogenetic analysis. Then, an experiment to elucidate Pi transport activity of the GmPTs was carried out using a yeast mutant defective in high-affinity Pi transport. Results showed that 12 of the 14 GmPTs were able to complement Pi uptake of the yeast mutant with Km values ranging from 25.7 to 116.3 µM, demonstrating that most of the GmPTs are high-affinity Pi transporters. Further results from qRT-PCR showed that the expressions of the 14 GmPTs differed not only in response to P availability in different tissues, but also to other nutrient stresses, including N, K and Fe deficiency, suggesting that besides functioning in Pi uptake and translocation, GmPTs might be involved in synergistic regulation of mineral nutrient homeostasis in soybean.
Conclusions
The comprehensive analysis of Pi transporter function in yeast and expression responses to nutrition starvation of Pht1 family genes in soybean revealed their involvement in other nutrient homeostasis besides P, which could help to better understand the regulation network among ion homeostasis in plants.
Introduction
As an essential but frequently less available nutrient for plant growth, phosphorus (P) is taken up by plants as orthophosphate (H2PO4 −, Pi) mainly through Pi transporters and driven by a proton gradient generated by plasma membrane H+-ATPases [1]. In native soil solution, Pi concentration is always less than 10 µM because it is easily bound by either soil organic matter or minerals [2], [3], [4], [5]. Meanwhile, the Pi concentration in the cytoplasm of plant cells is generally greater than 10 mM [6]. Therefore, plants must have specialized transporters to transport Pi from soil solution to plant cells against a large concentration gradient at the root-soil interface. Recent genome sequence analysis and experimental evidence indicated that plants contained a wide variety of Pi transporter families, including Pht1, Pht2, Pht3, Pht4, which were defined by protein sequence, structure, locations and functions [4], [5], [7], [8], [9], [10], [11].
Among the Pi transporter families in plants, Pht1 family is most widely studied. All the members in the Pht1 family have the same predicted structure, including 12 putative membrane-spanning domains, hydrophilic N- and C-terminals, a hydrophilic loop between transmembrane segments (TM) six and seven, a putative glycosylation site in TM10 and two cytoplasmic phosphorylation sites [12]. Since cloning of the first Pht1 family gene from Arabidopsis [13], many Pht1 genes have been isolated from a number of plant genomes, including Arabidopsis [14], graminaceous species [15], [16], [17], [18], [19], solanaceous species [20], [21], [22] and legumes [23], [24], [25], [26]. Most Pi transporter genes in the Pht1 family are expressed in roots, while a few are expressed in aerial parts, including leaves, stems, cotyledons, tubers, flowers, grains and seeds [14], [15], [19], [27], [28], implying their potential involvement in Pi internal translocation. Pi transporter genes in the Pht1 family from Arabidopsis and rice, containing 9 and 13 members, respectively, have been comprehensively studied and well characterized [7], [14], [16], [29]. All results indicate that there are distinct functions and different responses to P deficiency among Pht1 family genes.
In Arabidopsis, eight of nine Pi-transporter genes are expressed in roots. Fusion of the promoter regions from these genes with the GUS reporter gene indicates that four of them are highly expressed in the root epidermis and the expression is enhanced by P deficiency. Additionally, some members are expressed in shoot tissues, such as pollen grains, and thereby implying a wider role in Pi uptake and remobilization [14]. In rice, nine out of thirteen Pht1 transporter genes are expressed in both Pi-deprived roots and leaves. The transcript levels of OsPT2, OsPT3, OsPT6 and OsPT7 are significantly enhanced by P deficiency in roots. The expressions of OsPT1 and OsPT8 are abundant in both roots and leaves at two P levels [29], [30]. For legumes, Pi-transporters in the Pht1 family in Medicago truncatula have been well studied. Among them, MtPT1, MtPT2, MtPT3 and MtPT5 are highly expressed in Pi-deprived roots, but less with addition of high Pi [24]. In Lotus japonicus, 3 Pi transporters genes in Pht1 family have been isolated [31].
Soybean (Glycine max (L.) Merr.) is one of the most widely grown leguminous crops in the world. However, soybean production is limited by various environmental factors, especially by low P availability in soils [32]. It might help us to find some new approaches to improve the P efficiency of soybean through understanding the detailed characteristics of Pht1 genes. Compared to the Pht1 genes in Arabidopsis and rice, much less work has been done in soybean. Recently, two members from the soybean Pht1 family (GmPT1 and GmPT2) were reported to be plasma membrane proteins, and complementation analysis in yeast indicated that they might be constitutively expressed low affinity Pi transporters [23]. Therefore, these two Pi transporters might not be critically involved in response to P deficiency. The discovery and functional characterization of Pi transporters responsible for plant Pi uptake under limited P conditions should be more important to provide a clearer understanding of how plants coordinate the use of Pi to support growth and development.
Plant growth in soils can also be constrained by other nutrients rather than P, and therefore plants might evolve adaptive mechanisms to cope with multiple nutrient stresses. However, only a few studies have reported on interactions among P and other nutrients. For example, coincident potassium (K) and P deprivation induced the transcriptions of a MAP kinase gene, transcription factors and nutrient transporters in tomato [33]. After long term low P treatment, some iron (Fe) and sulfur (S) transporters were up regulated in Arabidopsis [34]. Cross-talks between P and Fe have also been demonstrated in rice [35]. The expression of AAR6 was up-regulated by N, P, or K deprivation, indicating that shared nutrient signaling transduction pathways might exist in higher plants [36]. As the most responsive gene family to Pi starvation, Pht1 family genes are likely to be involved in those shared pathways. But up to date, there have been no reports about regulation of Pht1 family genes by nutrients other than P.
In this study, 14 Pi transporter genes from the Pht1 family (GmPTs) were isolated from the soybean genome. A yeast Pi uptake-defective mutant was used to characterize the Pi uptake kinetics of GmPTs through radioactive 33P uptake analysis. Tissue specificity and regulation by P as well as N, K and Fe starvation of all 14 GmPTs were analyzed through quantitative RT-PCR (qRT-PCR).
Results
Soybean Pi Transporter Genes in the Pht1 Family
A search of the Phytozome soybean genome database (http://www.phytozome.net/soybean) yielded a total of 14 sequences identified as being related to high-affinity Pi transporters. According to the recommended nomenclature for plant Pi transporters (http://www.botanik.uni-koeln.de/bucher_ppnomenclature.html), these 14 identified genes were named as GLYma;Pht1;1 through GLYma;Pht1;14, with the order being based on their chromosome locations. For simplification, the Pi transporters are called GmPT1 through GmPT14 in this report (Table 1). BLAST analysis against the Pfam database (http://www.sanger.ac.uk/resources/databases/pfam.html) showed that they all belonged to the MFS family. The full-length cDNAs and amino acid sequences of these 14 Pi transporters were available in the Phytozome website. All 14 GmPTs exhibited a high degree of homology and were comparable in length, calculated molecular weight and theoretical pI value (Table 1). The distribution of GmPTs on soybean chromosomes is uneven and involves only 8 of 20 chromosomes. Chromosome 10 had the highest number of GmPTs genes with 4, followed by chromosome 20 with 3 (Table 1).
Table 1. Members of Pi transporter genes in the Pht1 family from soybean.
| Gene name | Accessionnumber | Locus tag. | aa | kD | pI |
| GmPT1 | FJ814697 | Glyma02g00840 | 533 | 58.47 | 8.31 |
| GmPT2 | FJ814696 | Glyma03g31950 | 539 | 59.27 | 8.52 |
| GmPT3 | FJ814701 | Glyma07g34870 | 516 | 58.31 | 8.64 |
| GmPT4 | JQ518269 | Glyma10g00950 | 533 | 58.42 | 8.54 |
| GmPT5 | FJ814694 | Glyma10g04230 | 521 | 57.31 | 8.63 |
| GmPT6 | FJ814693 | Glyma10g33020 | 502 | 55.44 | 9.28 |
| GmPT7 | FJ814695 | Glyma10g33030 | 536 | 58.73 | 8.34 |
| GmPT8 | FJ814700 | Glyma13g08720 | 519 | 57.64 | 8.56 |
| GmPT9 | FJ814698 | Glyma14g28780 | 525 | 58.24 | 8.33 |
| GmPT10 | FJ814699 | Glyma14g36650 | 529 | 58.19 | 7.91 |
| GmPT11 | JQ518270 | Glyma19g34710 | 539 | 59.27 | 8.25 |
| GmPT12 | FJ814692 | Glyma20g02660 | 506 | 56.78 | 8.63 |
| GmPT13 | FJ789662 | Glyma20g34610 | 536 | 58.63 | 7.63 |
| GmPT14 | JQ518271 | Glyma20g34620 | 527 | 58.11 | 8.93 |
Their accession number, locus tag on chromosomes, the respective numbers of amino acids (aa), the calculated molecular mass in kiloDaltons (kD), and theoretical pI value are given.
Like Pht1 transporters in other plant species, the PT genes in soybean encode proteins with comparable predicted secondary structures, with each being characterized by 12 hydrophobic domains presumably spanning the plasma membrane. Amino acid sequences of soybean Pi transporter homologs were aligned and compared with each other, and conserved amino acid residues were found (Figure S1). We calculated the relatedness of soybean Pht1 transporters using the DNAMAN computer program, with the results suggesting that the percentage of identical protein sequences ranges from 48% to 99% (Table S1). The highest percentage of amino acid sequence identity was found between GmPT2 and GmPT11 (99.27%), followed by GmPT6 and GmPT14 (98.23%). The lowest identity was observed between GmPT3 and GmPT10 (47.63%). GmPT3 and GmPT12 had a very high amino acid sequence identity of 93.89%, but both proteins had comparatively low amino acid sequence identity with all the other 12 Pht1 Pi transporter proteins.
Phylogenetic Analysis of GmPTs
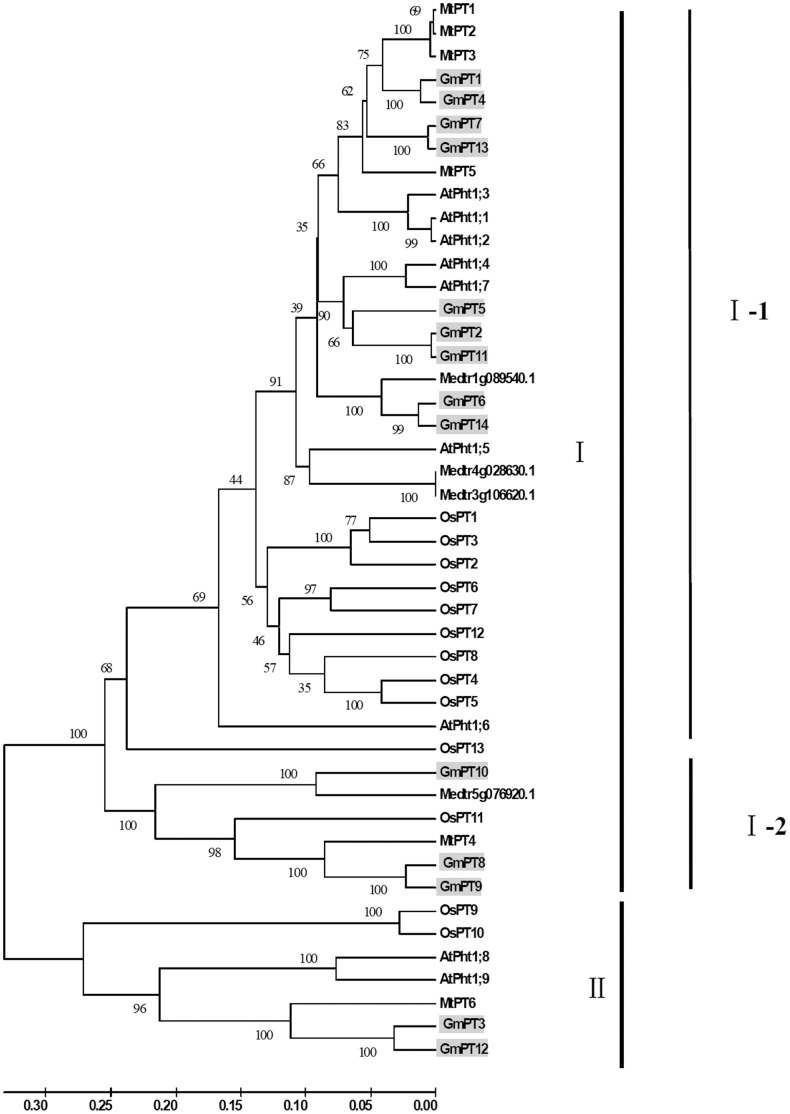
Combining GmPT1 through GmPT14 with Pi transporter protein sequences in rice, Arabidopsis and Medicago truncatula, we constructed a phylogenetic tree using neighbor-joining analysis in the MEGA 4.1 program (Figure 1). The phylogenetic results demonstrated that like the other three species, soybean GmPTs were divided into two distinct groups with strong bootstrap support. Group I contained 12 of the 14 GmPTs, 9 of 10 MtPTs, 7 of the 9 AtPhts and 11 of the 13 OsPTs. Group I could be further divided into two subgroups: I-1 and I-2. Subgroup I-1 contained 9 GmPTs, 7 MtPTs, 7 AtPhts and 10 rice Pi transporters. It is interesting to find that in subgroup I-2, GmPT8, GmPT9 and GmPT10 were clustered with the mycorrhiza-specific OsPT11 from rice [16] and MtPT4 from Medicago truncatula [24], [25], and no Arabidopsis AtPTs, implying possible roles for these three GmPTs in Arbuscular Mycorrhizal (AM) symbiosis. Group II only included a few Pi transporters, a pair from soybean (GmPT3 and GmPT12), a pair from Arabidopsis (AtPht1;8 and AtPht1;9), MtPT6 from Medicago truncatula and a pair from rice (OsPT9 and OsPT10). Both group I and group II contained Pht1 family Pi-transporters from all four species, indicating that divergence of Pi-transporters preceded divergence of dicots and monocots. By exploring the intron (the noncoding sequence between two coding sequences within a gene) and exon (the protein-coding region in the DNA) structures of GmPTs, we found the genes from the same group had the similar intron/exon structures, such as GmPT3 and GmPT12 in group II, their structures were totally different from those in Group I by characterized with three exons and two long introns, indicating the divergence of structures and functions of different GmPTs (Figure S2).
Figure 1. Phylogenetic tree of soybean, Arabidopsis, rice and Medicago plant Pi transporter proteins in Pht1 family.
Transporters and corresponding plant species are as follows: rice (Oryza sativa), OsPT1 through OsPT13 [16]; Arabidopsis (Arabidopsis thaliana), AtPht1;1 through AtPht1;9 [14]; Medicago (Medicago truncatula) MtPT1 through MtPT6 [68] and other four PT proteins obtained in Phytozome (http://www.phytozome.net/medicago), soybean (Glycine Max), GmPT1 through GmPT14 (this work).
Analysis of Pi Transport Activities of GmPTs in a Yeast Strain Defective in Pi-uptake
To analyze and compare the Pi transport activities of all the GmPTs, their coding regions were separately cloned into a yeast expression vector (p112A1NE), under the control of the yeast alcohol dehydrogenase promoter. The constructs were separately introduced into a yeast Pi transport mutant MB192, which lacks the function of the high-affinity Pi transporter gene PHO84. An empty vector was also transformed to be used as a control.
Because the Pht1 transporters are members of the H+/Pi symporter family, the pH dependence of GmPTs during Pi transport was assayed by measuring the optical density of the yeast cell lines at pH values ranging from 4 to 8. The pH optima for most of the yeast mutant cells carrying p112-GmPTs was 6, in comparison to the wild type, in which the pH optima ranged from 4 to 6. Therefore, the pH value was set as 6 in the subsequent studies.
The yeast MB192 strain grows poorly when supplied with limiting amounts of Pi. As shown in Figure 2, under low P conditions (20 µM), all the GmPT transformants grew much better than the control, suggesting that GmPTs can complement the yeast Pi-uptake mutant under Pi-limiting conditions (Figure 2).
Figure 2. Complementation of a yeast inorganic phosphate (Pi) transport mutant by GmPTs genes.
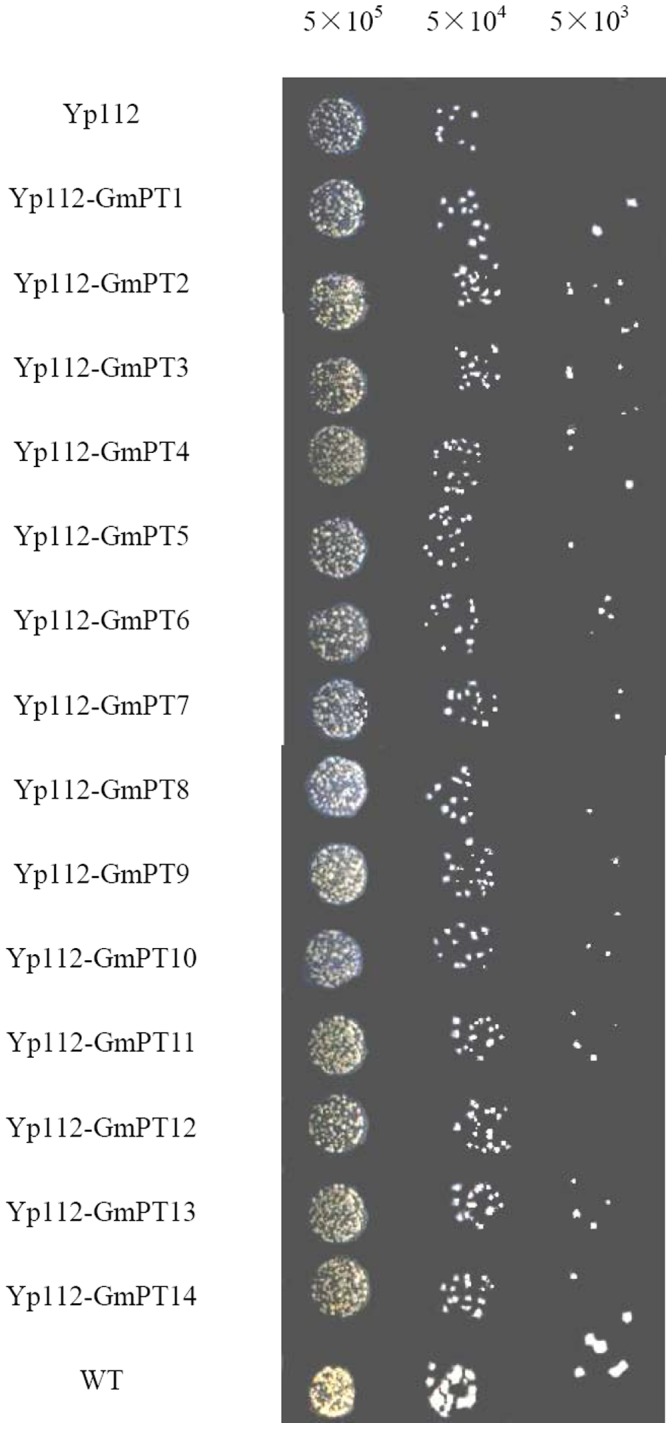
Yeast MB192 cells harboring either an empty expression vector (control) or the candidate Pht1 ORF(open reading frame), transformants were grown in YNB medium to an OD600 ≈0.8, then washed by 3% glucose with centrifugation at 1500 g, 4°C, and suspended in phosphate free YNB medium to OD600 ≈1.0. Different number cells (5×105, 5×104, 5×103) were applied to Pi-limiting medium (20 µM, pH 6.0) then incubated at 30°C for 3 d.
To make sure the effects of the expressions of GmPT genes in yeast on the growth of transformed yeast cells are Pi-deficiency dependent, but not just because of the expressions of GmPT genes, we analyzed the kinetic growth of Yp112-GmPTs transformants, wild type and empty vector control in normal YNB liquid medium with 2 mM Pi, and found that there were no significant growth differences between empty vector control and all the 14 Yp112-GmPTs transformants (Figure S3D), But under certain low P conditions, Yp112-GmPTs transformants always grew better than the empty vector control (Figure S3A, B, C), indicating the complementation of GmPTs was really Pi-deficiency dependent.
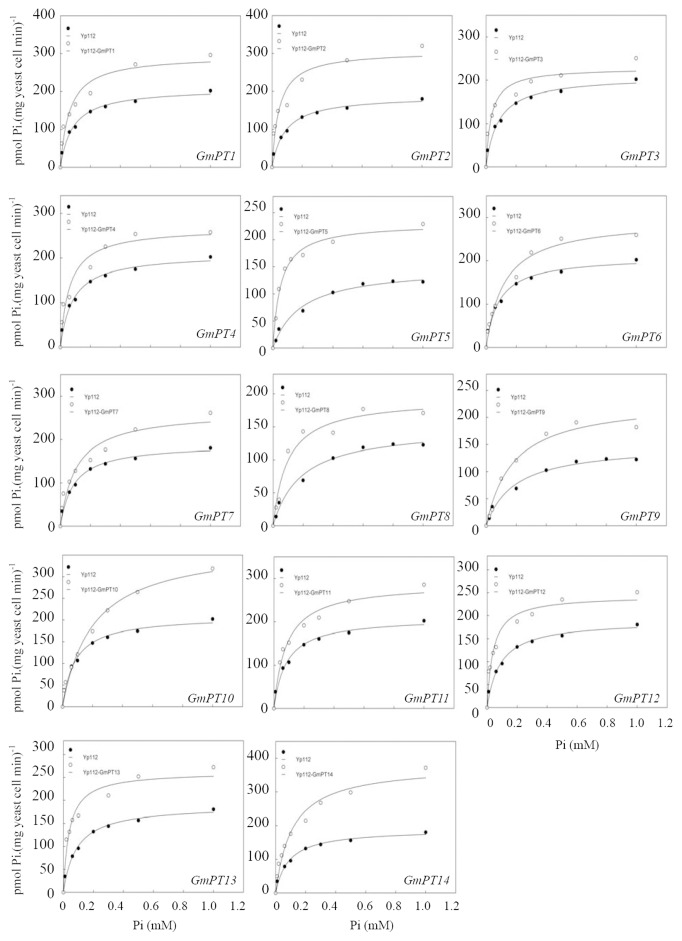
Furthermore, in order to determine the kinetic properties of GmPTs, 33P was employed in Pi-uptake experiments using transformed yeast cells. A Line weaver-Burk plot indicated that Pi uptake mediated by 12 GmPT genes followed Michaelis-Menten kinetics (Figure 3) with the estimated Km values ranged from 25.70±1.63 µM to 116.30±10.00 µM (mean±SE) (Table 2), while only GmPT6 and GmPT10 showed no difference when Pi concentration in the growth medium was less than 0.1 mM. The results from kinetic studies above demonstrated that most of the GmPTs were high-affinity Pi transporters.
Figure 3. Kinetic analysis of inorganic phosphate (Pi) uptake in yeast.
The non-linear regression of total Pi uptake by strain Yp112-GmPTs versus external Pi concentration at pH 6 were used to estimate the apparent Km value for Pi uptake. All the results were calculated from the three independent experiments.
Table 2. Kinetic parameter estimates of GmPTs-mediated inorganic phosphate (Pi) transport.
| Yeast | Km(µM) | Vmax(µM/g [dry weight]·min) |
| Yp112-GmPT1 | 67.30±15.60 | 298.30±24.40 |
| Yp112-GmPT2 | 44.00±12.90 | 295.00±20.90 |
| Yp112-GmPT3 | 30.00±8.60 | 226.70±16.30 |
| Yp112-GmPT4 | 65.30±13.30 | 282.30±43.28 |
| Yp112-GmPT5 | 25.70±1.63 | 231.30±18.80 |
| Yp112-GmPT6 | 153.00±44.00 | 281.70±37.60 |
| Yp112-GmPT7 | 105.30±20.00 | 247.00±24.50 |
| Yp112-GmPT8 | 46.00±5.60 | 231.50±5.50 |
| Yp112-GmPT9 | 88.00±8.90 | 240.30±7.10 |
| Yp112-GmPT10 | 231.00±15.60 | 400.00±25.00 |
| Yp112-GmPT11 | 79.00±8.30 | 271.30±45.00 |
| Yp112-GmPT12 | 45.30±14.53 | 245.30±15.60 |
| Yp112-GmPT13 | 32.30±1.60 | 265.70±32.50 |
| Yp112-GmPT14 | 116.30±10.00 | 373.00±14.10 |
| Yp112 | 127.00±8.50 | 154.70±3.60 |
Km and Vmax for yeast strain MB192 expressing the indicated GmPTs or carrying the empty expression vector (control) were determined at pH 6.0. The GmPTs mediated 33Pi uptake velocities, calculated according to their total Pi transport, following the Michaelis-Menten kinetics equation. Values shown are means ± SE for three independent experiments.
Expression Patterns of GmPTs as Regulated by P Availability in Different Tissues
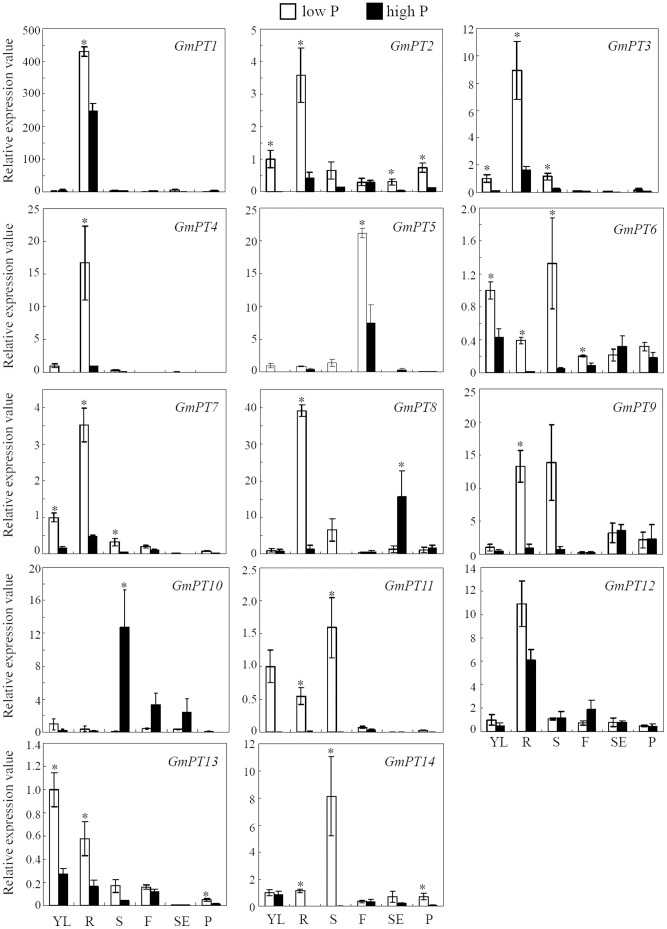
According to the nucleic acid sequences (Text S2), we designed gene-specific primers for the 14 GmPTs genes (Table S2). Tissue specificity and P response of the GmPTs were investigated using qRT-PCR. The main expression pattern under high P conditions was consistent with the results from the two soybean transcriptome atlases (Table S3) [37], [38], such as the expression levels of most GmPTs were low, and roots had the highest number of GmPTs genes to express in, indicating the important roles of Pi transporters in Pi transport and absorption in roots; GmPT5 mainly expressed in flowers, implying possible functions in Pi transport for source to sink (Figure 4).
Figure 4. Spatial Expression pattern analysis for the 14 GmPTs as related to P availability.
Plants were grown on low P (added 5 µM P as KH2PO4, open bars) and high P (added 500 µM P as KH2PO4, closed bars) conditions. Young leaves (YL), roots (R), stems (S) and flowers (F) were sampled 18 days after treatment initiation, and young pods (P) and seeds (SE) were sampled 29 days after treatment initiation. Each bar is the mean of three biological replications with standard error. Note: different scales are used in the graphs; asterisks indicate significant differences of GmPTs expression in certain tissues under low P and high P conditions in t-tests.
The expression of GmPLDZ (Glyma20g38200) which has been well demonstrated as a Pi starvation induced gene [39], was also analyzed presently. The results found that the GmPLDZ expression was highly enhanced under low P conditions, especially in leaves (Figure 5A), proving that the P treatments in the present study were sufficient for analyzing the responses of Pi starvation induced genes. Like Pi transporters in other plant species, the expressions of GmPTs, except GmPT10, were highly induced by P deficiency (Figure 4). Seven, including GmPT1, GmPT2, GmPT3, GmPT4, GmPT7 GmPT8 and GmPT12, were predominantly expressed in roots. GmPT5 and GmPT14 were mainly expressed in flowers and stems, respectively. GmPT9 and GmPT13 were highly induced in roots and stems, and young leaves and roots, respectively. GmPT6 and GmPT11 were expressed highly in young leaves, roots and stems (Figure 4). In addition, Pi starvation induced de novo synthesis of GmPT3 and GmPT4 in roots and GmPT11 in young leaves, roots and stems. In contrast, GmPT10 was expressed in stems, flowers and seeds at high P level. Finally, low or even undetectable expressions of GmPTs were observed in pods (Figure 4).
Figure 5. Expression of P, N, K or Fe responsive genes to different nutrient stresses.
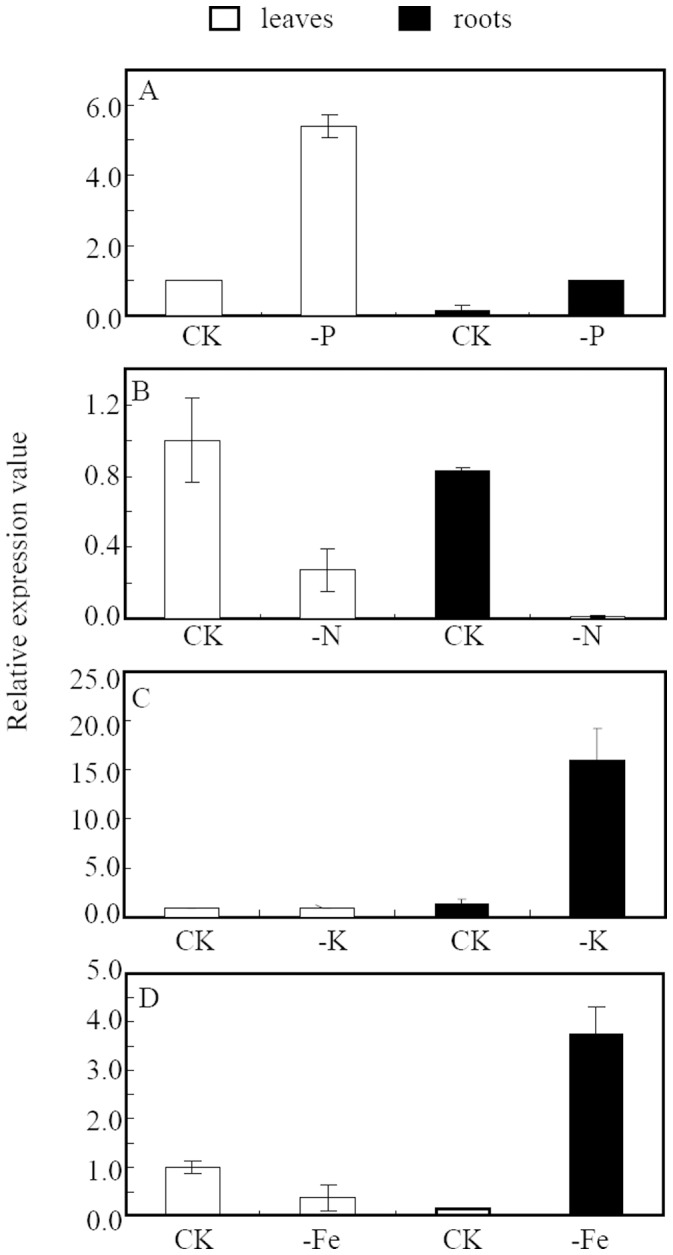
14-day old soybean seedlings were treated with N (−N), K (−K) and Fe (−Fe) deficiencies (see Experimental procedures for details). Seedlings grown under normal solution were used as controls (CK, added 500 µM P as KH2PO4). The expression levels in shoots and roots were analyzed by quantitative real-time PCR. Soybean gene PLDZ (Glyma20g38200) was used as low P responsive gene (A), NiR (Glyma02g14910) for low N treatment (B), HAK (Glyma3g42480) for low potassium treatment (C) and IRT (Glyma07g34930) for low Fe treatment (D), respectively. Each bar was the mean of three biological replications with standard error.
Responses of GmPTs to Nitrogen, Potassium and Iron Deficiency
To examine the potential induction of GmPT genes by other nutrient deficiencies, transcript abundance was assessed by qRT-PCR in soybean plants separately grown in nutrient solution deficient in nitrogen (N), potassium (K) or iron (Fe) for 14 days. Three marker genes, including GmHAK (Glyma3g42480, a K transporter) and GmIRT (Glyma07g34930, an iron transporter) which had been known respectively enhanced by K or Fe deficiency [40], [41], and GmNiR (Glyma02g14910, a nitrite reductase gene) which was repressed by N deficiency [42], were used to monitor the treatmental conditions. As expected, the expression of GmHAK, GmIRT and GmNiR was significantly regulated by K, Fe or N deficiency, respectively (Figure 5B, C, D).
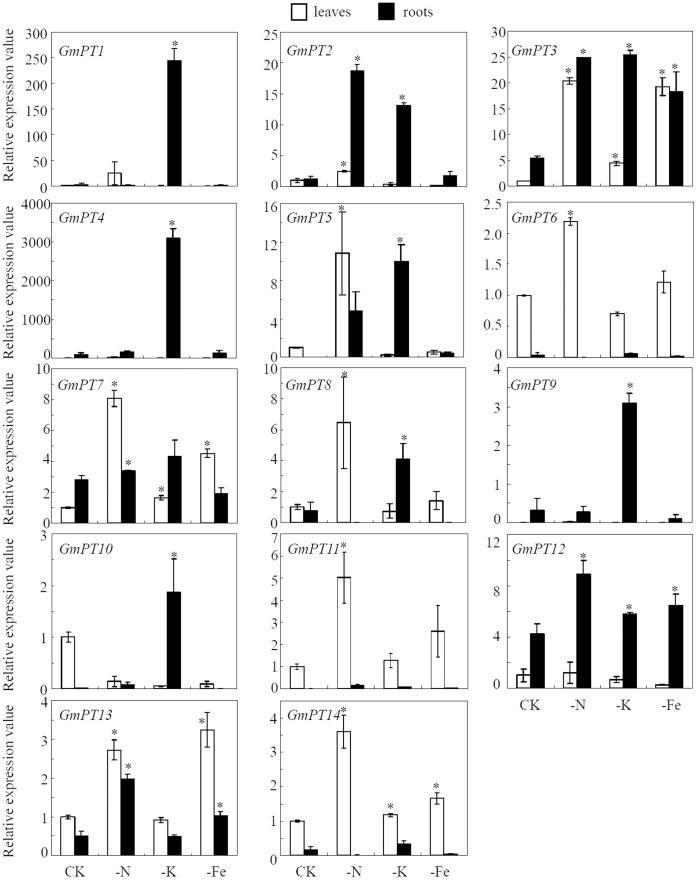
The expressions of GmPT genes in response to N, K, or Fe deficiency were shown in Figure 6. The 14 Pi transporter genes had very low expressions in leaves and roots under normal conditions. All of the gene expression levels were modified by one or more of the three stresses in different tissues. Under N deficiency conditions, the expressions of 10 genes were up-regulated more than 2-fold. Among them, GmPT6, GmPT7, GmPT8, GmPT11 and GmPT14 were increased in leaves, and GmPT12 was in roots. N deficiency enhanced the expressions of GmPT2, GmPT3, GmPT5 and GmPT13 in both leaves and roots. K deficiency enhanced the expressions of 8 GmPTs genes. GmPT3 was up-regulated in both leaves and roots, while the expressions of the other 7 GmPTs, including GmPT1, GmPT2, GmPT4, GmPT5, GmPT8, GmPT9 and GmPT10, were only enhanced in roots. Under Fe deficiency, GmPT3, GmPT12 and GmPT13 were up-regulated. The expression levels of the remaining GmPT genes were not altered significantly under Fe deficiency. Interestingly, GmPT3 was the only one gene with expression up-regulated by N, P or K deficiency simultaneously in both leaves and roots (Figure 6), suggesting that GmPT3 might be involved in an universal regulation network in response to multiple nutrient deficiencies.
Figure 6. Responses of GmPTs to different nutrient stresses.
Ten-day old soybean seedlings were treated with N (−N), K (−K) and Fe (−Fe) deficiencies (see Experimental procedures for details). Seedlings grown under normal solution were used as controls (CK, added 500 µM P as KH2PO4). Asterisks indicated the significant differences of GmPTs expression between nutrients deficient stresses and normal conditions in Student’s t-tests.
Discussion
Phosphorus is particularly critical for legume growth due to the huge demands for protein and oil synthesis, as well as, for biological N2 fixation [43]. However, low P availability is a worldwide constraint for crop growth in most soils. Therefore, to understanding Pi uptake and translocation in soybean can dramatically facilitate soybean adaptation to the low P soils. Phosphate transporter genes in the Pht1 family have been reported playing important roles in Pi uptake and translocation in plants [14], [16], [17], [19], [22], [24], [44], [45]. To date, information on this family is limited in soybean. In the current study, we identified and isolated 14 members of PTs in the Pht1 family from the soybean genome, and characterized their functions in Pi uptake using yeast mutants defective in high affinity Pi transport. The expression patterns in terms of tissue specificity, responses to P deficiency and other nutrient stresses were also analyzed.
Soybean Pi Transporter Genes in Pht1 Family
Like Pht1 family genes in other species, soybean PTs in the Pht1 family exhibited a high degree of homology, characterized by high protein identity (Table S1) and similar hydrophobic domains presumably spanning the plasma membrane (Figure S1). Interestingly, phylogenetic analysis of GmPT1 through GmPT14, along with Pi transporter protein sequences from Medicago truncatula, Arabidopsis, and rice, showed a similar pattern of evolutionary divergence within dicotyledons (Arabidopsis, Medicago and soybean) and monocotyledons (rice). These phylogenetic relationships among the GmPTs (Figure 1), may reflect biochemical and functional differences among different types of PTs.
With well-supported bootstrap values, GmPT8 and GmPT9 were clustered with the mycorrhiza-specific OsPT11 from rice [16] and MtPT4 from Medicago truncatula. Since OsPT11 is specifically induced by AM symbiosis, and MtPT4 is critical for AM symbiotic Pi transport and development, the absence of MtPT4 will lead to the inability of the fungus to proliferate within roots, and termination of symbiosis [25]. We speculate that, given the phylogenetic proximity, GmPT8 and GmPT9 are likely to play important roles in the symbiosis between soybean and AM fungus. GmPT10 was classified into the same subgroup with GmPT8 and GmPT9, though with a weaker bootstrap value than that between GmPT8 and GmPT9, implying a possible role for GmPT10 in symbiosis with AM fungi, but its functions might be different from those of GmPT8 and GmPT9 in AM roots.
GmPT3 and GmPT12, which had relatively lower protein sequence identity with the other GmPTs (Table S1), were classified into the group II (Figure 1) of Pht1 genes. By exploring gene structures, we found these two genes shared a similar and slightly unusual structure with a relatively large intron in the coding sequence and more exons than the other GmPTs, which had only one or two exons (Figure S2). These structural differences might be related to the special functions of GmPT3 and GmPT12 differing from other GmPTs. Recent reports have indicated that the size of exon and intron, and their intergenic distance are correlated with gene expression levels and expression breadth in soybean [46]. The relationship between gene structures and functions of GmPTs needs to be further analyzed.
Functions of GmPTs in Pi Uptake
Three systems have been well accepted to functionally characterize plant Pi transporters, including the complementation of yeast mutants defective in Pi transport, monitoring Pi uptake using Xenopus laevis oocytes and ectopic expression of Pi transporters in plant suspension cells [15], [28], [30], [47], [48], [49]. The yeast mutants defective in Pi transporters are most widely used for characterizing the kinetic properties of many PTs from different plants. With complemented yeast mutants, kinetic studies allow for quantifying the affinity of transporters [15], [47], [50]. However, few whole Pht1 families in crops have been functionally analyzed [15], [30]. The yeast Pi transport mutant MB192, which lacks function of the high-affinity Pi transporter gene PHO84, has been used to analyze Pi transporters from several crop species [30], [51]. In the present study, we used the MB192 yeast system to work on kinetic analysis of all 14 GmPTs, and found that 12 of 14 GmPTs acted as high-affinity Pi transporters (Table 2, Figure 3), implying their functions in high-affinity uptake and transport of Pi from low P soils. However, among the five reported Pht1 family genes in Medicago truncatula, only MtPT5 showed high affinity for Pi uptake [24], [26]. The possible reason why we could identify so many high-affinity Pi transporters here using the yeast system is that due to the existence of native Pi transport systems, the expression of high-affinity Pi transporters in yeast cells might display altered transport properties. Some high-affinity Pi transporters, such as AtPHT1;1 and HvPHT1;1, were not able to complement the yeast mutant. We also found that among the tested GmPTs, although GmPT6 and GmPT10 could complement the Pi absorption of the yeast mutant MB192 to a certain extent (Figure 2), but their Km values were higher than that of the tested mutant based on the kinetic analysis (Figure 3, Table 2). Therefore, we still characterized them as low-affinity Pi transporters. However, there is not a defined Km value for categorizing the Pi affinity of PTs, and the external Pi concentrations used here were limited, further works are needed to test weather these two transporters have dual-affinity for Pi uptake.
Characterization of the GmPTs in Response to P Availability
Recently, two soybean transcriptome atlases providing a record of high-resolution soybean gene expressions have been reported by two groups using next generation sequencing technique [37], [38]. The expression patterns of the 14 GmPTs with normal Pi supply in the present study are well consistent with those atlases. Most of the GmPTs showed low expressions in most tissues, and some were undetectable (Figure 4) [37], [38]. Differently, we also found that GmPT10 was mainly expressed in stems, flowers and seeds in high P conditions, implying it might be a low-affinity Pi transporter functioning in internal Pi translocation. The differences among our results and the previously reported transcriptome atlases might be due to the different sampling stages and growth systems. In addition to work on normal growth conditions, we tested the responses of the 14 GmPTs to Pi starvation, and found that most of the Pht1 genes had elevated transcript levels under low P conditions, while a few of them were differentially expressed between roots and aerial parts of the plants as previously reported [13], [14], [17], [24], [28], [47], [50]. Thirteen GmPTs were induced or increased under low P conditions in soybean (Figure 4), indicating their roles in soybean adaptation to low P availability. Six GmPTs were mainly expressed in roots, and the others were differently expressed in stems, flowers and seeds. As a whole, these results suggest pivotal functions for GmPTs in both Pi acquisition and translocation. Low or no expressions of GmPTs were detected in pods (Figure 4), indicating rare Pi exchange occurred or other Pi transporters function in this organ. The differential expressions of individual genes in different plant organs imply that there might be additional functions for the respective genes other than Pi uptake.
Involvement of GmPTs in N, K and Fe Signals
Previous reports strongly suggest that there might be crosstalks among ion signals in response to different nutrient stresses, the expressions of ion transporters might be involved in a process that facilitates mineral nutrient homeostasis [52], [53], [54], [55]. Wang et al [55] used a high-density array from tomato roots to test the expression profiling of 1,280 genes in N, K or Fe deficiency, and found the expressions of some Pi, K and Fe transporter genes were up-regulated by all three nutrient deficiencies. This suggests some transporter genes might be involved in the coordinated and coregulated uptake of these essential nutrient elements. Nitrogen, P, K, and Fe deficiencies are well known as important limiting factors to agricultural production [56], but no research has been reported on the responses of Pi transporters to N, K or Fe deficiency. Therefore, studying expressions of GmPTs genes under nutrient deficiencies other than P deficiency could reveal possible functions of Pi transporters in multiple mineral nutrient homeostasis.
The rice PHO1 gene family has been well documented to play important roles in Pi translocation from roots to shoots. Furthermore, one of the PHO1 family genes was up-regulated by N starvation [57], implying crosstalks exsiting between P and N signals in rice. Recent work on the NLA (N limitation adaptation) gene in Arabidopsis indicates that there is an antagonistic crosstalk between N and P deficiency [58]. It has been reported that NLA is involved in adaptive responses to low N conditions, where nla mutant plants display abrupt early senescence. Further analysis found the two suppressors of the nla mutation can impart the nla mutant phenotype to NLA wild type plants, and these suppressors have been proved to be the Pi transport-related genes, PHF1 and PHT1.1. In addition, NLA expression is regulated by the low-Pi induced microRNA miR827, suggesting that Pi transporters could directly affect plant N nutrition. In the present study, we found that most of the soybean PTs genes were significantly up-regulated under N deficiency, indicating that at least some of the Pi transporters are involved in N signaling pathways in soybean. Increased expression of a high-affinity Pi transporter gene was also found under low K in barley [59], showing the existence of interactions between P and K signals. This is also supported by our results in which 8 GmPTs were regulated by K deficiency (Figure 6). The underlying molecular mechanisms and pathways involved in these crosstalks need to be further researched.
Iron is the most studied nutrient element for interactions with P due to their strong precipitation [60]. It is well accepted that P and Fe deficiency have similar effects on the differentiation of epidermal cells, and subsequently affect root growth, including lateral root and root hair formation [61], [62], [63]. In Arabidopsis, primary root growth was inhibited by P deficiency due to Fe toxicity in the root tip [64]. But no P and Fe interactions related to Pi transporters has been reported. Here we found that the expressions of 5 GmPTs highly responded to Fe deficiency (Figure 6), and thus provided the first evidence that Pi transporter genes might be involved in interactions between P and Fe signaling.
All together, we conclude that there are strong interactions among N, P, K and Fe signals, and Pi transporter genes might be involved in cross-talks for sensing the changes of N, P, K and Fe status in soybean.
Materials and Methods
Computational Identification of the Pht1 Family Members of Pi Transporters in Soybean
The nucleic acid sequences of all 9 and 13 members of PTs in the Pht1 family in Arabidopsis [14] and rice (Oryza sativa) [16], respectively, were used as query sequences to BLAST search the Phytozome soybean genome database (http://www.phytozome.net/soybean). The predicted sequences revealed there were in total 14 members of the Pht1 gene family in soybean. The nucleic acid and amino sequences of the 14 Pi transporters were retrieved from the Phytozome website (Text S1, S2). Protein molecular weights and theoretical pI values were calculated using Compute pI/Mw tool (http://www.expasy.org/tools/pi_tool.html). Sequence identity was performed using DNAMAN version 6.0 (Lynnon Biosoft Company). Sequence alignment was performed with ClustalW [65]. The membrane-spanning domains of GmPT1 through GmPT14 were predicted by TopPred (http://www.cbib.u-bordeaux2.fr/pise/toppred.html). A phylogenetic tree based on entire protein sequence alignments using ClustalW was constructed by the neighbor-joining method with 1000 bootstrap replicates in the MEGA 4.1 program (http://www.megasoftware.net/mega4/mega41.html). Complete deletion was used to deal with gaps or missing data in sequences. The distance between sequences was estimated after Poisson correction. The distance between sequences was estimated after Poisson correction. Primers in Table S4 and Table S5 were used to amply the ORF and DNA sequences of GmPTs, respectively, the gained ORF and DNA sequences were used to construct gene structures through Gene Display Server (http://gsds.cbi.pku.edu.cn/index.php).
Yeast Manipulations
Saccharomyces cerevisiae MB192 (MATa pho3–1 pho84::HIS3 ade2 leu2-3, 112 his3-532, trp1-289 ura3-1, 2 can1) defective in the high-affinity Pi transporter gene PHO84 by insertion of an HIS3 DNA fragment [66], and the expression vector, p112A1NE, were used to functional complementation assay of GmPTs following the protocol described previously. The full coding regions of each of the 14 GmPTs were produced by RT-PCR. PCR primers were designed to introduce unique restriction sites at the 5′ and 3′ ends of the genes (Table S4). The cDNA amplicons were cloned into the yeast expression plasmid p112A1NE to create Yp112-GmPTs. According to Dohmen et al [67], these constructs and empty vector (as control) were transformed into the MB192. Yp112-GmPTs transformants were tested for their ability to complement the growth defect of MB192. Transformed yeast strains grew in yeast nitrogen base liquid (YNB) medium to the logarithmic phase (when the absorbance at 600 nm was 0.8 at 30°C), and then were harvested and washed (centrifugation at 1500 g, 4°C, 5 min) three times with Pi-free YNB medium (containing an equivalent concentration of potassium chloride rather than potassium phosphate). The collected pellets were suspended in the same medium and incubated at 30°C until absorbance at 600 nm was 1.0, as preparation for the next experiments. For pH-dependent Pi uptake experiments, different extracellular pH values in the range of 4.0–7.0 were used. For 33P uptake experiments in yeast, about 1 mg fresh yeast cell samples were used following the modified method as previously described [15]. Eight different concentrations of Pi (10 µM, 20 µM, 60 µM, 100 µM, 200 µM, 300 µM, 500 µM and 1000 µM) were used to assay Pi uptake by intact Saccharomyces cerevisiae cells by the addition of 2 µL of [33P]orthophosphate to 50 µL aliquots of cells, were incubated with shaking at 30°C for 5 min, Pi uptake was stopped by the addition of 1 mL cooled tris-succinate, harvested, then suspended in 25 mM Tris-succinate (pH 6) solution, and washed three times (centrifugation at 1500 g, 4°C, 5 min) with 3% glucose. Radioactivity was measured by a Beckman LS 6500 Scintillation Counter. The data were analyzed using the software SIGMAPLOT (v10.0) to determine the Km. For growth experiments on YNB solid medium, different numbers of transformant cells (5×105, 5×104, 5×103) calculated by blood cell counting plate methods were plated on pH 6.0 Pi limited (20 µM) YNB agar plates and culture at 30°C, 3 days. There were three independent biological experiments for each measurement. For growth experiments in YNB liquid medium, 100 µL cells in the logarithmic phase (OD600≈0.90) were subjected to 3.5 mL YNB liquid medium with different Pi concentrations and incubated at 30°C, OD 600 were measured every 5 hours up to 25 hours.
Plant Materials and Growth Conditions
Soybean cv. HN66 was employed in this study. For expression analysis of GmPTs in different tissues responding to P availability, soybean plants were nutrient solution cultured in a greenhouse. One week after germination, the seedlings were transplanted into the full-strength nutrient solution containing 500 µM KH2PO4, 3000 µM KNO3, 2000 µM Ca(NO3)2, 250 µM MgSO4, 25 µM MgCl2, 12.5 µM H3BO3, 1 µM MnSO4, 1 µM ZnSO4, 0.25 µM CuSO4, 0.25 µM (NH4)6Mo7O24 and 25 µM Fe-Na-EDTA. The seedlings were grown for 10 days till the first trifoliate leaves fully expanded and then treated with two P supplies (5 µM and 500 µM P). At 18 d after treatment, roots, stems, leaves and flowers were separately harvested. At 29 d after treatment, young pods and seeds were separately harvested. All tissue samples were stored at −80°C for RNA extraction.
To elucidate the responses of the GmPTs to the other nutrient deficiencies, ten-day old seedlings precultured in full-strength nutrient solution as described above were treated under −N, −P −K, and -Fe conditions for 14 days, respectively. For −N treatment, KNO3, Ca(NO3)2 and (NH4)6Mo7O24 was replaced by 1500 µM K2SO4, 2000 µM CaSO4 and 0.25 µM Na2MoO4, respectively. For -K treatment, KNO3 and KH2PO4 was replaced by 1500 µM Ca(NO3)2 and 500 µM NaH2PO4, respectively. For -Fe treatment, Fe-EDTA was fully withdrawn. Plants continuously grown in the full-strength nutrient solution were sampled as control (CK). Each treatment had three biological replicates. Leaves and roots were separately collected for total RNA extraction and qRT-PCR analysis.
Quantitative Real-time RT-PCR Analysis
For qRT-PCR analysis, the soybean housekeeping gene TefS1 (encodes the elongation factor EF-1a: X56856) was used as a reference gene. The optimal primer sequences for GmPTs and TefS1 were listed in Table S2. Total RNA was extracted from soybean plants using RNAiso™ Plus reagent (TaKaRa) according to the manufacturer’s instructions. RNA samples were treated with RNase-free DNaseI (Invitrogen) to remove the contaminating genomic DNA before synthesizing the first strand cDNA using the MMLV-reverse transcriptase (Promega, USA) according to the protocol from the supplier. qRT-PCR was carried out in a 20 µL volume containing 2 µL 1∶50 diluted reverse transcription product, 0.2 µM primers, and 10 µL SYBR® Premix EX Taq™ (TaKaRa). All the reactions were done on a DNA Engine Opticon 2 Continuous Fluorescence Detection System (MJ Research Inc., Waltham, MA). Reaction conditions for thermal cycling were: 95°C for 1 min, 40 cycles of 95°C for 15 s, 58–60°C for 15 s, and 72°C for 30 s. The annealing temperature (58–60°C) was adjusted to suit the amplification of individual GmPTs. Fluorescence data were collected during the cycle at 72°C.
Data Analysis
All the data were analyzed statistically using Microsoft Excel 2003 (Microsoft Company, USA) for calculating mean and standard error. Comparisons of gene expressions in different tissues or responses to different nutrient deficiencies were performed using the student t-test in the Microsoft Excel 2003.
Supporting Information
Alignment of amino acid sequences of the Pht1 family phosphate transporters in soybean. Sequence alignment was performed with the ClustalW program [65]. Identical and similar amino acids are shaded in black and grey, respectively. The membrane spanning domains of GmPTs predicted by TopPred (http://www.cbib.u-bordeaux2.fr/pise/toppred.html) are under lined and numbered by roman numerals (I–XII).
(TIF)
Schematic diagram of intron/exon structure of GmPT genes. The thin line represents the introns and the open boxes indicate the exons of the respective genes.
(TIF)
Kinetic growth profiles of yeast transformants. Yeast strains, including WT, empty vector control or Yp112-GmPTs transformants, grew in the logarithmic phase (OD600≈0.90), then 100 µL different yeast cells were subjected to 3.5 mL YNB liquid medium with different Pi concentrations and incubated at 30°C, OD 600 were measured every 5 hours up to 25 hours. The Pi concentrations were selected according to their Km values.
(TIF)
Percentage of protein sequences identity among the 14 soybean phosphate transporters in Pht1 family.
(DOC)
Genes and gene-specific primers used for quantitative real-time PCR experiments.
(DOC)
Expression pattern of soybean GmPTs in the common used tissues in reference 37, 38. The numbers presented in the table are normalized Illumina-Solexa reads number coming from the according experiment.
(DOC)
Primers used to generate the expression vectors in yeast complementary assays (restriction site sequences are underlined).
(DOC)
Primers used to generate DNA sequences of GmPTs.
(DOC)
Protein sequences of GmPTs.
(TXT)
Nucleic acid sequences of GmPTs.
(TXT)
Acknowledgments
We thank Ms. Xinxin Li and Mr. Chengchen Li for sampling assistance. Drs. Xiurong Wang, Jiang Tian and Jinxiang Wang for helpful discussions and comments.
Funding Statement
This work was jointly supported by the grants from the National Natural Science Foundation of China (Grant No. 30890131) and National Key Basic Research Special Funds of China (2011CB100301). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ullrich-Eberius CI, Novacky A, Fischer E, Luttge U (1981) Relationship between energy-dependent phosphate uptake and the electrical membrane potential in Lemna gibba G1. Plant Physiol 67: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holford I (1997) Soil phosphorus: its measurement, and its uptake by plants. Aust J Soil Res 35: 227–240. [Google Scholar]
- 3.Marschner H (1995) Mineral nutrition of higher plants. San Diego, CA: Academic Press. 889 p.
- 4. Raghothama K (1999) Phosphate acquisition. Annu Rev of Plant Biol 50: 665–693. [DOI] [PubMed] [Google Scholar]
- 5. Schachtman DP, Reid RJ, Ayling S (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mimura T (1999) Regulation of phosphate transport and homeostasis in plant cells. Int rev cytol 191: 149–200. [Google Scholar]
- 7. Liu F, Chang XJ, Ye Y, Xie WB, Wu P, et al. (2011) Comprehensive Sequence and Whole-Life-Cycle Expression Profile Analysis of the Phosphate Transporter Gene Family in Rice. Molecular Plant 4: 1105–1122. [DOI] [PubMed] [Google Scholar]
- 8. Guo B, Jin Y, Wussler C, Blancaflor E, Motes C, et al. (2008) Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol 177: 889–898. [DOI] [PubMed] [Google Scholar]
- 9. Knappe S, Flügge UI, Fischer K (2003) Analysis of the plastidic phosphate translocator gene family in Arabidopsis and identification of new phosphate translocator-homologous transporters, classified by their putative substrate-binding site. Plant Physiol 131: 1178–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Picault N, Hodges M, Palmieri L, Palmieri F (2004) The growing family of mitochondrial carriers in Arabidopsis. Trends Plant Sci 9: 138–146. [DOI] [PubMed] [Google Scholar]
- 11. Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216: 23–37. [DOI] [PubMed] [Google Scholar]
- 12. Karandashov V, Bucher M (2005) Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci 10: 22–29. [DOI] [PubMed] [Google Scholar]
- 13. Muchhal US, Pardo JM, Raghothama K (1996) Phosphate transporters from the higher plant Arabidopsis thaliana. Proc Natl Acad Sci U S A 93: 10519–10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mudge SR, Rae AL, Diatloff E, Smith FW (2002) Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J 31: 341–353. [DOI] [PubMed] [Google Scholar]
- 15. Ai P, Sun S, Zhao J, Fan X, Xin W, et al. (2009) Two rice phosphate transporters, OsPht1; 2 and OsPht1; 6, have different functions and kinetic properties in uptake and translocation. Plant J 57: 798–809. [DOI] [PubMed] [Google Scholar]
- 16. Paszkowski U, Kroken S, Roux C, Briggs SP (2002) Rice phosphate transporters include an evolutionarily divergent gene specifically activated in arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 99: 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schünmann P, Richardson A, Smith F, Delhaize E (2004) Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.). J Exp Bot 55: 855–865. [DOI] [PubMed] [Google Scholar]
- 18. Schünmann PHD, Richardson AE, Vickers CE, Delhaize E (2004) Promoter analysis of the barley Pht1;1 phosphate transporter gene identifies regions controlling root expression and responsiveness to phosphate deprivation. Plant Physiol 136: 4205–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagy R, Vasconcelos M, Zhao S, McElver J, Bruce W, et al. (2006) Differential regulation of five Pht1 phosphate transporters from maize (Zea mays L.). Plant Biol (Stuttg) 8: 186–197. [DOI] [PubMed] [Google Scholar]
- 20. Liu C, Muchhal US, Uthappa M, Kononowicz AK, Raghothama KG (1998) Tomato phosphate transporter genes are differentially regulated in plant tissues by phosphorus. Plant Physiol 116: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagy R, Drissner D, Amrhein N, Jakobsen I, Bucher M (2009) Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol 181: 950–959. [DOI] [PubMed] [Google Scholar]
- 22. Chen A, Hu J, Sun S, Xu G (2007) Conservation and divergence of both phosphate-\and mycorrhiza-regulated physiological responses and expression patterns of phosphate transporters in solanaceous species. New Phytol 173: 817–831. [DOI] [PubMed] [Google Scholar]
- 23. Wu Z, Zhao J, Gao R, Hu G, Gai J, et al. (2011) Molecular Cloning, Characterization and Expression Analysis of Two Members of the Pht1 Family of Phosphate Transporters in Glycine max . PLoS ONE 6: e19752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Versaw WK, Pumplin N, Gomez SK, Blaylock LA, et al. (2008) Closely related members of the Medicago truncatula PHT1 phosphate transporter gene family encode phosphate transporters with distinct biochemical activities. J Biol Chem 283: 24673–24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ (2007) A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci 104: 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrison MJ, Dewbre GR, Liu J (2002) A phosphate transporter from Medicago truncatula involved in the acquisition of phosphate released by arbuscular mycorrhizal fungi. The Plant Cell Online 14: 2413–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karthikeyan AS, Varadarajan DK, Mukatira UT, D’Urzo MP, Damsz B, et al. (2002) Regulated expression of Arabidopsis phosphate transporters. Plant Physiol 130: 221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rae AL, Cybinski DH, Jarmey JM, Smith FW (2003) Characterization of two phosphate transporters from barley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol Biol 53: 27–36. [DOI] [PubMed] [Google Scholar]
- 29.Ai P, Sun S, Zhao J, Xu G (2009) Regulation and function of Pht1 family phosphate transporters in rice. UC Davis, The Proceedings of the International Plant Nutrition Colloquium XVI, International Plant Nutrition Colloquium. Retrieved from: http://escholarship.org/uc/item/3657w1q3.
- 30. Jia H, Ren H, Gu M, Zhao J, Sun S, et al. (2011) The phosphate transporter gene OsPht1; 8 is involved in phosphate homeostasis in rice. Plant Physiol 156: 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maeda D, Ashida K, Iguchi K, Chechetka SA, Hijikata A, et al. (2006) Knockdown of an arbuscular mycorrhiza-inducible phosphate transporter gene of Lotus japonicus suppresses mutualistic symbiosis. Plant Cell Physiol 47: 807. [DOI] [PubMed] [Google Scholar]
- 32. Bureau M, Mederski H, Evans C (1953) The effects of phosphatic fertilizer materials and soil phosphorus level on the yield and phosphorus uptake of soybeans. Agron J 45: 150–154. [Google Scholar]
- 33. Wang YH, Garvin DF, Kochian LV (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. Evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol 130: 1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Misson J, Raghothama KG, Jain A, Jouhet J, Block MA, et al. (2005) A genome-wide transcriptional analysis using Arabidopsis thaliana Affymetrix gene chips determined plant responses to phosphate deprivation. Proc Natl Acad Sci U S A 102: 11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wasaki J, Yonetani R, Kuroda S, Shinano T, Yazaki J, et al. (2003) Transcriptomic analysis of metabolic changes by phosphorus stress in rice plant roots. Plant Cell Environ 26: 1515–1523. [Google Scholar]
- 36. Coello P, Polacco JC (1999) ARR6, a response regulator from Arabidopsis, is differentially regulated by plant nutritional status. Plant Sci 143: 211–220. [Google Scholar]
- 37. Libault M, Farmer A, Joshi T, Takahashi K, Langley RJ, et al. (2010) An integrated transcriptome atlas of the crop model Glycine max, and its use in comparative analyses in plants. Plant J 63: 86–99. [DOI] [PubMed] [Google Scholar]
- 38. Severin AJ, Woody JL, Bolon YT, Joseph B, Diers BW, et al. (2010) RNA-Seq Atlas of Glycine max: A guide to the soybean transcriptome. BMC Plant Biol 10: 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li M, Welti R, Wang X (2006) Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylglycerol accumulation in phosphorus-starved plants. Plant Physiol 142: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gierth M, Mäser P, Schroeder JI (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137: 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci 93: 5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang R, Guegler K, LaBrie ST, Crawford NM (2000) Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. The Plant Cell Online 12: 1491–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sánchez-Calderón L, Chacon-López A, Pérez-Torres CA, Herrera-Estrella L (2010) Phosphorus: Plant Strategies to Cope with its Scarcity. In: Hell R, Mendel RR, editors, Cell Biology of Metals and Nutrients. Plant Cell Monographs 17: 173–198. [Google Scholar]
- 44. Nagy R, Karandashov V, Chague V, Kalinkevich K, Tamasloukht MB, et al. (2005) The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J 42: 236–250. [DOI] [PubMed] [Google Scholar]
- 45. Rausch C, Daram P, Brunner S, Jansa J, Laloi M, et al. (2001) A phosphate transporter expressed in arbuscule-containing cells in potato. Nature 414: 462–470. [DOI] [PubMed] [Google Scholar]
- 46. Woody JL, Severin AJ, Bolon YT, Joseph B, Diers BW, et al. (2011) Gene expression patterns are correlated with genomic and genic structure in soybean. Genome 54: 10–18. [DOI] [PubMed] [Google Scholar]
- 47. Daram P, Brunner S, Persson BL, Amrhein N, Bucher M (1998) Functional analysis and cell-specific expression of a phosphate transporter from tomato. Planta 206: 225–233. [DOI] [PubMed] [Google Scholar]
- 48. Mitsukawa N, Okumura S, Shirano Y, Sato S, Kato T, et al. (1997) Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc Natl Acad Sci 94: 7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miller A, Zhou J (2000) Xenopus oocytes as an expression system for plant transporters. BBA-BIOMEMBRANES 1465: 343–358. [DOI] [PubMed] [Google Scholar]
- 50. Leggewie G, Willmitzer L, Riesmeier JW (1997) Two cDNAs from potato are able to complement a phosphate uptake-deficient yeast mutant: identification of phosphate transporters from higher plants. Plant Cell Online 9: 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, et al. (2010) A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J Biol Chem 285: 26532–26544. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52. Grossman A, Takahashi H (2001) Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Ann Rev Plant Biol 52: 163–210. [DOI] [PubMed] [Google Scholar]
- 53. Hammond JP, Bennett MJ, Bowen HC, Broadley MR, Eastwood DC, et al. (2003) Changes in gene expression in Arabidopsis shoots during phosphate starvation and the potential for developing smart plants. Plant Physiol 132: 578–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kobae Y, Hata S (2010) Dynamics of periarbuscular membranes visualized with a fluorescent phosphate transporter in arbuscular mycorrhizal roots of rice. Plant Cell Physiol 51: 341. [DOI] [PubMed] [Google Scholar]
- 55. Wang YH, Garvin DF, Kochian LV (2002) Rapid induction of regulatory and transporter genes in response to phosphorus, potassium, and iron deficiencies in tomato roots. Evidence for cross talk and root/rhizosphere-mediated signals. Plant Physiol 130: 1361–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kochian LV (2000) Molecular physiology of mineral nutrient acquisition, transport, and utilization. In: Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, 1204–1249.
- 57. Secco D, Baumann A, Poirier Y (2010) Characterization of the rice PHO1 gene family reveals a key role for OsPHO1;2 in phosphate homeostasis and the evolution of a distinct clade in dicotyledons. Plant Physiol 152: 1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kant S, Peng M, Rothstein SJ (2011) Genetic Regulation by NLA and MicroRNA827 for Maintaining Nitrate-Dependent Phosphate Homeostasis in Arabidopsis. PLoS Genet 7(3): e1002021 doi:101371/journalpgen1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith FW, Cybinski DH, Rae AL (1999) Regulation of expression of genes encoding phosphate transporters in barley roots. In: Gissel–Nielsen G, Jensen A, editors. Plant nutrition–molecular biology and genetics. 145–150.
- 60. Dalton C, Iqbal K, Turner D (1983) Iron phosphate precipitation in Murashige and Skoog media. Physiol Plantarum 57: 472–476. [Google Scholar]
- 61. López-Bucio J, Cruz-Ramirez A, Herrera-Estrella L (2003) The role of nutrient availability in regulating root architecture. Curr Opin in Plant Biol 6: 280–287. [DOI] [PubMed] [Google Scholar]
- 62. Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, et al. (2005) A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiol 138: 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schmidt W, Schikora A (2001) Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiol 125: 2078–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG (2008) The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol 147: 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bun-Ya M, Nishimura M, Harashima S, Oshima Y (1991) The PHO84 gene of Saccharomyces cerevisiae encodes an inorganic phosphate transporter. Mol Cell Biol 11: 3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dohmen RJ, Strasser AWM, Höner CB, Hollenberg CP (1991) An efficient transformation procedure enabling long-term storage of competent cells of various yeast genera. Yeast 7: 691–692. [DOI] [PubMed] [Google Scholar]
- 68. Grunwald U, Guo W, Fischer K, Isayenkov S, Ludwig-Müller J, et al. (2009) Overlapping expression patterns and differential transcript levels of phosphate transporter genes in arbuscular mycorrhizal, Pi-fertilised and phytohormone-treated Medicago truncatula roots. Planta 229: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of amino acid sequences of the Pht1 family phosphate transporters in soybean. Sequence alignment was performed with the ClustalW program [65]. Identical and similar amino acids are shaded in black and grey, respectively. The membrane spanning domains of GmPTs predicted by TopPred (http://www.cbib.u-bordeaux2.fr/pise/toppred.html) are under lined and numbered by roman numerals (I–XII).
(TIF)
Schematic diagram of intron/exon structure of GmPT genes. The thin line represents the introns and the open boxes indicate the exons of the respective genes.
(TIF)
Kinetic growth profiles of yeast transformants. Yeast strains, including WT, empty vector control or Yp112-GmPTs transformants, grew in the logarithmic phase (OD600≈0.90), then 100 µL different yeast cells were subjected to 3.5 mL YNB liquid medium with different Pi concentrations and incubated at 30°C, OD 600 were measured every 5 hours up to 25 hours. The Pi concentrations were selected according to their Km values.
(TIF)
Percentage of protein sequences identity among the 14 soybean phosphate transporters in Pht1 family.
(DOC)
Genes and gene-specific primers used for quantitative real-time PCR experiments.
(DOC)
Expression pattern of soybean GmPTs in the common used tissues in reference 37, 38. The numbers presented in the table are normalized Illumina-Solexa reads number coming from the according experiment.
(DOC)
Primers used to generate the expression vectors in yeast complementary assays (restriction site sequences are underlined).
(DOC)
Primers used to generate DNA sequences of GmPTs.
(DOC)
Protein sequences of GmPTs.
(TXT)
Nucleic acid sequences of GmPTs.
(TXT)