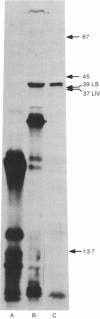
Abstract
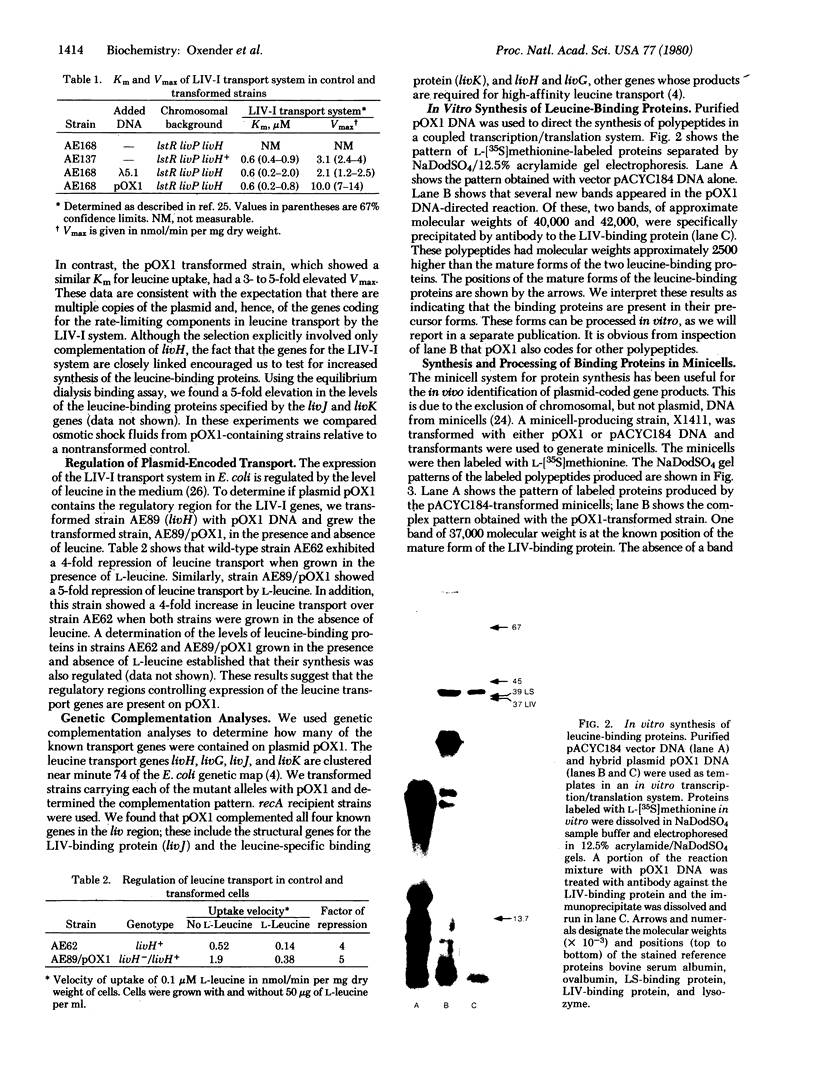
The four genes encoding the components of the high-affinity branched-chain amino acid transport systems in Escherichia coli (livH, livG, livJ, and livK) have been cloned into lambda phage and subsequently into the plasmid vector pACYC184. The presence of the four structural genes and their accompanying regulatory regions on the resultant plasmid, pOXI, was confirmed by genetic complementation and analysis and by transport studies carried out on the appropriate transformed mutant strains. When pOX1 DNA was used to direct an in vitro transcription/translation system, four major polypeptide products were produced. Immunoprecipitation with antibody directed against the LIV-binding protein identified the two leucine-binding proteins as products of in vitro synthesis. The binding proteins were produced in precursor forms and had molecular weights approximately 2500 higher than the processed, mature forms. A minicell-producing strain transformed with plasmid pOX1 produced the binding proteins in the processed form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Oxender D. L. Escherichia coli transport mutants lacking binding protein and other components of the branched-chain amino acid transport systems. J Bacteriol. 1977 Apr;130(1):384–392. doi: 10.1128/jb.130.1.384-392.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. J., Oxender D. L. Genetic separation of high- and low-affinity transport systems for branched-chain amino acids in Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):168–174. doi: 10.1128/jb.136.1.168-174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron J. R., Panasenko S. M., Lehman I. R., Davis R. W. In vitro construction of bacteriophage lambda carrying segments of the Escherichia coli chromosome: selection of hybrids containing the gene for DNA ligase. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3416–3420. doi: 10.1073/pnas.72.9.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Production, properties and utility of bacterial minicells. Curr Top Microbiol Immunol. 1975;69:1–84. doi: 10.1007/978-3-642-50112-8_1. [DOI] [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Klopotowski T., Iaccarino M. Multiplicity of isoleucine, leucine, and valine transport systems in Escherichia coli K-12. J Bacteriol. 1974 Feb;117(2):382–392. doi: 10.1128/jb.117.2.382-392.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Mayo M. M., Quay S. C. Leucine binding protein and regulation of transport in E. coli. J Supramol Struct. 1977;6(3):419–431. doi: 10.1002/jss.400060315. [DOI] [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport proteins. Biomembranes. 1974;5:25–79. doi: 10.1007/978-1-4684-7389-6_2. [DOI] [PubMed] [Google Scholar]
- Rahmanian M., Claus D. R., Oxender D. L. Multiplicity of leucine transport systems in Escherichia coli K-12. J Bacteriol. 1973 Dec;116(3):1258–1266. doi: 10.1128/jb.116.3.1258-1266.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Josefsson L. G. Precursors of three exported proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1209–1212. doi: 10.1073/pnas.75.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Selker E., Brown K., Yanofsky C. Mitomycin C-induced expression of trpA of Salmonella typhimurium inserted into the plasmid ColE1. J Bacteriol. 1977 Jan;129(1):388–394. doi: 10.1128/jb.129.1.388-394.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Wood J. M. Leucine transport in Escherichia coli. The resolution of multiple transport systems and their coupling to metabolic energy. J Biol Chem. 1975 Jun 25;250(12):4477–4485. [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]