Abstract
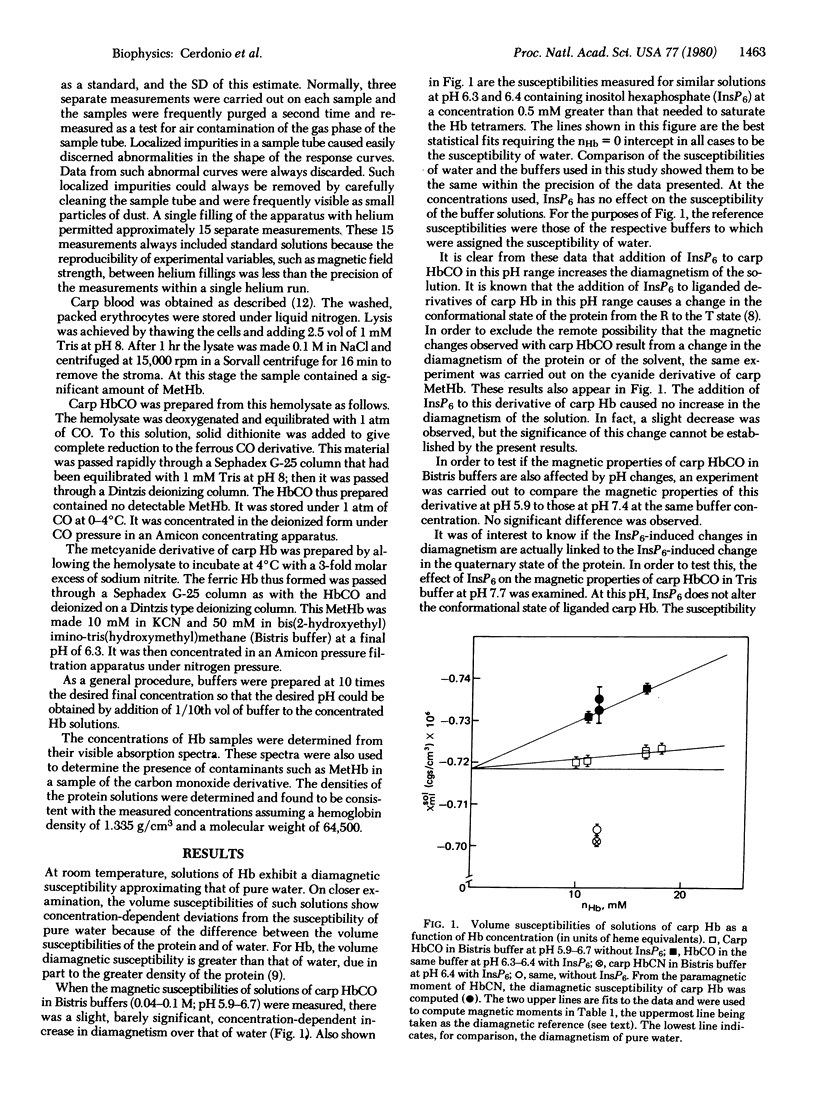
Deionized carp carbon monoxide hemoglobin in distilled water or in bis(2-hydroxyethyl)imino-tris(hydroxymethyl)methane or Tris buffer exhibits a slight but significant paramagnetism. This is most clearly demonstrated by the decrease in this paramagnetism that is caused by the addition of inositol hexaphosphate to this protein in the former buffer at pH 6.3-6.4. No such effect is seen when inositol hexaphosphate is added to carp cyanomethemoglobin, demonstrating that the change observed with carbon monoxide derivative is not due to a modification in the diamagnetic properties of the protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert Y., Banerjee R. Magnetic susceptibility measurements of deoxygenated hemoglobins and isolated chains. Biochim Biophys Acta. 1975 Sep 9;405(1):144–154. doi: 10.1016/0005-2795(75)90324-4. [DOI] [PubMed] [Google Scholar]
- Cerdonio M., Congiu-Castellano A., Calabrese L., Morante S., Pispisa B., Vitale S. Room-temperature magnetic properties of oxy- and carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4916–4919. doi: 10.1073/pnas.75.10.4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerdonio M., Congiu-Castellano A., Mogno F., Pispisa B., Romani G. L., Vitale S. Magnetic properties of oxyhemoglobin. Proc Natl Acad Sci U S A. 1977 Feb;74(2):398–400. doi: 10.1073/pnas.74.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina B., Ascoli F., Brunori M. Spectral changes and allosteric transition in trout haemoglobin. Nature. 1975 Aug 28;256(5520):761–762. doi: 10.1038/256761a0. [DOI] [PubMed] [Google Scholar]
- Iizuka T., Kotani M. Analysis of thermal equilibrium between high-spin and low-spin states in ferrihemoglobin complexes. Biochim Biophys Acta. 1969 Dec 23;194(2):351–363. doi: 10.1016/0005-2795(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Knowles F. C., McDonald M. J., Gibson Q. H. The origin of the Adams-Schuster difference spectrum of oxyhemoglobin. Biochem Biophys Res Commun. 1975 Sep 16;66(2):556–563. doi: 10.1016/0006-291x(75)90546-x. [DOI] [PubMed] [Google Scholar]
- Messana C., Cerdonio M., Shenkin P., Noble R. W., Fermi G., Perutz R. N., Perutz M. F. Influence of quaternary structure of the globin on thermal spin equilibria in different methemoglobin derivatives. Biochemistry. 1978 Aug 22;17(17):3652–3662. doi: 10.1021/bi00610a035. [DOI] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Sanders J. K., Chenery D. H., Noble R. W., Pennelly R. R., Fung L. W., Ho C., Giannini I., Pörschke D., Winkler H. Interactions between the quaternary structure of the globin and the spin state of the heme in ferric mixed spin derivatives of hemoglobin. Biochemistry. 1978 Aug 22;17(17):3640–3652. doi: 10.1021/bi00610a034. [DOI] [PubMed] [Google Scholar]
- Tan A. L., De Young A., Noble R. W. The pH dependence of the affinity, kinetics, and cooperativity of ligand binding to carp hemoglobin, Cyprinus carpio. J Biol Chem. 1972 Apr 25;247(8):2493–2498. [PubMed] [Google Scholar]
- Tan A. L., Noble R. W., Gibson Q. H. Conditions restricting allosteric transitions in carp hemoglobin. J Biol Chem. 1973 Apr 25;248(8):2880–2888. [PubMed] [Google Scholar]
- Tan A. L., Noble R. W. The effect of inositol hexaphosphate on the allosteric properties of carp hemoglobin. J Biol Chem. 1973 Nov 10;248(21):7412–7416. [PubMed] [Google Scholar]


