Abstract
Aging human lens crystallins are progressively modified by yellow glycation, oxidation, and cross-linked carbonyl compounds that have deleterious properties on protein structure and stability. In order to test the hypothesis that some of these compounds originate from oxidized vitamin C, we have overexpressed the human vitamin C transporter 2 (hSCVT2) in the mouse lens. We find that levels of ascorbic and dehydroascorbic acid are highly elevated compared to the wild type and that the lenses have accumulated yellow color and advanced Maillard reaction products identical with those of the human lens. Treatment of the mice with nucleophilic inhibitors can slow down the process, opening new avenues for the pharmacological prevention of senile cataractogenesis.
Keywords: glycation, ascorbic acid, crystallin, cross-linking, aging
Introduction
The aging human lens, by virtue of having little to no protein turnover, is susceptible to accumulation of damage to its proteins, the crystallins. During early development, the crystallins undergo a set of postsynthetic modifications that include phosphorylation, deamidation, and enzyme-mediated protein truncation. 1–3 While, however, the phosphorylation status appears to be little changed once fiber-like cells are differentiated, deamidation is an ongoing process that participates in chemical modification of side-chain residues resulting from oxidation, cleavage of the peptide backbone through reactive oxygen species, and covalent modification of amino acid residues.4 This is associated with enhanced risk of crystalline aggregation.5
In contrast, senescence-related changes typically relate to stochastic forms of damage, i.e., post-translations by low-molecular weight compounds. All processes occur throughout life. While they are unlikely to occur rapidly enough to trigger cataract formation per se, they are thought to predispose lens crystallins toward aggregation and formation of light scattering high-molecular-weight aggregates. In that regard, unequivocal evidence for age-related accumulation of multimeric protein cross-links in the aging human lens has been reported.6
The Maillard reaction is the reaction that is commonly observed during cooking or baking foods. It involves reactive carbonyl compounds from reducing sugars, methyglyoxal, oxidized lipids, or ascorbic acid (ASA) oxidation products, which, upon reaction with proteins, lead to the formation of yellow-colored protein adducts and cross-links. The Maillard reaction proceeds throughout aging in the human lens and other tissues.7 Because of the high reactivity of ASA degradation products with proteins and its presence in high concentrations in the human lens, we and others have postulated that it is, in part, responsible for the progressive damage to the aging human lens crystallins. 8–10 However, it has not been possible to unequivocally implicate vitamin C in this aging process because the products formed are not specific for this vitamin.
To confirm the role of vitamin C in lens ag, we took advantage of the fact that the mouse lens has very low levels of the vitamin and engineered a mouse that selectively overexpresses the human vitamin C transporter 2 (hSVCT2)11 in both the epithelium and fiber-like lens cells. Consequently, lenticular levels of vitamin C and its oxidation products dramatically increased, resulting in accelerated formation of several advanced glycation end products (AGEs) identical with those of the aging human lens. With the use of this humanized mouse model, we conducted a first intervention study with potential inhibitors to test the feasibility of preventing ascorbylation in aging lenses.
Study Design
Generation of Transgenic Mice
Mice were housed under diurnal lighting conditions and allowed free access to food and water. All animals were used in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmology and Vision Research. The hSVCT2 driven by αA crystallin promoter with chick δ1-crystallin enhancer was microinjected into fertilized eggs of B6SJLF1 mice, and the eggs were then transferred into the oviducts of pseudopregnant female mice (Transgenic Animal Model Core, University of Michigan, Ann Arbor, MI). hSVCT2 transgenic mice were identified via PCR screening from genomic DNA extracted from tails. Transgenic mice were crossbred to C57BL/6 gene background by at least eight generations.
Aging Study Design
Transgenic and age-matched control mice, 10 mice per group, were maintained on a standard mouse diet (Prolab 5P75 Isopro 3000; LabDiet, Richmond, IN). Mice were killed at 6, 9, and 12 months. Eyes were removed from mice and decapsulated to release lenses.
Intervention Study Design
Pyridoxamine (PM) dihydrochloride, DL-penicillamine (PA), aminoguanidine (AG), and nucleophilic compounds NC-I and NC-II were all purchased from Sigma Company (St. Louis, MI). The mouse diet with 0.1% w/w potential inhibitors listed above was produced by Bio-Serv using standard diet Isopro 3000. Transgenic and age-matched control mice, 10 mice per group, were maintained on a standard mouse diet or special medical diet started at 2 months and continued until 9months of age. The body weight and food intake were monitored monthly. Nine-month-old mice were sacrificed, and eyes were removed and decapsulated to release the lenses.
Measurement of Ascorbic Acid and Dehydroascorbic Acid
ASA and dehydroascorbic acid (DHA) concentrations were determined based on dimethyl-o-phenylene-diamine derivatization as previously described, 12 with few modifications.36 In brief, after mice were killed, eyes were removed and decapsulated immediately. Mouse lenses were homogenized in 200µL of 10% cold trichloroacetic acid and kept on ice for 10 min.
After oxidation with 10 µL of 0.01 M iodine in 2.7% potassium iodide, 20µL of 0.01 M thiosulfate was added to reduce excess iodine. Samples were then derivatized by adding 50µL of sodium phosphate buffer (pH 5.4) and 20µL of dimethyl-o-phenylene-diamine (1 mg/mL in 0.1M HCl). The derivatized samples were injected into an HPLC and separated by C18 reverse phase. The derivative was detected by using a fluorescent detector with excitation at 360 nm and emission at 440 nm. For quantitation of DHA, the lens extract was directly derivatized under the same conditions but without the oxidation step.
Measurement of Advanced Glycation End Products
Several AGEs were analyzed in both aging and intervention studies. Pentosidine, carboxymethyllysine (CML), carboxyethyllysine (CEL), furosine, and vesperlysine A were analyzed after hydrolysis of lens tissue with 6 M HCL. CML, CEL, and furosine were determined with a gas chromatography/mass spectrometry method as reported by a previous study.13 Vesperlysine A was determined by two-step HPLC as reported previously. 14 K2P and fluorescence measurements were completed after enzymatic digestion with a series protease following a previous report.15 Two types of fluorescence were recorded at λ335/385 and λ370/440.
Statistical Analysis
All values were expressed as mean±SE. Statistical significance of difference in mean values was assessed by repeated measures ANOVA or Student’s t-test. Only P values of <0.05 were considered statistically significant.
Results
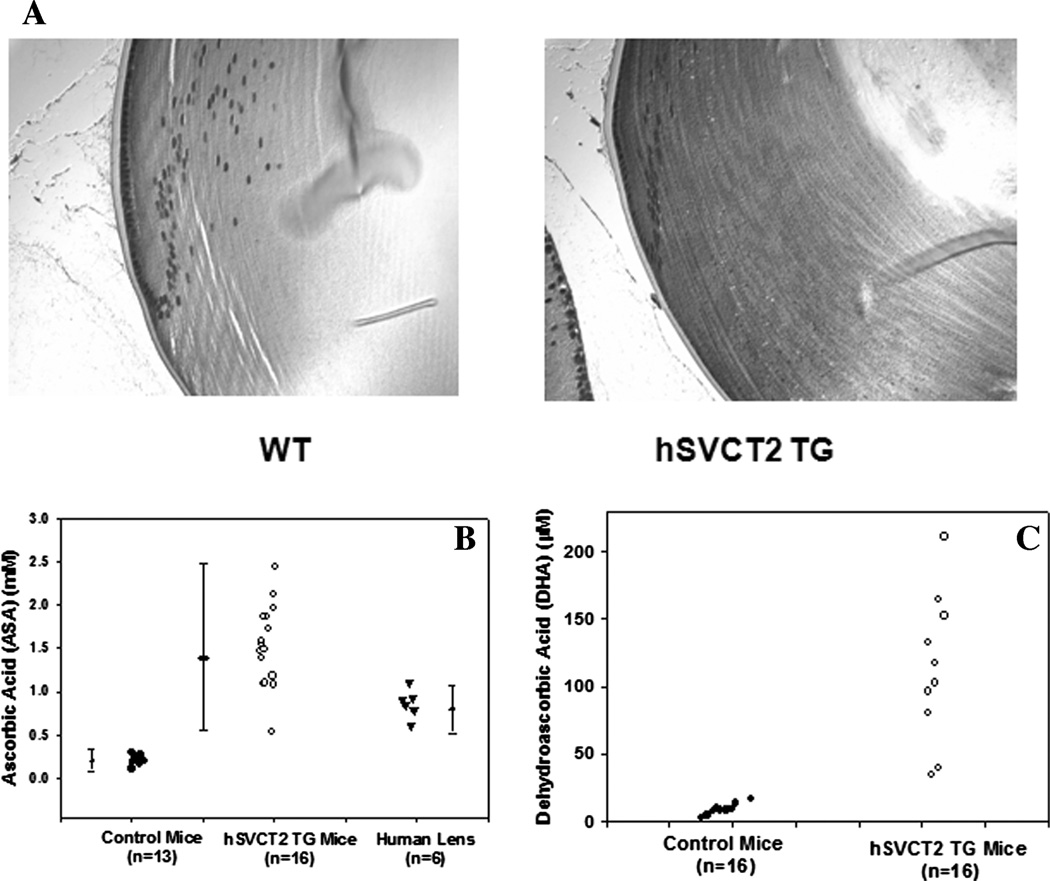
Expression of hSVCT2 in lens epithelium and fiber cells leads to increased lenticular levels of ASA and DHA. Evidence of lens-specific expression of the transporter was obtained in several ways. First, immunohistochemical staining with an hSVCT2 antibody (Alpha Diagnostic International, San Antonio, TX) revealed staining throughout the epithelium and the fiber-like cells of the transgenic-mouse lens, whereas only the lens epithelium of the wild type was positive (Fig. 1A).
FIGURE 1.
Effects of hSVCT2 overexpression in mouse lens epithelial and fiber cells on uptake and lenticular levels of vitamin C and dehydroascorbic acid (DHA). (A) Immunohistochemical staining of a typical control (left) and transgenic (right) lens with an antibody against the C-terminal portion of hSVCT2 reveals a diffuse presence of hSVCT2 along the cell membrane in cortical layers of the lens. (B) ASA concentration in 16 lenses from hSVCT2 mice, 13 controls from the same litter, and six human lenses. (C) DHA concentration in 10 lenses from hSVCT2 and control mice. WT = wild type; TG = transgenic.
Finally, lenticular levels of ASA and DHA measured by HPLC in trichloroacetic acid extract were five-fold to 15-fold elevated. ASA increased from approximately 0.2 mM to 0.5–2.5 mM, and DHA increased from 10µM to 40–250 µM in the transgenic lenses (Fig. 1B and C). ASA levels are shown for comparison in human lenses obtained at autopsy and stored frozen (Fig. 1B). Because of auto-oxidation during storage, these ascorbate levels are likely to be at the lower end. Most importantly, in contrast to previous lens-specific transgenes,16 sustained age-related expression of the hSVCT2 transgene persisted throughout the 12-month testing period (data not shown).
Expression of hSVCT2 in Mouse Lens Leads to Increased Levels of Ascorbic Acid–derived Advanced Ascorbylation End Products
The incubation of ASA and other reducing sugars with proteins under oxidative conditions is known to result in the formation of various protein-bound fluorophores and cross-links, such as pentosidine and vesperlysine A, as well as the colorless, glycoxidation, lipoxidation product CML, all of which have been documented in the aging human lens.14,17 In addition, a lysine–lysine cross-link named K2P was recently identified in the human lens as a major fluorophore and UVA-active protein modification.18 K2P could be synthesized from the reaction of ASA with human lens proteins.19 All these compounds, including protein-bound fluorescence, were assayed at 6, 9, and 12 months of age either in the enzymatic digest (K2P and protein-bound fluorescence) or acid hydrolyzate of the mouse lens crystallins (pentosidine, vesperlysine A, and CML) following established procedures. The glucose-derived Amadori product fructose–lysine (assayed as furosine) and the methylglyoxal (MGO)-derived CEL were also measured. Protein-bound fluorescence at λ335/385 nm and λ370/440 nm was significantly increased in the transgenic versus the wild-type lenses and increased with age (P = 0.0001) (Table 1). The fluorescent cross-links K2P, pentosidine, and vesperlysine A were all significantly increased in the transgenic lenses at all time points (P =0.001 to P = 0.0001) (Table 1). CML was also increased, although to a lesser degree (P = 0.001 or less) (Table 1). CEL was barely increased (Table 1), suggesting that MGO is likely only mildly elevated, although other markers of MGO levels will be needed to confirm this conclusion. Similarly, furosine was not increased (Table 1), excluding the presence of high lenticular glucose concentrations as a cause for the elevated AGEs in the transgenic lenses.
TABLE 1.
Lens protein bound fluorescence and advanced glycation end products (AGEs) in transgenic-mouse model and wild-type mouse at age 6, 9, and 12 months
| Wild-type mouse lens |
Transgenic-mouse lens |
Human lens |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fluorescence and AGEs |
6 mos | 9 mos | 12 mos |
P value with age |
6 mos | 9 mos | 12 mos |
P value with age |
Reported Human lens results with age |
Ref. |
| λ335/385(unit/unit protein) | 6.57 ± 2.02 | 9.11 ± 0.97 | 13.91 ± 1.07 | 0.05 | 15.04 ± 2.53 | 18.97 ± 3.36 | 45.50 ± 11.17 | 0.0001 | Significant | 35 |
| λ370/440(unit/unit protein) | 5.20 ± 1.76 | 5.88 ± 1.02 | 6.95 ± 1.53 | N.S | 8.16 ± 3.41 | 10.89 ± 3.37 | 17.59 ± 4.20 | 0.0001 | Significant | 36 |
| K2P(pmol/µmol leu equi) | 0.00 | 0.10 ± 0.00 | 0.11 ± 0.0 | N.S | 3.08 ± 1.33 | 3.50 ± 1.51 | 19.94 ± 4.27 | 0.0001 | Significant | 18 |
| CML(µmol/mol lysine) | 159.73 ± 13.05 | 228.82 ± 30.47 | 253.03 ± 35.81 | 0.01 | 367.35 ± 76.96 | 444.41 ± 101.14 | 583.33 ± 105.37 | 0.005 | Significant | 14 |
| CEL(µmol/mol lysine) | 205.37 ± 6.71 | 48.10 ± 5.11 | 53.30 ± 9.75 | N.S | 86.58 ± 14.32 | 65.04 ± 18.80 | 77.62 ± 28.72 | N.S | Significant | 37 |
| Furosine(µmol/mol lysine) | 1035.95 ± 175.88 | 1383.21 ± 267.32 | 1250.16 ± 263.30 | N.S | 1323.13 ± 319.68 | 1391.42 ± 272.52 | 1138.92 ± 215.27 | N.S | N.S | 14 |
| Vesperlysine A (pmol/µmol leu equi) | 0 | 0 | 0 | N.S | 2.93 ± 1.35 | 10.35 ± 1.59 | 14.17 ± 2.29 | 0.008 | Significant | 14 |
| Pentosidine (pmol/µmol leu equi) | 0.0006 ± 0.0 | 0.056 ± 0.053 | 0.111 ± 0.071 | N.S | 0.44 ± 0.27 | 1.13 ± 0.48 | 1.94 ± 0.79 | 0.0001 | Significant | 9 |
| Lens discoloration | no | no | no | no | mild | yellow | Yellow around 55 yrs | 20 | ||
Abbreviations: CEL, carboxyethyllysine; CML, carboxymethyllysine; K2P, ;N.S., not significant.
Finally, we noticed in the course of this work that the 12-month-old transgenic lenses had acquired a yellow color similar to that observed in older human lenses (Fig. 2).
FIGURE 2.
Macroscopic appearance of the hSVCT2 transgenic-mouse lenses at 12 months. The transgenic lenses are colored yellow. All mouse lenses were photographed in the same dish to allow accurate comparison between wild-type and transgenic lenses. A fresh 67-year-old human lens obtained at autopsy is shown for comparison. (In color in Annals online.)
Effect of Inhibitors on Ascorbic Acid-derived Advanced Glycation End Products
The incubation of ASA and other reducing sugars with proteins under oxidative conditions is known to result in the formation of various protein cross-links. This has been unequivocally demonstrated in our transgenic mice. This humanized mouse model condensed into 12 months the age-related lens discoloration process that usually develops over several decades in the human; thus, we expect it to be a useful model for testing several inhibitors to prevent the ascorbylation process in vivo.
Toward this end, we have tested five candidate inhibitors in a 7-month-long intervention study. NC-I was able to significantly decrease the lysine–arginine cross-link pentosidine by more than 50% (Table 2) (P <0.04) and suppressed by 60% the increase in the lysine-modified AGE product CML (Table 2) (P <0.007). NC-II-treated mice showed significantly decreased pentosidine level (Table 2) (P <0.003) but no change in CML. NC-I but not NC-II tended to decrease the lysine-linked modification CEL. It should be noted that the latter was only mildly elevated, as previously observed,15 suggesting that MGO formation is not an important pathway in the oxidative degradation of ASA. Animals treated with AG, PM, NC-I, and NC-II showed improvements of lysine–lysine cross-link compoundK2P. Because of large standard deviations, none of them reached significance. Animals treated with AG, PM, and pentosidine showed no significant improvement in any of the other measured AGEs.
TABLE 2.
Inhibitors effect on ascorbylation in transgenic-mouse compared to wild-type mouse lens
| Fluorescence and AGEs | WT | TG | PD | AG | PA | NC-I | NC-II |
P value compare to TG |
|---|---|---|---|---|---|---|---|---|
| λ335/385(unit/unit protein) | 9.10 ± 0.97 | 17.54 ± 2.28 | 16.87 ± 5.52 | 17.36 ± 3.26 | 15.51 ± 3.95 | *13.41 ± 2.41 | **13.24 ± 2.19 | *0.04 **0.004 |
| λ370/440(unit/unit protein) | 5.88 ± 1.02 | 8.76 ± 1.26 | 8.66 ± 1.17 | 8.51 ± 1.70 | 7.97 ± 2.25 | *6.63 ± 1.19 | **6.53 ± 1.00 | *0.05 **0.02 |
| K2P(pmol/µmol leu equi) | 50.10 ± 0.00 | 2.11 ± 1.21 | 0.98 ± 0.48 | 0.91 ± 0.50 | 2.02 ± 1.43 | 1.10 ± 0.36 | 1.33 ± 0.51 | N.S |
| CML(µmol/mol lysine) | 156.00 ± 13.05 | 224.68 ± 30.21 | 223.21 ± 21.17 | 226.34 ± 56.46 | 226.66 ± 74.91 | **183.70 ± 22.68 | 210.83 ± 36.08 | **0.007 |
| CEL(µmol/mol lysine) | 48.09 ± 5.11 | 62.02 ± 5.98 | 61.11 ± 14.45 | 62.02 ± 8.24 | 61.12 ± 5.94 | 52.61 ± 14.39 | 59.70 ± 11.74 | N.S |
| Pentosidine(pmol/µmol leu equi) | 0.055 ± 0.053 | 0.795 ± 0.366 | 0.725 ± 0.341 | 0.532 ± 0.203 | 0.637 ± 0.143 | *0.401 ± 0.120 | **0.380 ± 0.149 | *0.04 **0.003 |
| Furosine(µmol/mol lysine) | 1383.21 ± 267.32 | 1331.91 ± 351.85 | 1235.71 ± 426.73 | 1597.49 ± 376.74 | 1566.04 ± 274.86 | 1168.56 ± 356.43 | 1408.13 ± 533.32 | N.S |
| GSH | 2.46 ± 0.12 | 2.10 ± 0.09 | 2.03 ± 0.07 | 2.15 ± 0.13 | 2.15 ± 0.11 | 2.16 ± 0.16 | 2.01 ± 0.07 | N.S |
Abbreviations: AG, aminoguanidine; GSH, glutathione; NC-1, nucleophilic compound I; NC-2, nucleophilic compound II; PA, penicillamine; PD, pyridoxamine; TG, transgenic; WT, wild type.
Discussion
The above data strongly implicate vitamin C in at least one form of the chemical processes that affect the aging human lens crystallins. Furthermore, the hSVCT2–δenαA mouse is an animal model capable of reproducing, within a very short time, the yellow discoloration and crystallin modifications that increase over several decades in the human lens. These findings suggest that a substantial part of the yellowing process to aging human lens crystallins likely arises from vitamin C. These two statements and their significance for the aging human lens and cataractogenesis need to be examined. At the outset, it should be noted that cataracts, i.e., the formation of light-scattering protein aggregates, can occur at any age as a result of mutations in any of the lens proteins that are critically involved in lens transparency. Thus, chemical modification of lens crystallins is not needed for opacification to occur, and, in the absence of overt cataractogenic conditions and risk factors, the healthy lens can remain transparent for decades while being progressively discolored. Concerning this latter process, however, several epidemiological and clinical studies have revealed a strong association among lens color, lens fluorescence, and the nuclear sclerosis that accompany age-onset cataract formation.20,21 It is, therefore, reasonable to postulate that the accumulation of protein modifications by vitamin C oxidation and other Maillard reaction products may predispose lens crystallins toward destabilization and aggregation, as supported by several in vitro studies.22–24
PM and AG are two AGE inhibitors that have been extensively studied both in vitro and in vivo.25–27 Studies revealed that PM and AG can dramatically inhibit AGE formation in diabetic animals. PA, a thiol compound, is another potential AGE inhibitor and antioxidant that has been shown to inhibit AGEs formation and nitric oxide synthase activity in cultured rabbit proximal tubular epithelial cells28 as well as AGE formation in bovine eyes incubated with glucose or glucose-6-phosphate.29 Both NC-I and NC-II have a guanidino group in the structure that can trap dicarbonyl compounds and block AGE formation. In addition, guanidino compounds can also serve as free radical scavengers.30 Increased guanidino compounds were found present in brain from hyperargininemia patients.31,32 However, their role in this condition as well as in normal humans is still unclear.32
We decided to choose these five prototypic inhibitors for our first pharmacological intervention in the δenαA-hSVCT2 transgenic mouse. NC-I and NC-II stood out showing significant inhibition of AGE formation by ascorbylation. NC-I and NC-II also showed significant improvement of protein-bound fluorescence at both bands of λex/em 335/385nm and 370/440 nm. Other inhibitors, PM, AG, and PA had no significant effect. All five inhibitors showed potential reduction of the lysine–lysine cross-link K2P with, however, large standard deviations such that none of them reached significance. A previous study on K2P levels in the human lens showed that levels remain quite low until middle age and significantly increase at late age.33 This was also confirmed in our previous δenαA-hSVCT2 transgenic-mouse aging study.15 K2P was maintained at a fairly low level from 6 to 9 months and dramatically increased at 12 months. For budgetary considerations, we chose to stop the intervention at 9 months of age. This may explain the high standard deviations in some of the assays, and it is, therefore, possible that other inhibitors besides NC-I and NC-II may have significant effects beyond 9 months. Similar to a previous study, CEL level was only mildly elevated and not significantly affected by treatment, except for a tendency of decreased NC-I. If MGO is indeed a CEL precursor, it is not a significant ASA oxidation product. Finally, the glucose indicator furosine also was not significantly increased in the transgenic lenses, confirming that is an unlikely source of the AGEs measured in this study. PM and AG are two widely studied AGE inhibitors. Surprisingly, they were unable to inhibit formation of most AGEs in spite of data showing they can definitely block the in vitro ascorbylation.34 To our knowledge, there are no in vitro inhibition data on ascorbylation by PA. However, based on its ability to inhibit fluorescence in experiments involving glucose,29 PA may still be able to block ascorbylation in vitro.
The discrepant effects of NC-I and NC-II versus PM, AG, or PA may be, in part, linked to their ability to reach and be taken up by the lens. In fact, two effective inhibitors support our hypothesis. NC-I and NC-II have been shown elsewhere penetrating through the blood barrier. NC-I and NC-II may be able to achieve millimolar concentrations in the lens and thus better trap dicarbonyl compounds. The glutathione level (GSH) was unchanged with inhibitor treatment (data not shown), suggesting that two inhibitors did not change the redox balance, and the GSH was efficient at maintaining ASA in a reduced form.
Acknowledgments
We thank the National Eye Institute (NEY-07099) and the Visual Sciences Research Center Grant (NE 1 EY-11373) for support of these studies.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Schey KL, Fowler JG, Schwartz M JC. Complete map and identification of the phosphorylation site of bovine lens major intrinsic protein. Invest. Ophthalmol. Vis. Sci. 1997;38:2508–2515. [PubMed] [Google Scholar]
- 2.Takemoto L, Boyle D. Increased deamidation of asparagine during human senile cataractogenesis. Mol. Vis. 2000;6:164–168. [PubMed] [Google Scholar]
- 3.Srivastava OP, Srivastava K, Harrington V. Age-related degradation of betaA3/A1-crystallin in human lenses. Biochem. Biophys. Res. Commun. 1999;258:632–638. doi: 10.1006/bbrc.1999.0506. [DOI] [PubMed] [Google Scholar]
- 4.Takemoto L. Deamidation of Asn-143 of gamma S crystallin from protein aggregates of the human lens. Curr. Eye. Res. 2001;22:148–153. doi: 10.1076/ceyr.22.2.148.5524. [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Srivastava OP. Effect of deamidation of asparagine 146 on functional and structural properties of human lens alphaB-crystallin. Invest. Ophthalmol. Vis. Sci. 2004;45:206–214. doi: 10.1167/iovs.03-0720. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava OP, Kirk MC, Srivastava K. Characterization of covalent multimers of crystallins in aging human lenses. J. Biol. Chem. 2004;279:10901–10909. doi: 10.1074/jbc.M308884200. [DOI] [PubMed] [Google Scholar]
- 7.Baynes JW. From life to death–the struggle between chemistry and biology during aging: the Maillard reaction as an amplifier of genomic damage. Biogerontology. 2000;1:235–246. doi: 10.1023/a:1010034213093. [DOI] [PubMed] [Google Scholar]
- 8.Bensch KG, Fleming JE, Lohmann W. The role of ascorbic acid in senile cataract. Proc. Natl. Acad. Sci. USA. 1985;82:7193–7196. doi: 10.1073/pnas.82.21.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagaraj RH, Sell DR, Prabhakaram M, et al. High correlation between pentosidine protein crosslinks and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. USA. 1991;88:10257–10261. doi: 10.1073/pnas.88.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng R, Lin B, Lee KW, Ortwerth BJ. Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim. Biophys. Acta. 2001;1537:14–26. doi: 10.1016/s0925-4439(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 11.Hediger MA. New view at C. Nat. Med. 2002;8:445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 12.Tessier F, Birlouez-Aragon I, Tjani C, Guilland JC. Validation of a micromethod for determining oxidized and reduced vitamin C in plasma by HPLC-fluorescence. Int. J. Vitam. Nutr. Res. 1996;66:166–170. [PubMed] [Google Scholar]
- 13.Dunn JA, Mccance DR, Thorpe SR, et al. Age-dependent accumulation of N epsilon-(carboxymethyl) lysine and N epsilon-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- 14.Tessier F, Obrenovich M, Monnier VM. Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 1999;274:20796–20804. doi: 10.1074/jbc.274.30.20796. [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Reneker LW, Obrenovich ME, et al. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc. Natl. Acad. Sci. USA. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cammarata PR, Zhou C, Chen G, et al. A transgenic animal model of osmotic cataract. Part 1: over-expression of bovine Na+/inositol cotransporter in lens fibers. Invest. Ophthalmol. Vis. Sci. 1999;40:1727–1737. [PubMed] [Google Scholar]
- 17.Dunn JA, Ahmed MU, Murtiashaw MH, et al. Reaction of ascorbate with lysine and protein under autoxidizing conditions: formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry. 1990;29:10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- 18.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. Structure elucidation of a novel yellow chromophore from human lens protein. J. Biol. Chem. 2004;279:45441–45449. doi: 10.1074/jbc.M405664200. [DOI] [PubMed] [Google Scholar]
- 19.Cheng R, Feng Q, Ortwerth BJ. LC-MS display of the total modified amino acids in cataract lens proteins and in lens proteins glycated by ascorbic acid in vitro. Biochim. Biophys. Acta. 2006;1762:533–543. doi: 10.1016/j.bbadis.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Chylack LT, JR, Wolfe JK, Friend J, et al. Quantitating cataract and nuclear brunescence, the Harvard and LOCS systems. Optom. Vis. Sci. 1993;70:886–895. doi: 10.1097/00006324-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Siik S, Chylack LT, JR, Friend J, et al. Lens autofluorescence and light scatter in relation to the lens opacities classification system, LOCS III. Acta Ophthalmol. Scand. 1999;77:509–514. doi: 10.1034/j.1600-0420.1999.770504.x. [DOI] [PubMed] [Google Scholar]
- 22.Nagaraj RH, Oya-Ito T, Padayatti PS, et al. Enhancement of chaperone function of alpha-crystallin by methylglyoxal modification. Biochemistry. 2003;42:10746–10755. doi: 10.1021/bi034541n. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson JE, JR, Lou MF, Gracy RW. Ascorbic acid mediated alteration of alpha-crystallin secondary structure. Curr. Eye. Res. 1995;14:163–166. doi: 10.3109/02713689508999929. [DOI] [PubMed] [Google Scholar]
- 24.Prabhakaram M, Ortwerth BJ. The glycation and cross-linking of isolated lens crystallins by ascorbic acid. Exp. Eye. Res. 1992;55:451–459. doi: 10.1016/0014-4835(92)90118-c. [DOI] [PubMed] [Google Scholar]
- 25.Stitt A, Gardiner TA, Alderson NL, et al. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- 26.Tanimoto M, Gohda T, Kaneko S, et al. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-A(y)/Ta mice. Metabolism. 2007;56:160–167. doi: 10.1016/j.metabol.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 27.Lin YT, Tseng YZ, Chang KC. Aminoguanidine prevents fructose-induced arterial stiffening inWistar rats: aortic impedance analysis. Exp. Biol. Med. (Maywood) 2004;229:1038–1045. doi: 10.1177/153537020422901008. [DOI] [PubMed] [Google Scholar]
- 28.Verbeke P, Perichon M, Friguet B, Bakala H. Inhibition of nitric oxide synthase activity by early and advanced glycation end products in cultured rabbit proximal tubular epithelial cells. Biochim. Biophys. Acta. 2000;1502:481–494. doi: 10.1016/s0925-4439(00)00071-5. [DOI] [PubMed] [Google Scholar]
- 29.Stevens A. The effectiveness of putative anti-cataract agents in the prevention of protein glycation. J. Am. Optom. Assoc. 1995;66:744–749. [PubMed] [Google Scholar]
- 30.Nakai K, Kadiiska MB, Jiang JJ, et al. Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin. Proc. Natl. Acad. Sci. USA. 2006;103:4616–4621. doi: 10.1073/pnas.0510352103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Deyn PP, Marescau B, D’Hoogem R, et al. Guanidino compound levels in brain regions of non-dialyzed uremic patients. Neurochem. Int. 1995;27:227–237. doi: 10.1016/0197-0186(95)00041-6. [DOI] [PubMed] [Google Scholar]
- 32.Mizutani N, Hayakawa C, Ohya Y, et al. Guanidino compounds in hyperargininemia. Tohoku J. Exp. Med. 1987;153:197–205. doi: 10.1620/tjem.153.197. [DOI] [PubMed] [Google Scholar]
- 33.Cheng R, Feng Q, Argirov OK, Ortwerth BJ. K2P-a novel cross-link from human lens protein. Ann. N.Y. Acad. Sci. 2005;1043:184–194. doi: 10.1196/annals.1333.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argirova M, Argirov O. Inhibition of ascorbic acid-induced modifications in lens proteins by peptides. J. Pept. Sci. 2003;9:170–176. doi: 10.1002/psc.451. [DOI] [PubMed] [Google Scholar]
- 35.Nagaraj RH, Prabhakaram M, Ortwerth BJ, Monnier VM. Suppression of pentosidine formation in galactosemic rat lens by an inhibitor of aldose reductase. Diabetes. 1994;43:580–586. doi: 10.2337/diab.43.4.580. [DOI] [PubMed] [Google Scholar]
- 36.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. USA. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed MU, Brinkmann Frye E, Degenhardt TP, et al. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 1997;324(Pt 2):565–570. doi: 10.1042/bj3240565. [DOI] [PMC free article] [PubMed] [Google Scholar]