Abstract
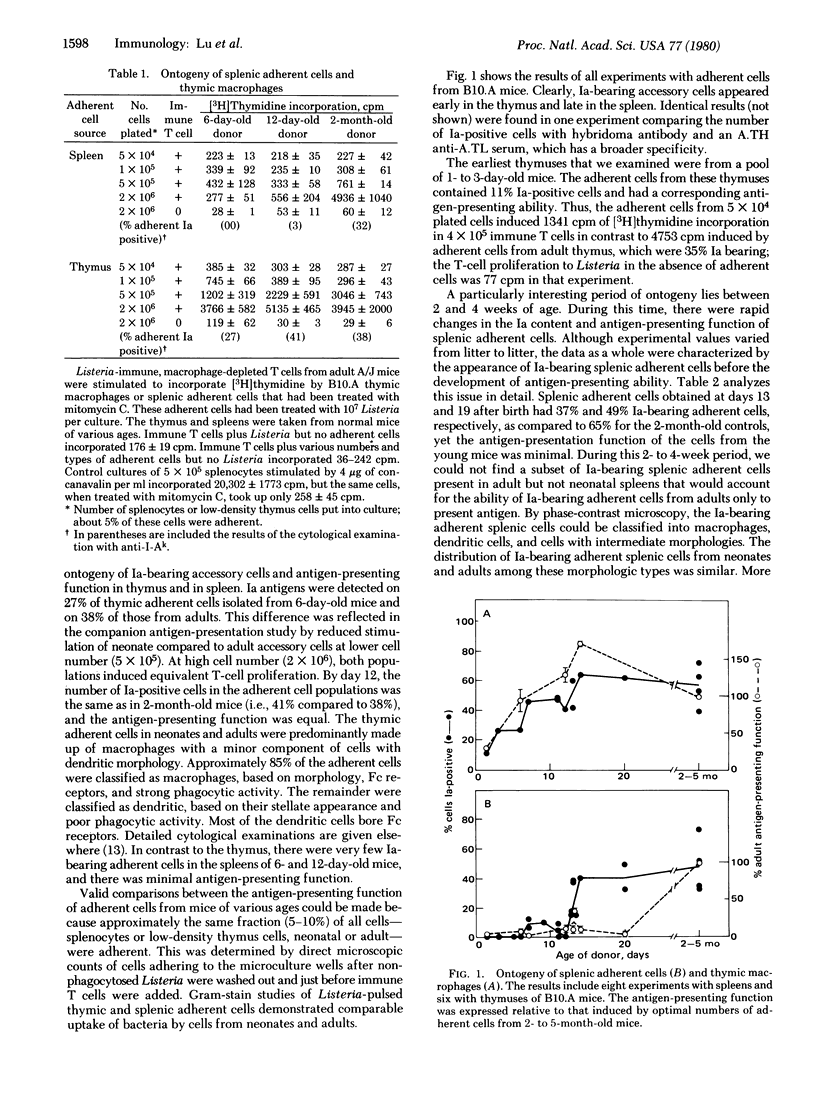
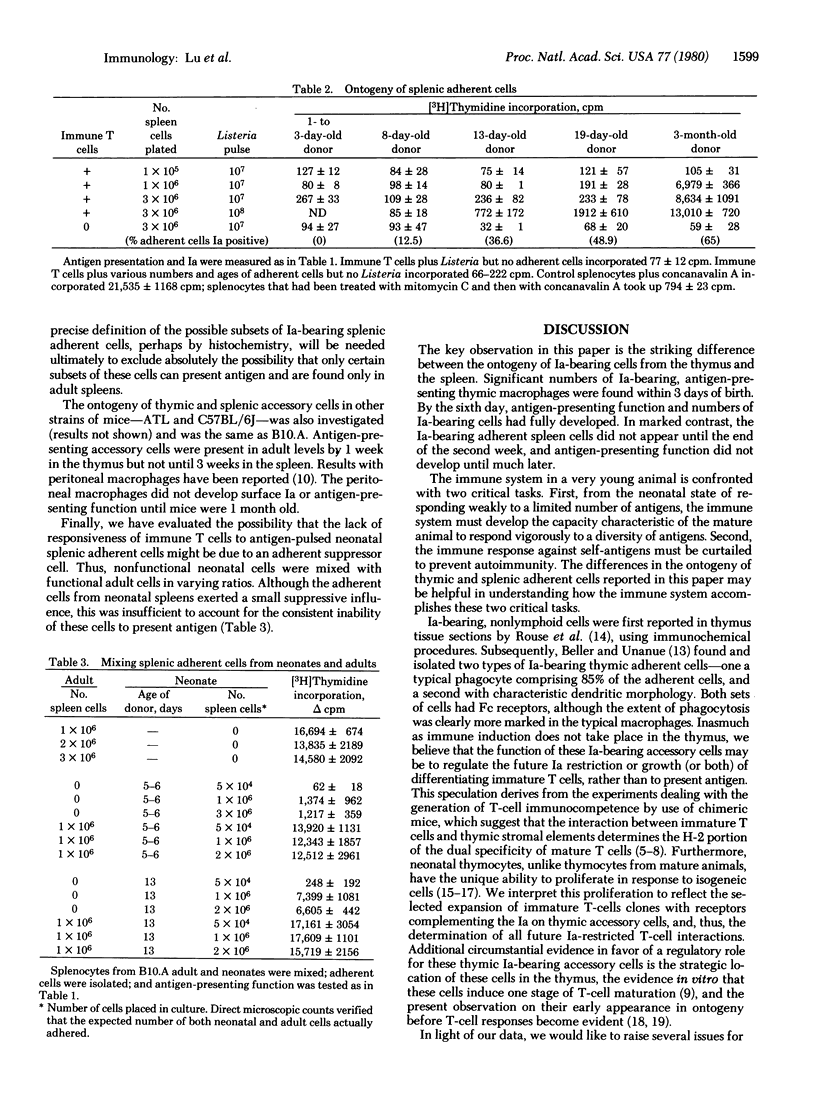
The ontogeny of Ia-bearing accessory cells was studied in mice. Ia-bearing adherent cells from the thymus, consisting predominantly of macrophages, were found from birth. These adherent cells were able to present antigen, as measured by their ability to induce immune T-cell proliferation. In contrast, Ia-bearing adherent cells from the spleen were not found until the second week of life, and their antigen-presentation function was not present until later. The differential ontogeny of Ia-bearing accessory cells at these sites may be important in both development of immune competence and the restriction of autoimmunity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrenbrecht S. Normal development of the thymus-dependent limb of humoral immune responses in mice. Eur J Immunol. 1973 Aug;3(8):506–511. doi: 10.1002/eji.1830030811. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. IA antigens and antigen-presenting function of thymic macrophages. J Immunol. 1980 Mar;124(3):1433–1440. [PubMed] [Google Scholar]
- Beller D. I., Unanue E. R. Thymic macrophages modulate one stage of T cell differentiation in vitro. J Immunol. 1978 Nov;121(5):1861–1864. [PubMed] [Google Scholar]
- Bevan M. J., Fink P. J. The influence of thymus H-2 antigens on the specificity of maturing killer and helper cells. Immunol Rev. 1978;42:3–19. doi: 10.1111/j.1600-065x.1978.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Cowing C., Pincus S. H., Sachs D. H., Dickler H. B. A subpopulation of adherent accessory cells bearing both I-A and I-E or C subregion antigens is required for antigen-specific murine T lymphocyte proliferation. J Immunol. 1978 Nov;121(5):1680–1686. [PubMed] [Google Scholar]
- Farr A. G., Dorf M. E., Unanue E. R. Secretion of mediators following T lymphocyte-macrophage interaction is regulated by the major histocompatibility complex. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3542–3546. doi: 10.1073/pnas.74.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Howe M. L., Goldstein A. L., Battisto J. R. Isogeneic lymphocyte interaction: recognition of self antigens by cells of the neonatal thymus. Proc Natl Acad Sci U S A. 1970 Oct;67(2):613–619. doi: 10.1073/pnas.67.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Adachi T. Generation of specific helper cells and suppressor cells in vitro for the IgE and IgG antibody responses. J Immunol. 1976 Jul;117(1):40–47. [PubMed] [Google Scholar]
- Lu C. Y., Calamai E. G., Unanue E. R. A defect in the antigen-presenting function of macrophages from neonatal mice. Nature. 1979 Nov 15;282(5736):327–329. doi: 10.1038/282327a0. [DOI] [PubMed] [Google Scholar]
- Mosier D. E. Ontogeny of mouse lymphocyte function. I. Paradoxical elevation of reactivity to allogeneic cells and phytohemagglutinin in BALB-c fetal thymocytes. J Immunol. 1974 Jan;112(1):305–310. [PubMed] [Google Scholar]
- Pierres M., Germain R. N. Antigen-specific T cell-mediated suppression. IV. Role of macrophages in generation of L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT)-specific suppressor T cells in responder mouse strains. J Immunol. 1978 Oct;121(4):1306–1314. [PubMed] [Google Scholar]
- Pilarski L. M. Ontogeny of cell-mediated immunity. I. Early development of alloantigen-specific cytotoxic T-cell precursors in postnatal mice. J Exp Med. 1977 Sep 1;146(3):887–892. doi: 10.1084/jem.146.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E., Karnovsky M. J. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972 Sep 1;136(3):466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Rouse R. V., van Ewijk W., Jones P. P., Weissman I. L. Expression of MHC antigens by mouse thymic dendritic cells. J Immunol. 1979 Jun;122(6):2508–2515. [PubMed] [Google Scholar]
- Schwartz R. H., Yano A., Paul W. E. Interaction between antigen-presenting cells and primed T lymphocytes: an assessment of Ir gene expression in the antigen-presenting cell. Immunol Rev. 1978;40:153–180. doi: 10.1111/j.1600-065x.1978.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulation of lymphocyte functions by the macrophage. Immunol Rev. 1978;40:227–255. doi: 10.1111/j.1600-065x.1978.tb00408.x. [DOI] [PubMed] [Google Scholar]
- Von Boehmer H., Adams P. B. Syngeneic mixed lymphocyte reaction between thymocytes and peripheral lymphoid cells in mice: strain specificity and nature of the target cell. J Immunol. 1973 Feb;110(2):376–383. [PubMed] [Google Scholar]
- Waksman B. H. Tolerance, the thymus, and suppressor T cells. Clin Exp Immunol. 1977 Jun;28(3):363–374. [PMC free article] [PubMed] [Google Scholar]
- Waldmann H., Pope H., Beetles C., Davies A. J. The influence of thymus on the development of MHC restrictions exhibited by T-helper cells. Nature. 1979 Jan 11;277(5692):137–138. doi: 10.1038/277137a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Haas W., Jerne N. K. Major histocompatibility complex-linked immune-responsiveness is acquired by lymphocytes of low-responder mice differentiating in thymus of high-responder mice. Proc Natl Acad Sci U S A. 1978 May;75(5):2439–2442. doi: 10.1073/pnas.75.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]


