Abstract
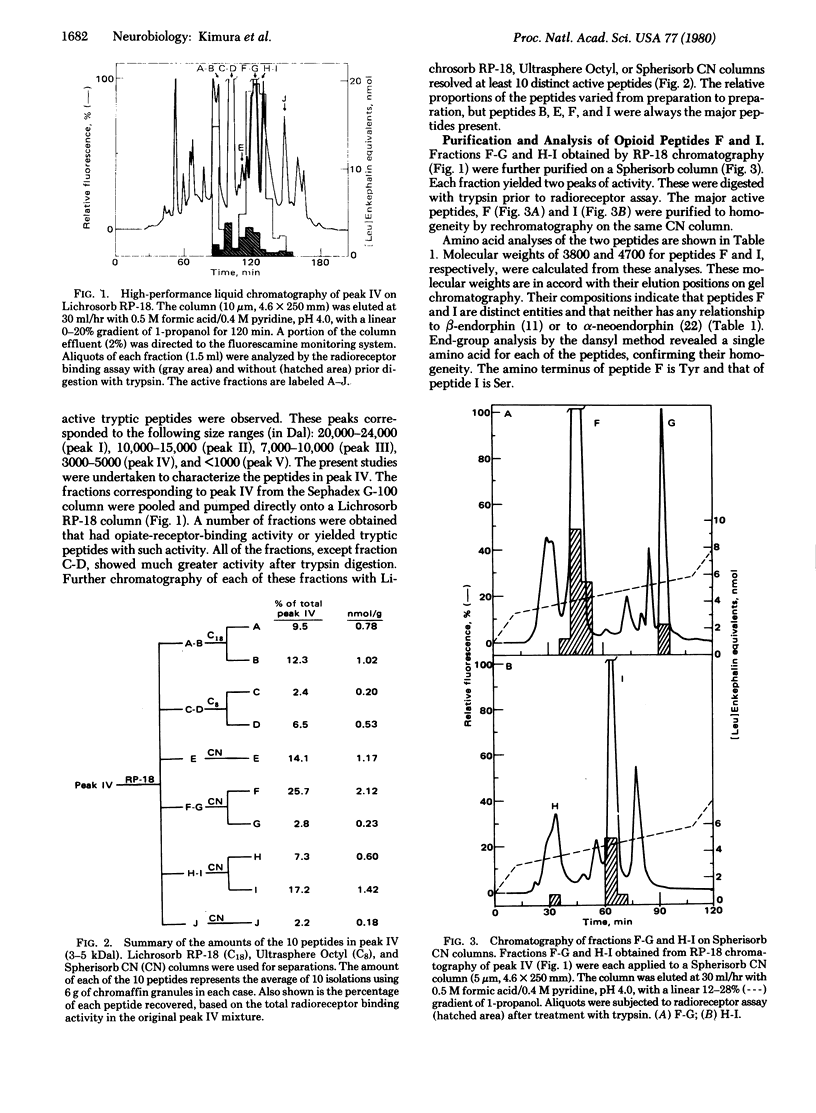
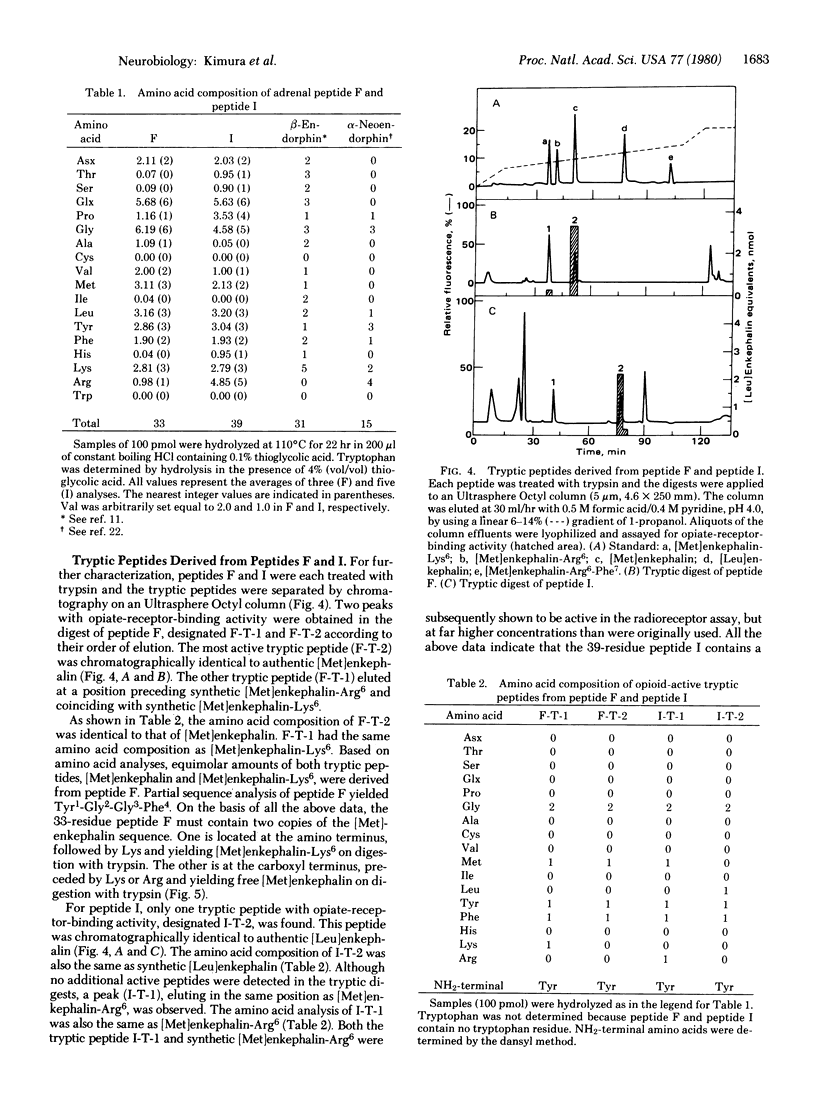
Adrenal chromaffin granules contain at least 10 peptides, ranging in size from 3 to 5 kilodaltons, that yield, upon digestion with trypsin, peptides that show specific binding to opiate receptors. All are distinctly different from beta-endorphin. Two of these peptides have been purified to homogeneity and subjected to chemical analysis. One is apparently a [Met]enkephalin precursor containing two copies of the [Met]enkephalin sequence. The other peptide contains both [Leu]enkephalin and [Met]enkephalin sequences and is presumably a common precursor of the two forms of enkephalin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Stein S., Stone J., Udenfriend S. Automatic Monitoring of primary amines in preparative column effluents with fluorescamine. Anal Biochem. 1975 Aug;67(2):438–445. doi: 10.1016/0003-2697(75)90316-4. [DOI] [PubMed] [Google Scholar]
- Gerber L. D., Stein S., Rubinstein M., Wideman J., Udenfriend S. Binding assay for opioid peptides with neuroblastoma x glioma hybrid cells: specificity of the receptor site. Brain Res. 1978 Jul 28;151(1):117–126. doi: 10.1016/0006-8993(78)90954-x. [DOI] [PubMed] [Google Scholar]
- Kangawa K., Matsuo H. alpha-Neo-endorphin : a "big" Leu-enkephalin with potent opiate activity from porcine hypothalami. Biochem Biophys Res Commun. 1979 Jan 15;86(1):153–160. doi: 10.1016/0006-291x(79)90394-2. [DOI] [PubMed] [Google Scholar]
- Kimura S., Lewis R. V., Gerber L. D., Brink L., Rubinstein M., Stein S., Udenfriend S. Purification to homogeneity of camel pituitary pro-opiocortin, the common precursor of opioid peptides and corticotropin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1756–1759. doi: 10.1073/pnas.76.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I., Childers S., Snyder S. H. Met- and Leu-enkephalin immunoreactivity in separate neurones. Nature. 1979 Nov 22;282(5737):407–410. doi: 10.1038/282407a0. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stein S., Gerber L. D., Rubinstein M., Udenfriend S. High molecular weight opioid-containing proteins in striatum. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4021–4023. doi: 10.1073/pnas.75.8.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. V., Stein S., Udenfriend S. Separation of opioid peptides utilizing high performance liquid chromatography. Int J Pept Protein Res. 1979 May;13(5):493–497. doi: 10.1111/j.1399-3011.1979.tb01911.x. [DOI] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S., Rossier J., Stein S., Udenfriend S. Putative enkephalin precursors in bovine adrenal medulla. Biochem Biophys Res Commun. 1979 Aug 13;89(3):822–829. doi: 10.1016/0006-291x(79)91852-7. [DOI] [PubMed] [Google Scholar]
- Li C. H., Chung D. Isolation and structure of an untriakontapeptide with opiate activity from camel pituitary glands. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1145–1148. doi: 10.1073/pnas.73.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier J., Vargo T. M., Minick S., Ling N., Bloom F. E., Guillemin R. Regional dissociation of beta-endorphin and enkephalin contents in rat brain and pituitary. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5162–5165. doi: 10.1073/pnas.74.11.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultzberg M., Lundberg J. M., Hökfelt T., Terenius L., Brandt J., Elde R. P., Goldstein M. Enkephalin-like immunoreactivity in gland cells and nerve terminals of the adrenal medulla. Neuroscience. 1978;3(12):1169–1186. doi: 10.1016/0306-4522(78)90137-9. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S., Böhlen P., Stone J., Dairman W., Udenfriend S. Amino acid analysis with fluorescamine at the picomole level. Arch Biochem Biophys. 1973 Mar;155(1):202–212. doi: 10.1016/s0003-9861(73)80022-0. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Lewis R. V., Kimura S., Rossier J., Gerber L. D., Brink L., Stein S., Udenfriend S. Isolation of the opioid heptapeptide Met-enkephalin [Arg6,Phe7] from bovine adrenal medullary granules and striatum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6680–6683. doi: 10.1073/pnas.76.12.6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Yang H. Y., Fratta W., Hong J. S., DiGiulio A. M., Costa E. Detection of two endorphin-like peptides in nucleus caudatus. Neuropharmacology. 1978 Jun;17(6):433–438. doi: 10.1016/0028-3908(78)90023-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Pisano J. J. High-performance liquid chromatography of amino acid derivatives. Methods Enzymol. 1977;47:45–51. doi: 10.1016/0076-6879(77)47007-1. [DOI] [PubMed] [Google Scholar]