Abstract
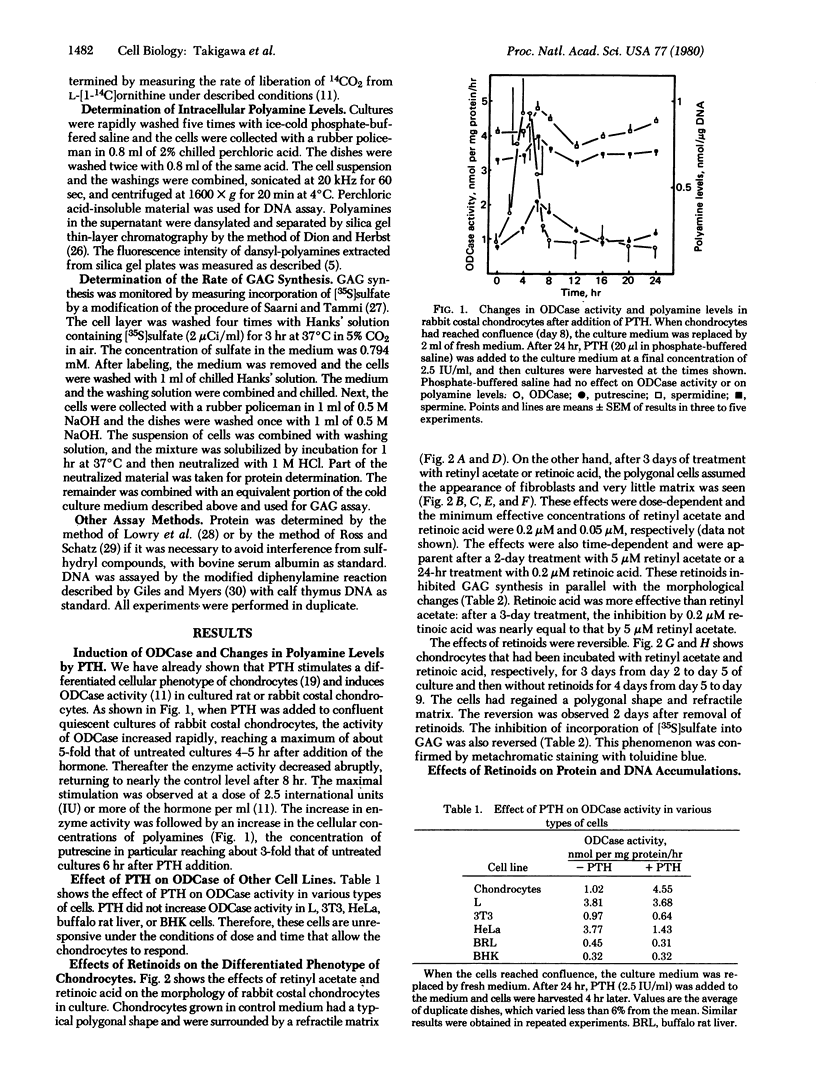
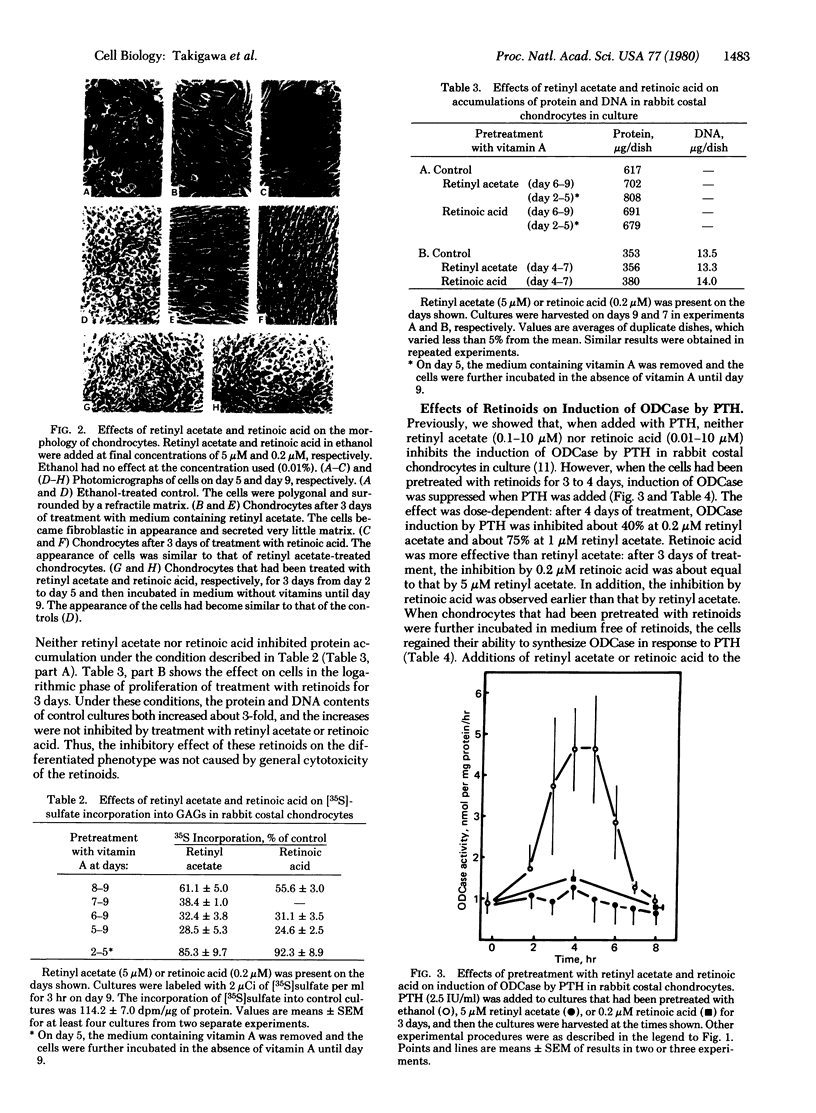
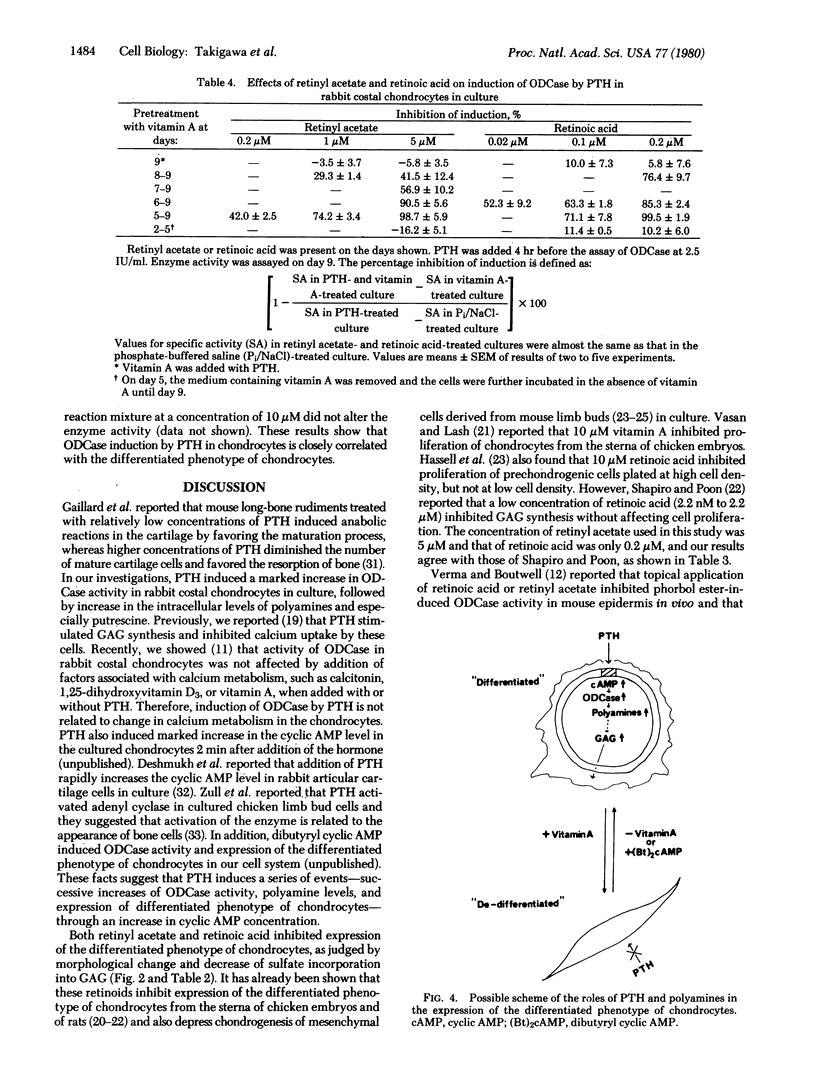
The activity of ornithine decarboxylase (OD-Case:L-ornithine carboxy-lyase, EC 4.1.1.17) in rabbit costal chondrocytes in culture increased markedly after addition of parathyroid hormone (PTH), reaching a maximum 4 to 5 hr after PTH addition. The increase in ODCase activity was followed by increase in the intracellular concentrations of polyamines, especially putrescine, which increased in 6 hr to about 3-fold that of untreated cultures. The induction of ODCase by PTH was not observed in L, 3T3, HeLa, buffalo rat liver, or BHK cells. Retinyl acetate and retinoic acid both inhibited expression of the differentiated phenotype of chondrocytes by rabbit costal chondrocytes in culture within 3 days after their addition, as judged by morphological change and decrease in sulfate incorporation into glycosaminoglycans but did not inhibit cell proliferation. PTH could not induce an increase in ODCase in de-differentiated cells that had been pretreated with retinyl acetate or retinoic acid for 3 days. but 4 days after removal of the retinoids, these de-differentiated cells regained the ability to synthesize ODCase in response to PTH. These facts suggest that the induction of ODCase and the formation of putrescine by PTH are good markers of the differentiated phenotype of cultured chondrocytes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byus C. V., Wicks W. D., Russel D. H. Induction of ornithine decarboxylase in Reuber H35 rat hepatoma cells. J Cyclic Nucleotide Res. 1976 Jul-Aug;2(4):241–250. [PubMed] [Google Scholar]
- Conroy P. D., Simms D. M., Pointon J. J. Occurrence of ornithine decarboxylase and polyamines in cartilage. Biochem J. 1977 Feb 15;162(2):347–350. doi: 10.1042/bj1620347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikuhara Y., Tamada F., Takigawa M., Takeda Y., Mori Y. Changes in polyamine metabolism of rat liver after administration of D-galactosamine. Favorable effects of putrescine administration on galactosamine-induced hepatic injury. Gastroenterology. 1979 Jul;77(1):123–132. [PubMed] [Google Scholar]
- Deshmukh K., Kline W. G., Sawyer B. D. Effects of calcitonin and parathyroid hormone on the metabolism of chondrocytes in culture. Biochim Biophys Acta. 1977 Aug 25;499(1):28–35. doi: 10.1016/0304-4165(77)90225-2. [DOI] [PubMed] [Google Scholar]
- Fillingame R. H., Morris D. R. Polyamine accumulation during lymphocyte transformation and its relation to the synthesis, processing, and accumulation of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4479–4487. doi: 10.1021/bi00746a028. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Pennypacker J. P., Lewis C. A. Chondrogenesis and cell proliferation in limb bud cell cultures treated with cytosine arabinoside and vitamin A. Exp Cell Res. 1978 Mar 15;112(2):409–417. doi: 10.1016/0014-4827(78)90223-9. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Zardi L., Russell D. H., Baserga R. Changes in polyamine metabolism in WI38 cells stimulated to proliferate. Exp Cell Res. 1975 Jan;90(1):8–14. doi: 10.1016/0014-4827(75)90350-x. [DOI] [PubMed] [Google Scholar]
- Inoue H., Kato Y., Takigawa M., Adachi K., Takeda Y. Effect of DL-alpha-hydrazino-delta-aminovaleric acid, an inhibitor of ornithine decarboxylase, on polyamine metabolism in isoproterenol-stimulated mouse parotid glands. J Biochem. 1975 Apr;77(4):879–893. doi: 10.1093/oxfordjournals.jbchem.a130796. [DOI] [PubMed] [Google Scholar]
- Kato Y., Nasu N., Takase T., Suzuki F. Demonstration of somatomedin activity of "multiplication-stimulating activity" in rabbit costal chondrocytes in culture. J Biochem. 1978 Oct;84(4):1001–1004. doi: 10.1093/oxfordjournals.jbchem.a132180. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis C. A., Pratt R. M., Pennypacker J. P., Hassell J. R. Inhibition of limb chondrogenesis in vitro by vitamin A: alterations in cell surface characteristics. Dev Biol. 1978 May;64(1):31–47. doi: 10.1016/0012-1606(78)90058-1. [DOI] [PubMed] [Google Scholar]
- Lichti U., Slaga T. J., Ben T., Patterson E., Hennings H., Yuspa S. H. Dissociation of tumor promoter-stimulated ornithine decarboxylase activity and DNA synthesis in mouse epidermis in vivo and in vitro by fluocinolone acetonide, a tumor-promotion inhibitor. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3908–3912. doi: 10.1073/pnas.74.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Pennypacker J. P., Lewis C. A., Hassell J. R. Altered proteoglycan metabolism in mouse limb mesenchyme cell cultures treated with vitamin A. Arch Biochem Biophys. 1978 Mar;186(2):351–358. doi: 10.1016/0003-9861(78)90445-9. [DOI] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Rath N. C., Reddi A. H. Changes in ornithine decarboxylase activity during matrix-induced cartilage, bone and bone marrow differentiation. Biochem Biophys Res Commun. 1978 Mar 15;81(1):106–113. doi: 10.1016/0006-291x(78)91636-4. [DOI] [PubMed] [Google Scholar]
- Ross E., Schatz G. Assay of protein in the presence of high concentrations of sulfhydryl compounds. Anal Biochem. 1973 Jul;54(1):304–306. doi: 10.1016/0003-2697(73)90280-7. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Byus C. V., Manen C. A. Proposed model of major sequential biochemical events of a trophic response. Life Sci. 1976 Nov 1;19(9):1297–1305. [PubMed] [Google Scholar]
- Saarni H., Tammi M. A rapid method for separation and assay of radiolabeled mucopolysaccharides from cell culture medium. Anal Biochem. 1977 Jul;81(1):40–46. doi: 10.1016/0003-2697(77)90596-6. [DOI] [PubMed] [Google Scholar]
- Shapiro S. S., Poon J. P. Effect of retinoic acid on chondrocyte glycosaminoglycan biosynthesis. Arch Biochem Biophys. 1976 May;174(1):74–81. doi: 10.1016/0003-9861(76)90325-8. [DOI] [PubMed] [Google Scholar]
- Solursh M., Meier S. The selective inhibition of mucopolysaccharide synthesis by vitamin A treatment of cultured chick embryo chondrocytes. Calcif Tissue Res. 1973;13(2):131–142. doi: 10.1007/BF02015403. [DOI] [PubMed] [Google Scholar]
- Suzuki F., Yoneda T., Shimomura Y. Calcitonin and parathyroid-hormone stimulation of acid mucopolysaccharide synthesis in cultured chondrocytes isolated from growth cartilage. FEBS Lett. 1976 Nov;70(1):155–158. doi: 10.1016/0014-5793(76)80747-8. [DOI] [PubMed] [Google Scholar]
- Suzuki H. [Vibration syndrome of vibrating tool users in a factory of steel foundry. Part 1. Complained symptoms (author's transl)]. Sangyo Igaku. 1978 Sep;20(5):261–268. doi: 10.1539/joh1959.20.261. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Inoue H., Gohda E., Asada A., Takeda Y., Mori Y. The role of putrescine in cell proliferation of the skin of mice induced by ethylphenylpropriolate. Exp Mol Pathol. 1977 Oct;27(2):183–196. doi: 10.1016/0014-4800(77)90029-6. [DOI] [PubMed] [Google Scholar]
- Takigawa M., Watanabe R., Ishida H., Asada A., Suzuki F. Induction by parathyroid hormone of ornithine decarboxylase in rabbit costal chondrocytes in culture. J Biochem. 1979 Jan;85(1):311–314. doi: 10.1093/oxfordjournals.jbchem.a132326. [DOI] [PubMed] [Google Scholar]
- Vasan N. S., Lash J. W. Chondrocyte metabolism as affected by vitamin A. Calcif Tissue Res. 1975 Dec 18;19(2):99–107. doi: 10.1007/BF02563995. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Boutwell R. K. Vitamin A acid (retinoic acid), a potent inhibitor of 12-O-tetradecanoyl-phorbol-13-acetate-induced ornithine decarboxylase activity in mouse epidermis. Cancer Res. 1977 Jul;37(7 Pt 1):2196–2201. [PubMed] [Google Scholar]
- Verma A. K., Rice H. M., Shapas B. G., Boutwell R. K. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity in mouse epidermis by vitamin A analogs (retinoids). Cancer Res. 1978 Mar;38(3):793–801. [PubMed] [Google Scholar]
- Yamasaki Y., Ichihara A. Induction of ornithine decarboxylase in cultured mouse L cells. I. Effects of cellular growth, hormones, and actinomycin D. J Biochem. 1976 Sep;80(3):557–562. doi: 10.1093/oxfordjournals.jbchem.a131311. [DOI] [PubMed] [Google Scholar]
- Zull J. E., Krug S., Abel D., Caplan A. I. Development of parathyroid hormone- and calcitonin-activated adenylate cyclases in embryonic chicken limb and in cultured cells from embryonic chicken limb. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3871–3875. doi: 10.1073/pnas.75.8.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]



