Abstract
Rising climate temperatures in the future are predicted to accelerate the microbial decomposition of soil organic matter. A field microcosm experiment was carried out to examine the impact of soil warming in freshwater wetlands on different organic carbon (C) pools and associated microbial functional responses. GeoChip 4.0, a functional gene microarray, was used to determine microbial gene diversity and functional potential for C degradation. Experimental warming significantly increased soil pore water dissolved organic C and phosphorus (P) concentrations, leading to a higher potential for C emission and P export. Such losses of total organic C stored in soil could be traced back to the decomposition of recalcitrant organic C. Warming preferentially stimulated genes for degrading recalcitrant C over labile C. This was especially true for genes encoding cellobiase and mnp for cellulose and lignin degradation, respectively. We confirmed this with warming-enhanced polyphenol oxidase and peroxidase activities for recalcitrant C acquisition and greater increases in recalcitrant C use efficiency than in labile C use efficiency (average percentage increases of 48% versus 28%, respectively). The relative abundance of lignin-degrading genes increased by 15% under warming; meanwhile, soil fungi, as the primary decomposers of lignin, were greater in abundance by 27%. This work suggests that future warming may enhance the potential for accelerated fungal decomposition of lignin-like compounds, leading to greater microbially mediated C losses than previously estimated in freshwater wetlands.
INTRODUCTION
The average global surface temperature has increased by 0.74°C since 1850 and is likely to increase by another 1.1 to 6.4°C by the end of this century (43). Decomposition of soil organic matter strongly responds to global warming (6). For open-water regions in freshwater ecosystems, decreased amounts of soil organic carbon (C) associated with nutrient release from soil-microorganism complexes greatly contributes to C dynamics as well as nutrient balance (38, 44). Meanwhile, phosphorus (P) is the key element causing eutrophication and subsequent water quality deterioration (39). Wetlands are well regarded as productive and biologically diverse ecosystems; in particular, the nutrient composition of shallow surface water can be altered by a range of biogeochemical processes (45). Understanding C and nutrient biogeochemical cycling in wetlands in response to global warming and the associated ecological feedback to the biosphere has gained great attention worldwide.
The global change-driven loss of dissolved C is highly correlated to the decomposition of organic C pools stored in soil. Organic C pools can be divided into labile and recalcitrant C fractions, the proportions of which vary according to the heterogeneity of soil with different intrinsic turnover times (14). Labile fractions constitute a small amount of total organic C (TOC) pools and have relatively fast turnover rates, while recalcitrant fractions represent larger portions of the pools and have relatively slow turnover rates (37). The decomposition of labile organic C pools can be equally as sensitive as or even less sensitive than more resistant organic C pools to temperature. For instance, a clear-cut test with changes in stable isotope composition in vegetation indicated that the decomposition of young and old soil organic C might be equally responsive to changes in climate (13), while a 14C isotopic profile (26) and a three-pool model (25) demonstrated that the nonlabile organic C pools may be expected to be of particular importance to C dynamics under warming. This is because recalcitrant compounds with relatively low decomposition rates and high activation energies are inherently more sensitive to rising temperature than labile ones (11). Wetlands contain one-third of the global organic C (19), and even small changes in organic C pools due to altered environmental conditions in these ecosystems may have substantial effects on global C dynamics (12). However, which fractions of the wetland organic C pools are more sensitive to degradation under elevated temperature and how this may affect dissolved organic C (DOC) export are still being debated.
Microorganisms mediate decomposition of soil C pools and drive Earth's biogeochemical cycling (15, 51). In response to warming, microbial biomass may decrease, especially when labile C pools are depleted. The reduction in readily available substrate, in turn, leads to the downregulation of microbial functions (17), lessening the microbial impact on the global C budget. Other studies (5, 40), however, suggested that microbial biomass may either remain steady or even increase, which would accelerate global C cycling and the microbially mediated C feedbacks to climate warming. The destiny of microbial biomass and associated labile C pools in this response remains under debate. Aside from the alteration in microbial biomass, recent studies have also shown that warming can also greatly impact microbial community composition and physiology (8, 16, 17, 49). Such shifts in microbial community composition were also always combined with altered microbial physiology and functioning. For instance, higher copy numbers of fungal genes in warmed samples (8, 50) led to the adjustment of strategies to use C as a resource for microbial growth (2), which suggested a greater C utilization efficiency than in bacteria (31). However, these altered microbial responses to experimental warming based on phylogenetic analysis have not been well explored with regard to the impact of potential changes in microbial community functional and metabolic processes on wetland soil C cycling.
In the past decade, substantial experimental warming research on microbial responses to warming with associated C biogeochemical cycling has been conducted on soil in terrestrial ecosystems such as forests, grass prairies, plateaus, and arctic lands (32, 40, 41, 49). However, the genetic linkage of C dynamics with associated microbial functions in freshwater ecosystems subjected to warming is poorly studied. In this work, a microcosm ecosystem was developed in May 2008 simulating warming scenarios to investigate biogeochemical dynamics in subtropical wetlands (50). In addition to conventional analysis, a comprehensive functional gene microarray, GeoChip 4.0, containing 83,992 50-mer oligonucleotide probes (21, 34) was used to gain insight into soil microbial changes, including microbial gene diversity, and associated ecological function shifts when communities were subjected to warming. We observed how microorganisms mediated the response of different organic C fractions to experimental warming, particularly through the functional potential of detected C-degrading genes. We hypothesized that the accelerated decomposition of recalcitrant organic C pools under experimental warming could be linked with increased microbial metabolic activity for recalcitrant C acquisition and the potential shifts in microbial community composition to a relative dominance of fungi.
MATERIALS AND METHODS
Microcosm configuration.
A custom-built novel microcosm (see Fig. S1 in the supplemental material) simulating climate warming under a minute scale for both daily and seasonal temperature variations was developed using separately monitored water bath jackets under both current ambient temperature conditions (control) and simulated warming conditions of 5°C above ambient temperature (warmed). Specifics regarding the configuration and corresponding operation of this microcosm system since May 2008 have been reported previously (50) and can be found in the supplemental material. This device used a water bath incubation method to maintain hydrological characteristics and a humid habitat for microbial growth, offering a high-resolution temperature comparison, good repeatability, and the capability to simulate realistic warming conditions.
Sample description.
The study sites (see Table S1 in the supplemental material) were located in the southern region of the Taihu Lake Basin and the Ningshao Plain, which has high spatial and temporal variability in sediment-water nutrient exchange. The basic physicochemical parameters of the three subtropical wetland soils selected in situ were previously described (50). In brief, YaTang (YT) wetland is in an advanced state of eutrophication and was classified as eutrophic, with the highest relative levels of organic matter, nutrients, and water content. XiaZhuhu (XZ) and XiXi (XX) wetlands are in a meso-eutrophic state although the organic matter and nutrient contents of XZ soil were nearly twice those of XX soil. Transparent polyvinyl chloride (PVC) columns (45.0 cm in height and 10.0 cm in internal diameter) were prefabricated in May 2008 to be filled with 20 cm of fresh soil and 20 cm of the corresponding overlying water. The details for preparing wetland columns with three replicates were described previously (50). For pore water sampling, a soil solution sampler (0.5-μm porous polyacrylonitrile hollow fiber; Chinese Academy of Sciences, Nanjing) was horizontally embedded into the soil in each column at a fixed depth of 5 cm. About 100 ml of overlying water and 30 ml of pore water were sampled from each wetland column at approximately 90-day intervals from the summer of 2010 to the winter 2010 to 2011 after approximately 2.5 years of warmed incubation. In addition, approximately 100 g of 0- to 5-cm fresh top soil was collected (16 December 2010) for each column from the experimental warming microcosm system. The sampling procedures for water and soil were previously described (50).
Water and soil biogeochemical analysis.
Soil pore water was immediately digested and measured spectrophotometrically using a continuous flow analyzer (Autoanalyzer III; Bran+Luebbe, Germany) set to 880 nm for P measurements. Dissolved organic C (9) was determined using a TOC analyzer (Shimadzu Scientific Instruments, Columbia, MD). Soil samples were centrifuged to remove pore water. One-third of each soil sample was stored at 4°C for 1 to 2 days and was subsequently used for microbial biomass measurement using a fumigation-extraction method as described by Wu et al. (47). In brief, soil-extractable C in fumigated and nonfumigated soil samples was extracted by 0.5 M K2SO4. The difference in extractable C amounts between the fumigated and nonfumigated soils was assumed to be the amount released from lysed soil microbes. Another portion of each pooled sample was stored at −15°C, freeze-dried, ground, sieved, and analyzed for TOC content according to standard methods (4). Organic C content was determined using KMnO4 oxidation (33). Three fractions of labile organic C in these wetland columns, highly labile organic C (HLOC), mid-labile organic C (MLOC), and labile organic C (LOC), were determined using 33 mmol liter−1, 167 mmol liter−1, and 333 mmol liter−1 of KMnO4, respectively. Recalcitrant organic C (RO-C) pools were calculated as the difference between the TOC and the LOC.
Laboratory incubation for soil respiration and enzyme activity assays.
For soil respiration measurements, each soil core was put into a glass with a chamber containing up to 10 ml of 2N NaOH solution as a CO2 absorbent, and respiration rates were measured every other day for the first week and then weekly for the remaining days during a 60-day incubation period (20). Extracellular enzyme activity, including polyphenol oxidase and peroxidase involved in lignin degradation were analyzed under saturating conditions according to Li et al. (28). These measures represent potential enzyme activities indicative of overall enzyme concentrations (46) which are used to evaluate the actual rates of enzymatic reactions under natural in situ conditions. Briefly, both enzymes were assayed spectrophotometrically at 430 nm using 1,2,3-benzenetriol [4-(2-pyridylazo)-pyrogallol (PAPG)] as the substrate, followed by quantification of a red oxidation product of PAPG. For polyphenol oxidase assays, 10 ml of 1% PAPG was added to 1 g of soil and mixed to complete homogenization before incubation in the dark at 30°C for 3 h. After incubation, 4 ml of citric and phosphoric acid buffer (pH 4.5) was added. For peroxidase assays, 10 ml of 1% PAPG plus 2 ml of 0.5% H2O2 was added to 1 g of soil and mixed to complete homogenization before incubation in the dark at 30°C for 1 h. After incubation, 2.5 ml of 0.5 mol liter−1 HCl was added. For both enzymes, 35 ml of diethyl ether was used to absorb the oxidized reaction product; subsequently, absorbance of the solution was measured.
DNA extraction, amplification, and labeling.
DNA extraction was conducted as described previously (34). Aliquots of DNA (100 ng) from each sample were amplified by whole community genome amplification with a TempliPhi kit (GE Healthcare, Piscataway, NJ) using a modified reaction buffer (48). The amplified DNA (2 μg) was then labeled with Cy3 using random primers and the Klenow fragment of DNA polymerase I (34). Labeled DNA was then dried in a SpeedVac (at 45°C for 45 min; ThermoSavant). Sample tracking control (NimbleGen, Madison, WI) was used to confirm sample identity, and hybridization buffer (7.32 μl) was added to the samples (34).
GeoChip 4.0 hybridization and data processing.
The functional gene microarray (GeoChip 4.0) in this study was developed by the Institute for Environmental Genomics (University of Oklahoma). For hybridization, an HX12 mixer (NimbleGen) was placed onto the array using NimbleGen's precision mixer alignment tool. The array was preheated to 42°C on a hybridization station (MAUI; BioMicro Systems, Salt Lake City, UT) for at least 5 min, after which 6.8-μl samples were loaded onto the array surface and hybridized by mixing for approximately 16 h. After hybridization, the arrays were scanned with a laser power of 100% and a 100% photomultiplier tube (MS 200 Microarray Scanner; NimbleGen). Low-quality spots were removed prior to statistical analysis, as described previously (21). Spots were scored as positive if the signal-to-noise ratio (SNR) was >2.0 and the coefficient of variation (CV) of the background was <0.8; these positive spot data were used for the subsequent analysis. The abundance of the detected genes indicated by signal intensities was the relative gene abundance by GeoChip 4.0 analysis, which indicated the relative shifts in microbial community composition differences between the two treatments.
Statistical analysis.
Three-factor, repeated measure analysis of variance (ANOVA) tests were performed to show the effect of geographic variation (site), experimental warming, and sampling date on DOC and P concentrations in soil pore water. Soil organic C fractions, microbial biomass, respiration, and enzyme activities were examined by two-way ANOVA with experimental warming and sampling sites as factors. Student-Newman-Keuls (SNK) analysis was conducted for multiple comparisons within the three sample sites (i.e., YT, XZ, and XX). Student's t tests were used to examine the effects of experimental warming. Detected gene biodiversity was calculated based on Simpson's index. Diversity indices and Mantel tests were carried out using R, version 2.9.1 (http://www.r-project.org/). A paired Student t test was further performed to examine significant changes in microbial gene diversities and community compositions at a P value of <0.05 or 0.01 using SPSS (version 15.0). The Spearman correlation was used to analyze correlations.
RESULTS
Dissolved carbon and phosphorus concentrations in soil pore water.
After a 2.5-year incubation, DOC concentrations in warmed soil pore water increased by 25.5% (XX) to 33.4% (YT) compared to the controls (Fig. 1A). The observed DOC concentration increased to 30.1 mg liter−1 in the summer of 2010 under warming (Fig. 1A, Aug). Geographic variation (site), experimental warming, and sampling date all had significant impacts on DOC concentrations as analyzed by ANOVA (Table 1); however, no possible interaction of these variables was significant (Table 1). Meanwhile, warming significantly (P < 0.01) increased P concentrations in soil pore water (Table 1 and Fig. 1B). The effect of warming on P concentration was highly dependent on the original nutrient status of soils (P = 0.014) (Table 1). A positive correlation between DOC and P concentration (R2 = 0.738; P = 0.037) under warming was also observed, indicating that the effects of warming on the C concentration may also impact P dynamics.
Fig 1.
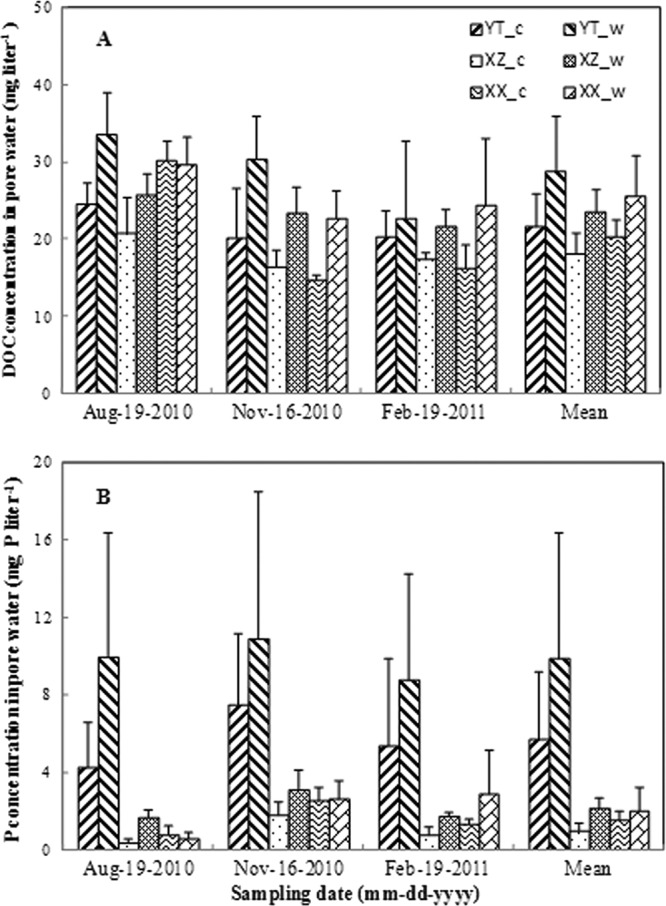
Dissolved organic carbon (DOC) and phosphorus (P) concentrations in soil pore water from YT, XZ, and XX wetland columns in the microcosm experiment under ambient temperature (control, c) and at 5°C above ambient temperature (warmed, w). The DOC and P concentrations measured here (i.e., on the dates indicated) were about 2.5 years from the start of the experiment in May 2008. Error bars are +1 standard deviation.
Table 1.
Results of three-factor, repeated measures ANOVA of soil pore water DOC and phosphorus concentrations to geographic variation, experimental warming, and sampling datea
| Substance |
P value by factor(s)b |
||||||
|---|---|---|---|---|---|---|---|
| Site | Warming | Date | Site × warming | Site × date | Warming × date | Site × warming × date | |
| DOC | 0.019 | <0.001 | 0.001 | 0.697 | 0.258 | 0.459 | 0.490 |
| P | <0.001 | 0.001 | 0.015 | 0.014 | 0.789 | 0.872 | 0.703 |
The DOC and P concentrations were measured in situ in YT, XZ, and XX wetland columns under warmed and control conditions (control, ambient temperature; warmed, ambient temperature + 5°C) in the microcosm experiment on three different sampling dates (i.e., 19 August 2010, 16 November 2010, and 19 February 2011).
Significant P values (<0.05) are in bold.
Soil organic carbon pools and MBC.
The average labile organic C pools as HLOC, MLOC, and LOC in tested wetlands under warming increased by 20.1%, 25.4%, and 6.59%, respectively (Table 2). For HLOC, the contents in soil increased by 1.66 mg g−1 (18.2%), 0.514 mg g−1 (8.87%), and 1.27 mg g−1 (58.7%) under warming conditions for YT, XZ, and XX wetland columns, respectively (P < 0.001) (Table 2). Similarly, significant increases were found in MLOC, but no significant differences in LOC were observed (P = 0.547) (Table 2). The recalcitrant organic C (RO-C) contributed to a large amount of total C (∼82.3%) compared to LOC (∼11.2%) and HLOC (∼6.7%) (Table 2). Warming significantly decreased RO-C content by 22.5 mg g−1 (12.0%) and TOC content by 25.3 mg g−1 (11.3%) in tested wetland columns (Table 2). Further analysis showed that the decrease in RO-C pools was significantly (P < 0.01) correlated with decreases in TOC, according to Spearman correlation analysis, implying that TOC variations in response to warming may be greatly influenced by recalcitrant organic C fractions. Microbial biomass C (MBC) in tested soils accounted for only a small portion of TOC (∼2.03%) and represented ∼12.1% of the labile organic C pools (Table 2). ANOVA with SNK analysis showed an increased microbial biomass C with experimental warming for YT (increased by 21.4%; P = 0.051) and XZ (increased by 31.1%; P = 0.009), along with XX wetland samples (increased by 29.9%; P = 0.067) (Table 2). Additionally, linear regression analysis across different wetlands verified a positive correlation (P < 0.01) between labile organic C fractions and microbial biomass (see Fig. S2 in the supplemental material); among three different labile organic C pools, HLOC pools may be most representative of a possible shift in microbial biomass because they had the highest adjusted R2 values (0.663 compared to 0.471 for LOC, for example).
Table 2.
Effects of warming on soil total organic carbon, highly labile organic carbon, mid-labile organic carbon pools, labile organic carbon, recalcitrant organic carbon, and soil microbial biomass carbon
| Condition and site | Mean amt of carbon by fraction (g kg−1 soil [SD])a |
|||||
|---|---|---|---|---|---|---|
| TOC | HLOC | MLOC | LOC | RO-C | MBC | |
| Control | ||||||
| YT | 133 (9.97) | 9.13 (0.23) | 13.0 (1.01) | 19.0 (4.20) | 111 (7.92) | 1.37 (0.01) |
| XZ | 54.8 (5.91) | 5.80 (0.32) | 8.55 (0.30) | 9.93 (0.43) | 44.9 (6.13) | 1.08 (0.02) |
| XX | 35.5 (1.67) | 2.14 (0.07) | 3.12 (0.23) | 3.70 (0.31) | 31.8 (1.69) | 0.97 (0.02) |
| Warmed | ||||||
| YT | 117 (2.62) | 10.8 (0.15) | 18.6 (0.89) | 20.5 (4.26) | 98.5 (6.98) | 1.66 (0.10) |
| XZ | 49.2 (3.55) | 6.31 (0.07) | 8.67 (0.13) | 9.93 (0.32) | 39.3 (3.40) | 1.42 (0.04) |
| XX | 31.8 (1.26) | 3.39 (0.07) | 3.64 (0.13) | 4.32 (0.16) | 27.4 (1.14) | 1.25 (0.11) |
| P valueb | 0.005 | <0.001 | <0.01 | 0.547 | 0.010 | <0.001 |
TOC, total organic carbon; HLOC, highly labile organic carbon; MLOC, mid-labile organic carbon; LOC, labile organic carbon; RO-C, recalcitrant organic carbon; MBC, soil microbial biomass carbon.
Determined by ANOVA. Significant P values (<0.05) are in bold.
Microbial respiration and metabolic activities.
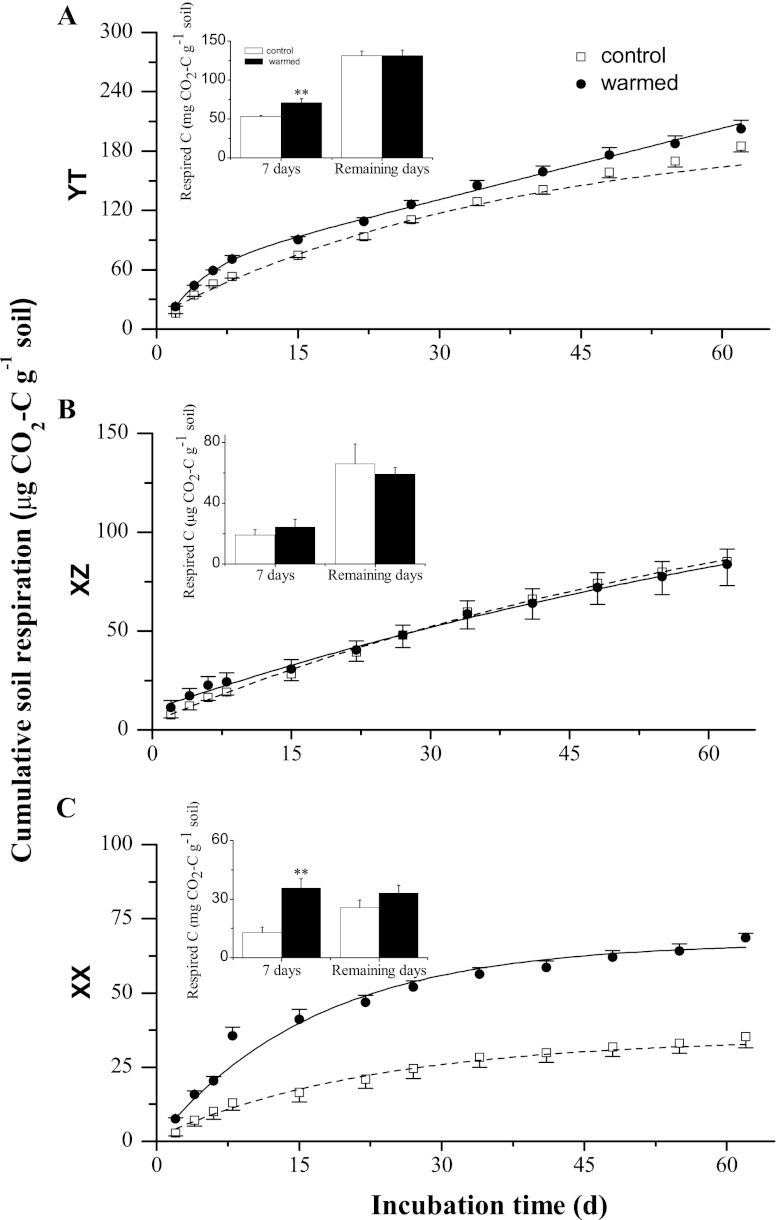
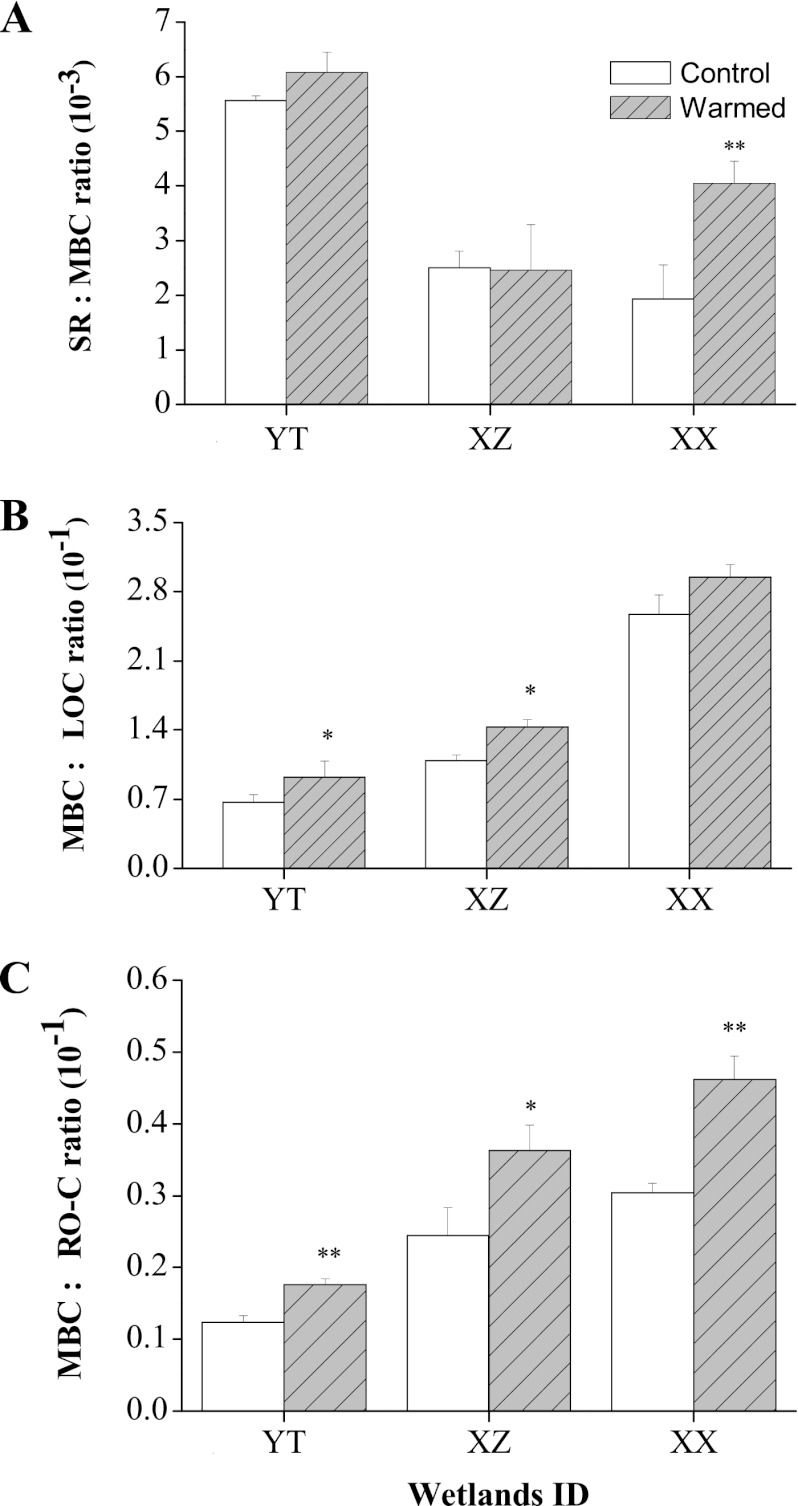
Cumulative soil respiration was generally greater in all warmed samples, with a range from 68.6 (XX) to 202 μg of CO2-C g−1 of soil (YT), than the level in the control (35.3 for XX to 185 μg of CO2-C g−1 for YT) (Fig. 2). About 22.3 (XZ) to 51.9% (XX) of the total CO2 was released from the soil organic C within the first 7 days of the 60-day incubation. Respiration increases of 17.3 (XX) to 28.2% (XZ) were observed for warmed soils compared to the control (Fig. 2, inset). After 7 days, soil respiration rates in warmed samples tended to decline over time, and no significant difference was found between any two treatments up to day 60 (Fig. 2). In addition, microbial metabolic activity estimated by soil microbial respiration to biomass C (2) was positively affected by experimental warming in the case of XX (Fig. 3A). Experimental warming increased C use efficiency (5) for both labile and recalcitrant pools. The labile carbon use efficiency (estimated by the MBC/LOC ratio) (Fig. 3B) significantly (P < 0.001) increased by 38.4%, 31.0%, and 14.5% under warming for YT, XZ, and XX, respectively, while the recalcitrant carbon use efficiency increased by 48.2%, 42.7%, and 51.6%, respectively (estimated by the MBC/RO-C ratio) (Fig. 3C). The XX wetland with the lowest relative soil organic matter content among the three tested wetlands seemed to have the highest carbon use efficiency (XX) (Fig. 3B and C), indicating that microorganisms in nutrient-poor wetlands may be relatively more efficient than those in nutrient-enriched wetlands (i.e., YT) at converting C to form new cells rather than releasing the C dioxide into the atmosphere.
Fig 2.
Cumulative soil respiration (μg of CO2-C g−1 of soil) over the 60-day incubation period at a constant temperature of 25°C for the YT, XZ, and XX wetlands under control (dotted regression lines) and warmed (solid regression lines) conditions. Differences in cumulative soil respiration for the initial 7 days and the remaining days are shown in the inset bar chart. Error bars are +1 standard deviation. Asterisks represent significant Student's t test differences between warmed and control samples (*, P < 0.05; **, P < 0.01).
Fig 3.
(A) Microbial metabolic activities expressed as the ratio of the soil respiration rate (SR; g of CO2-C kg−1 day−1) to microbial biomass carbon (MBC; g kg−1). (B) Labile carbon use efficiency by microbes expressed as the ratio of MBC to labile organic carbon (LOC; g kg−1). (C) Recalcitrant carbon use efficiency by microbes expressed as the ratio of MBC to recalcitrant organic carbon (RO-C; g kg−1) for tested subtropical wetland soils (i.e., YT, XZ, and XX) under experimental warming. Error bars show +1 standard deviation. Asterisks represent significant Student's t test differences between warmed and control samples (*, P < 0.05; **, P < 0.01).
Microbial gene diversity and community structure shifts.
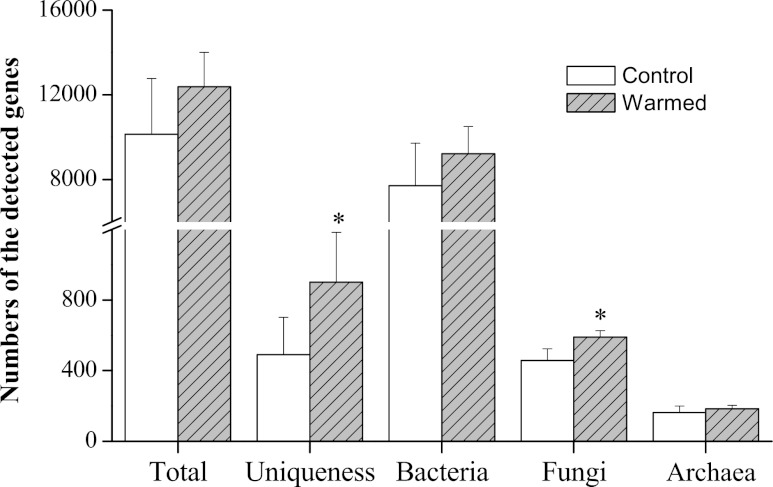
The number of total genes detected by GeoChip 4.0 encoding key soil enzymes was 25.5% greater in warmed samples than in the control (Fig. 4). Approximately 4.6 to 10.2% of detected genes were unique and were found only in warmed samples (P = 0.033) (see Table S2 in the supplemental material). The number of detected genes belonging to different phylogenetic groups was increased for bacteria (by 22.3%; P = 0.089) and fungi (by 27.2%; P = 0.047) in warmed samples; however, no significant or marginally significant differences (P = 0.242) were found for archaea (Fig. 4). The microbial abundance for fungus-related genes as indicated by normalized signal intensity was preferentially higher (P = 0.078) in warmed samples than in the controls. The microbial community structure showed changes in response to warming as well. Most of the bacteria-related genes were derived from Alpha-, Beta-, Gamma-, and Deltaproteobacteria, Actinobacteria, Firmicutes, and Cyanobacteria, while Ascomycota (∼78.9% of total fungus-related genes) and Basidiomycota (∼18.9%) were found to be the dominant phyla of fungi in warmed samples (see Fig. S3 in the supplemental material). Among these main phyla, the gene diversities of Proteobacteria, Ascomycota, and Basidiomycota were significantly (P < 0.05) increased under warming. In order to show the relationship between detected functional genes and soil biogeochemical properties, Mantel tests (Table 3) were performed. The results suggested that the microbial biomass and highly labile organic C were critical in influencing and shaping the microbial functional community structure (microbial biomass, rM = 0.580 and P 0.050, where rM is Mantel's r; for HLOC, rM = 0.448 and P = 0.046).
Fig 4.
Microbial community diversity of total detected genes (total), unique genes detected only in control or warmed samples (uniqueness), and main phylogenetic groups (bacteria, fungi, and archaea), as indicated by detected gene numbers under warmed and control conditions (control, ambient temperature; warmed, ambient temperature + 5°C) by GeoChip 4.0. The number of genes in each category is the average of total gene numbers from YT, XZ, and XX wetland samples. Error bars are +1 standard deviation. Asterisks represent significant Student's t test differences between warmed and control samples (*, P < 0.05; **, P < 0.01).
Table 3.
The relationship of detected functional genes involved in carbon cycling by GeoChip 4.0 and individual environmental variables
| Environmental variable | rMa | Pb |
|---|---|---|
| pH | 0.130 | 0.407 |
| Water content | 0.204 | 0.251 |
| C-relatedc | ||
| TOC | 0.197 | 0.318 |
| HLOC | 0.448 | 0.046 |
| MLOC | 0.099 | 0.409 |
| LOC | 0.194 | 0.312 |
| RO-C | 0.238 | 0.313 |
| MBC | 0.580 | 0.050 |
Mantel statistic. The signal intensities of carbon cycling genes for warmed and control soil samples were used as the first matrix; the normalized environmental variables were used as the second matrix.
Significant P values (<0.05) are in bold.
TOC, total organic carbon; HLOC, highly labile organic carbon; MLOC, mid-labile organic carbon; LOC, labile organic carbon; RO-C, recalcitrant organic carbon; MBC, soil microbial biomass carbon.
Microbial functions in degradation of carbon pools.
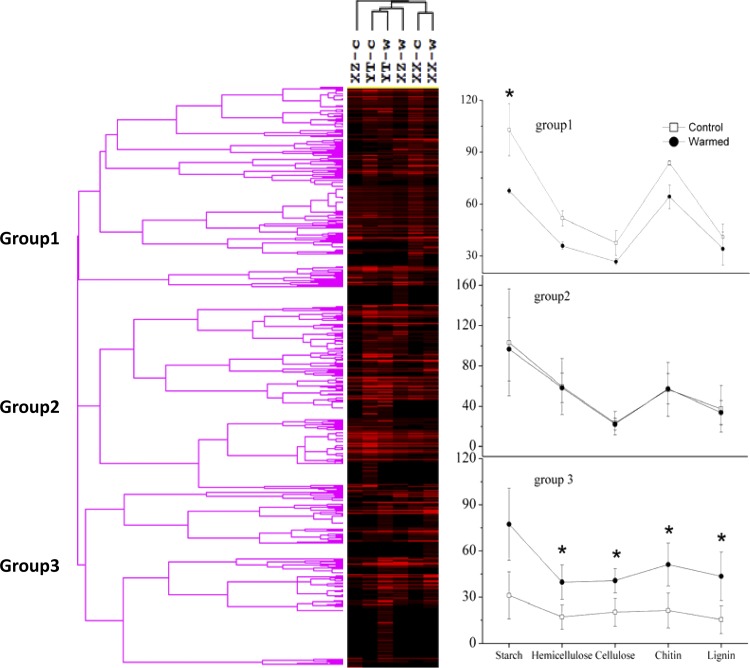
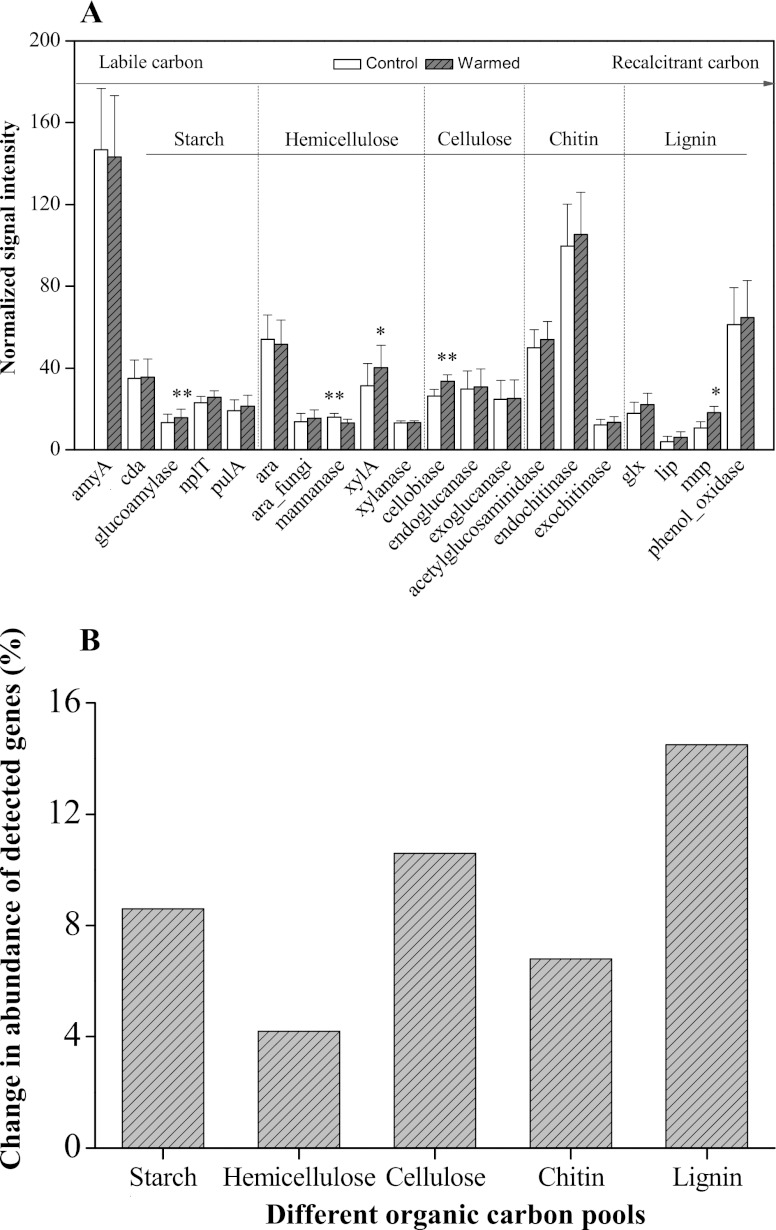
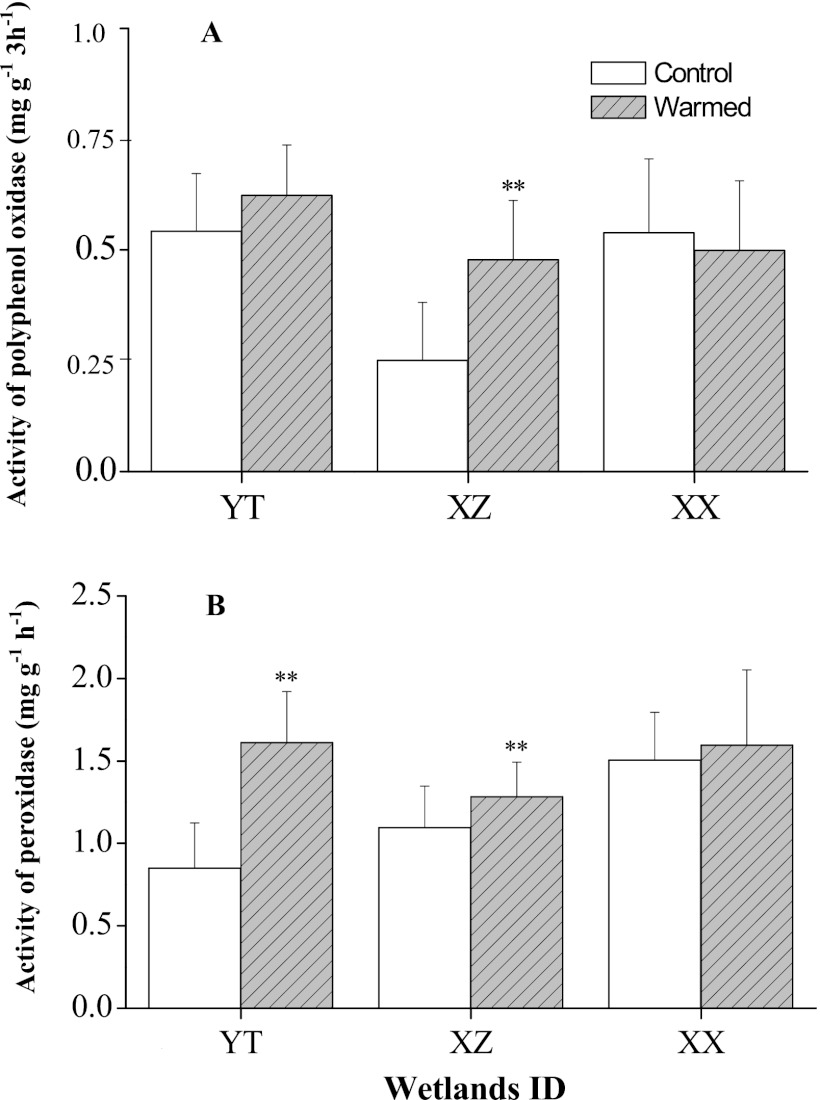
C-degrading genes measured by GeoChip 4.0 include a variety of genes responsible for degrading starch, hemicellulose, cellulose, chitin, and lignin. The complexity of C is presented in an increasing order, from labile to recalcitrant C. The relative abundance of each C-degrading gene represents the microbial functional potential for degrading different organic C fractions (Fig. 5 and 6). Experimental warming generally increased overall detected C-degrading genes, implying that a faster temperature-dependent decomposition of organic matter occurred in warmed soils. For hierarchical cluster analysis (Fig. 5), clustered genes in group 3 (31.4% of total detected C-degrading genes) showed significant increases (P < 0.05) in abundance under warming conditions for genes for key soil enzymes involved in different C degradation. Specific genes encoding glucoamylase for starch degradation, xylA for hemicellulose degradation, cellobiase for cellulose degradation (P = 0.010), and mnp for lignin degradation (P = 0.033) showed significantly (P < 0.01 or 0.05) higher abundance in warmed soils than in the controls (Fig. 6A). In contrast, genes encoding mannanase for hemicellulose degradation significantly (P < 0.01) decreased when subjected to warming. Hierarchical clustering analysis of mnp genes (Fig. 7) showed that warmed samples were clustered together and well separated from control samples. Microbial populations similar to Phanerochaete sordida (protein identification [ID] number, 21929222) and Trametes versicolor (protein ID number, 40714547) showed the strongest signal intensities (Fig. 7). Warming stimulated genes for degrading both labile and recalcitrant C but to different degrees. The relative abundance of detected genes increased by 8.6% and 4.2% for starch and hemicellulose degradation, respectively, while the abundance of detected genes increased by 14.5% and 6.8% for lignin and chitin degradation, respectively, under warming (Fig. 6B). Meanwhile, within the top 20 population/genes showing the highest normalized signal intensities (see Table S3 in the supplemental material), a large proportion of detected genes (more than 50%) were involved in degrading chitin and lignin. Particularly, genes derived from Trametes versicolor which are responsible for lignin degradation increased significantly (P < 0.05) in warmed samples (see Table S3). Genes derived from Ganoderma lucidum (protein ID number, 224037824) were detected in all of the warmed samples but were absent in the controls (see Fig. S4 in the supplemental material). Consistent with this, the potential activities of some key soil enzymes such as polyphenol oxidase and peroxidase involved in lignin degradation were generally enhanced under warming, especially for YT and XZ wetland samples (Fig. 8). In contrast, the abundance of starch-degrading genes clustered in group 3 did not significantly change and even decreased (P < 0.05) in group 1 under warming, as revealed by hierarchical cluster analysis (Fig. 5).
Fig 5.
Hierarchical cluster analysis of all C-degrading genes involved in carbon cycling by GeoChip 4.0. Results were generated in CLUSTER, version 3.0, using the Spearman rank correlation and the complete linkage method and visualized using TREEVIEW. Gray indicates signal intensities above background while black indicates signal intensities below background. Brighter red indicates higher signal intensities. YT-c, XZ-c, and XX-c represent control samples, and YT-w, XZ-w, and XX-w represent warmed samples. The abundance of detected genes, indicated by normalized signal intensities, involved in the degradation of different organic carbon pools (i.e., starch, hemicellulose, cellulose, chitin, and lignin) was plotted in three separate groups (group 1, group 2, and group 3). Genes clustered in group 3 showed a significant (P < 0.05) difference in abundance of detected genes between warmed and control samples. Error bars are ±1 standard deviation. Asterisks represent significant Student's t test differences between warmed and control samples (*, P < 0.05; **, P < 0.01).
Fig 6.
(A) Degradation of organic carbon pools (labile and recalcitrant fractions) indicated by normalized signal intensities of detected key genes involved in carbon degradation as determined by GeoChip 4.0 from tested samples in the microcosm experiment under warmed and control conditions (control, ambient temperature; warmed, ambient temperature + 5°C). The signal intensity for each gene was the average of the total signal intensity from YT, XZ, and XX wetland samples. The complexity of organic C fractions is presented in order from labile to recalcitrant C. Error bars are +1 standard deviation. Asterisks represent significant Student's t test differences between warmed and control samples (*, P < 0.05; **, P < 0.01). (B) Percent change in abundance of detected genes between warmed and control samples, indicated by the normalized signal intensities for decomposition of different organic carbon pools (starch, hemicellulose, cellulose, chitin, and lignin) subjected to experimental warming.
Fig 7.
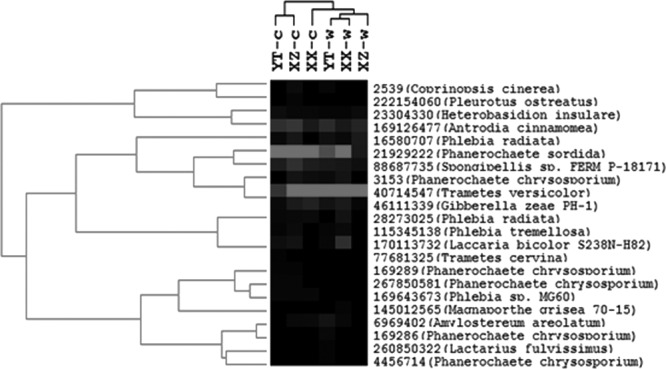
Hierarchical cluster analysis of mnp genes encoding manganese peroxidase, which is responsible for lignin degradation. Results were generated in CLUSTER, version 3.0, using the Spearman rank correlation and the complete linkage method and visualized using TREEVIEW. Gray indicates signal intensities above background while black indicates signal intensities below background. Lighter gray indicates higher signal intensity. YT-c, XZ-c, and XX-c represent control samples, and YT-w, XZ-w, and XX-w represent warmed samples. Warmed samples clustered together and were well separated from the controls. Protein identification numbers and organisms are given at right.
Fig 8.
Potential enzyme activities of polyphenol oxidase (A) expressed as mg g−1 3 h−1 and peroxidase (B) expressed as mg g−1 h−1 for tested subtropical wetland soils (i.e., YT, XZ, and XX) sampled on 16 December 2010 under experimental warming. Error bars are +1 standard deviation. Asterisks represent significant Student's t test differences between warmed and control samples (*, P < 0.05; **, P < 0.01).
DISCUSSION
Global warming is making it increasingly difficult for wetlands to effectively perform storage functions. A series of lab-scale soil incubation experiments simulating global warming using fixed temperatures to illustrate the impact of elevated temperature on C and nutrient cycling in ecosystems were reported previously (23, 30). However, these lab-scale experiments examined only a single temperature and do not reflect actual conditions under future global warming conditions; as such, the implications for ecosystem function are limited. Here, we conducted a field microcosm experiment with a high-resolution temperature control system. Through continuously comparing temperature differences at 2-min intervals between ambient and warming conditions, this system provided a more accurate simulation of realistic warming scenarios under expected future climate conditions. The present work, relating warming impacts on different types of organic matter to microbial functional responses, can assist in understanding microbial roles in ecological C cycling and microbially mediated feedbacks in freshwater wetlands to future climate warming.
Enhanced C and nutrient release associated with microbial responses under warming.
When taken together, the current data (Fig. 1 and Table 1) indicate that experimental warming enhanced the ecological role of wetland soil as a source for both DOC and P, leading to a relatively high potential of soil C emission and P transport to surface water bodies. This is consistent with findings (29) that a temperature increase of 2.1°C resulted in a 16% increase in DOC concentrations in forest soil baseflow. Experimental warming benefited microbial growth (Table 2), which is critical for the decomposition of organic matter and acceleration of nutrient cycling. On a global scale from colder arctic regions to the tropics, the amount of organic matter stored in soil substantially decreases, accompanied by an increase in microbial population density (22). This indicates that higher temperatures promote decomposition processes by increasing microbial populations in the soil. As one of the primary determinants of metabolic rates from the cellular level to global-scale ecosystems (1, 18), rising temperature increases microbial metabolic activity (Fig. 3A) and soil respiration rates (Fig. 2), thus enhancing soil C acquisition for energy. Temperature-dependent decomposition induced aggregate breakdown and liberated nutrients from soil-microorganism complexes (3, 36). Excessive P leached to water bodies (Fig. 1) may weaken the soil storage capacity, causing water quality deterioration in freshwater ecosystems.
Potential shifts in microbial function and structure for degrading recalcitrant C.
The response of different soil organic C fractions to warming is a major concern and source of uncertainty in projecting future climate change (25). GeoChip 4.0 analysis showed greater increases in genes for recalcitrant C than genes for labile C degradation, especially for lignin (Fig. 6B). This suggests that the microbial functional potential to biodegrade humus-like organic C was preferentially enhanced under warming. Humification refers to microbial transformation of organic matter into humus (51), and this process will probably be impaired by global warming because of changed microbial roles, such as the enhanced potential enzyme activities of polyphenol oxidase and peroxidase enzymes (Fig. 8), which are negatively correlated with the intensity of humification (28). Our results are supported by the similar findings of Li et al. (27), who showed that the relative losses of humified organic matter for manipulated soil from boreal forests under warming were disproportionately increased compared to loss of labile C. Generation of recalcitrant dissolved organic matter by the microbial carbon pump (24) is well regarded as a vital process for C sequestration in oceans. However, in this work we observed an ∼12.0% reduction in recalcitrant organic C pools with warming and a correlated, significant (P < 0.01) response in TOC although the amount of LOC remained almost unchanged (Table 2). Therefore, the warming-induced increase in DOC may be traced back to the recalcitrant organic C pools in wetlands. Genetic evidence validated our hypothesis of C loss derived from recalcitrant organic C.
Increased decomposition of recalcitrant organic C pools may be due to shifts in microbial community composition and structure to the relative dominance of fungi (Fig. 4; see also Fig. S3 in the supplemental material). Such an increase in the fungus-to-bacteria ratio indicated by detected genes agreed with findings of a previous investigation which used phospholipid fatty acid analysis in the same wetland columns (50). In the soil, fungus population numbers are smaller, but they dominate the soil biomass because fungi are more efficient than bacteria at converting available substrate C to form new cells (22). Experimental warming significantly increased the C use efficiency in tested wetlands (Fig. 3B and C) with greater increases in recalcitrant C use efficiency than labile C use efficiency (average percentage increases of 48% versus 28%, respectively). The relatively higher increment of recalcitrant C use efficiency suggested that the decomposition of recalcitrant C was preferentially accelerated under experimental warming. One possible reason for this is that most fungi can consume cellulose and lignin, which is more difficult to degrade and is decomposed at a lower rate (22). Similarly, Zhang et al. (49) and Clemmensen (10) observed increased fungal biomass and abundance in grassland and tundra soils, which may act as a major mechanism underlying warming-induced acclimatization of soil respiration. With respect to microbial roles in decomposition of different organic C pools, fungi, as the primary decomposer of lignin (42), could excrete a lignin-degrading enzyme which is responsible for oxidation of lignin. Simpson et al. (42) further found that future warming may accelerate lignin degradation and associated increases in the fungal community. Many factors may influence the fungal contribution to the microbial community under warming. For example, rising temperature benefits fungal growth because the filamentous nature of fungi makes them more tolerant of higher soil temperature than bacteria (49). Oxygen conditions in soils influence fungus diversity and the role of fungi in lignin oxidation since fungi rely on more aerobic conditions (49). In freshwater ecosystems, the hydrological cycling of water bodies is greatly impacted by rising temperature, which accelerates the evaporation and loss of surface water (7). The decreased depth of the water column facilitates the penetration of atmospheric oxygen through the water layer into the bottom soils. Moreover, warming-enhanced photosynthesis by microalga growth may also contribute to enhanced oxygen concentrations near the bottom of water sources. Therefore, fungal lignin oxidation is probably related to oxygen conditions in soil. However, future study is needed to confirm whether and how experimental warming impacts oxygen conditions in freshwater wetlands. In our study, since long-term C storage is determined by recalcitrant fractions (5), differential impacts of warming on genes/populations for degrading more recalcitrant C further implied that microbially mediated C losses may be greater in freshwater wetlands than previously estimated. As such, the fate of large releases of C from soils may accelerate global C and nutrient cycling in the future. However, because decomposition rates and microbial responses may vary less with different daily or seasonal dynamics (i.e., “native” temperature) than when soil is manipulated with alternatively perturbed temperature changes (25, 40), further analysis in situ with long-term monitoring should be established.
Linking microbial features to labile organic C.
LOC is a direct reservoir of readily available substrate and is important for ecosystem functioning (22). The values of the MBC/LOC ratio (ranging from 0.07 to 0.29) are consistently higher than the values of the MBC/RO-C ratio (ranging from 0.01 to 0.05) for both warmed and control samples (Fig. 3B and C), indicating that LOC is easily digested and utilized by microbes relative to use of recalcitrant C. Highly labile C pools also exerted considerable control on microbial functional potential (Table 3). If LOC is depleted under warming, microbial activity would be expected to decrease. We confirmed this in lab-incubated warmed soils which showed an initial burst of respiratory activity within the first 7 days of incubation but then had a greater decrease in respiration rate over the remaining 60-day incubation period. The warmed samples, with higher microbial biomass, will consume more readily available substrate C (i.e., labile C), leading to a greater decline in C substrate than in the control. A close look at the results from many soil warming experiments (17, 35) showed that rising temperature may cause a gradual decline of LOC. In contrast, our field warming experiments did not cause a decrease in the availability of labile organic C pools. Such maintenance of labile C pools may likely result from warming-enhanced organic inputs (such as microalga detritus) which are equal to or larger than decomposition of labile organic compounds. This is especially true for terrestrial grass ecosystems under experimental warming, as reported previously (5). Though water column primary productivity was not measured in this work, faster microalga growth was observed in warmed wetland columns. Increased organic carbon input from accelerated decomposition and transfer of microalga detritus into soil organic matter may compensate for the decreased labile organic fractions. The integration of microbial ecology, C biogeochemistry, and experimental warming along with associated biotic factors (e.g., substrate availability) should receive further investigation.
Summary.
Experimental warming enhanced the ecological role of soil in wetlands as a source of both DOC and P, posing a positive feedback for freshwater ecosystems. Experimental warming did not result in a depletion of labile organic C fractions but did lead to a decrease in recalcitrant C fractions. We confirmed this with functional microarray detection of C-degrading genes at the molecular level. Microorganisms may alter their populations/genes in response to warming to potentially function more effectively in decomposing recalcitrant pools, especially lignin. The abundance of detected C-degrading genes increased in the warmed samples by 8.6%, 4.2%, 6.8%, and 14.5% for starch, hemicellulose, chitin, and lignin degradation, respectively, suggesting that recalcitrant C fractions were susceptible to warming-induced degradation. The increased potential enzyme activities of polyphenol oxidase and peroxidase further suggest that warming-enhanced fungal decomposition of lignin may be prevalent in freshwater wetlands. The labile organic C pools exert great control on microbial biomass and potential functioning. A comprehensive evaluation of C dynamics in the phytoplankton-microbe-soil system under warming is needed to better understand the role of wetlands in climate warming.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (40701162) and the Zhejiang and Hangzhou Science and Technology Program, China (2009C33060; 20091633F06). This work was also partially supported by the U.S. Department of Energy, Biological Systems Research on the Role of Microbial Communities in Carbon Cycling Program (DE-SC0004601), and the Oklahoma Bioenergy Center.
Footnotes
Published ahead of print 24 August 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Allen AP, Gillooly JF. 2009. Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol. Lett. 12:369–384 [DOI] [PubMed] [Google Scholar]
- 2. Allison SD, Wallenstein MD, Bradford MA. 2010. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 3:336–340 [Google Scholar]
- 3. Angers DA, Eriksen-Hamel NS. 2008. Full-inversion tillage and organic carbon distribution in soil profiles: a meta-analysis. Soil Sci. Soc. Am. J. 72:1370–1374 [Google Scholar]
- 4. Bao S. 2000. Agro-chemical analysis of soil. China Agricultural Press, Beijing, China [Google Scholar]
- 5. Belay-Tedla A, Zhou XH, Su B, Wan SQ, Luo YQ. 2009. Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol. Biochem. 41:110–116 [Google Scholar]
- 6. Bond-Lamberty B, Thomson A. 2010. Temperature-associated increases in the global soil respiration record. Nature 464:579–582 [DOI] [PubMed] [Google Scholar]
- 7. Carter V. 1996. Technical aspects of wetlands: wetland hydrology, water quality, and associated functions. U.S. Geological Survey water supply paper 2425. U.S. Geological Survey, Reston, VA: http://water.usgs.gov/nwsum/WSP2425/hydrology.html [Google Scholar]
- 8. Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW. 2010. Soil microbial community responses to multiple experimental climate change drivers. Appl. Environ. Microbiol. 76:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chapin FS, et al. 2000. Arctic and boreal ecosystems of western North America as components of the climate system. Glob. Change Biol. 6:211–223 [DOI] [PubMed] [Google Scholar]
- 10. Clemmensen KE, Michelsen A, Jonasson S, Shaver GR. 2006. Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytol. 171:391–404 [DOI] [PubMed] [Google Scholar]
- 11. Conant RT, et al. 2008. Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Change Biol. 14:868–877 [Google Scholar]
- 12. Conant RT, et al. 2008. Experimental warming shows that decomposition temperature sensitivity increases with soil organic matter recalcitrance. Ecology 89:2384–2391 [DOI] [PubMed] [Google Scholar]
- 13. Conen F, Leifeld J, Seth B, Alewell C. 2006. Warming mineralises young and old soil carbon equally. Biogeosciences 3:515–519 [Google Scholar]
- 14. Davidson EA, Trumbore SE, Amundson R. 2000. Soil warming and organic carbon content. Nature 408:789–790 [DOI] [PubMed] [Google Scholar]
- 15. Falkowski PG, Fenchel T, Delong EF. 2008. The microbial engines that drive Earth's biogeochemical cycles. Science 320:1034–1039 [DOI] [PubMed] [Google Scholar]
- 16. Feng XJ, Simpson MJ. 2009. Temperature and substrate controls on microbial phospholipid fatty acid composition during incubation of grassland soils contrasting in organic matter quality. Soil Biol. Biochem. 41:804–812 [Google Scholar]
- 17. Frey SD, Drijber R, Smith H, Melillo J. 2008. Microbial biomass, functional capacity, and community structure after 12 years of soil warming. Soil Biol. Biochem. 40:2904–2907 [Google Scholar]
- 18. Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. 2001. Effects of size and temperature on metabolic rate. Science 293:2248–2251 [DOI] [PubMed] [Google Scholar]
- 19. Gorham E. 1991. Northern peatlands: role in the carbon-cycle and probable responses to climatic warming. Ecol. Appl. 1:182–195 [DOI] [PubMed] [Google Scholar]
- 20. Haney RL, Brinton WF, Evans E. 2008. Soil CO2 respiration: comparison of chemical titration, CO2 IRGA analysis and the Solvita gel system. Renew. Agr. Food Syst. 23:171–176 [Google Scholar]
- 21. He Z, et al. 2010. GeoChip 3.0 as a high-throughput tool for analyzing microbial community composition, structure and functional activity. ISME J. 4:1167–1179 [DOI] [PubMed] [Google Scholar]
- 22. Hoorman JJ, Islam R. 2010. Understanding soil microbes and nutrient recycling. Actinomycetes 107:40–500 [Google Scholar]
- 23. Jensen HS, Andersen FO. 1992. Importance of temperature, nitrate, and pH for phosphate release from aerobic sediments of 4 shallow, eutrophic lakes. Limnol. Oceanogr. 37:577–589 [Google Scholar]
- 24. Jiao NZ, et al. 2011. The microbial carbon pump and the oceanic recalcitrant dissolved organic matter pool. Nat. Rev. Microbiol. 9:555 doi:10.1038/nrmicro2386-c521625248 [Google Scholar]
- 25. Knorr W, Prentice IC, House JI, Holland EA. 2005. Long-term sensitivity of soil carbon turnover to warming. Nature 433:298–301 [DOI] [PubMed] [Google Scholar]
- 26. Leifeld J, Fuhrer J. 2005. The temperature response of CO2 production from bulk soils and soil fractions is related to soil organic matter quality. Biogeochemistry 75:433–453 [Google Scholar]
- 27. Li JW, Ziegler S, Lane C, Billings SA. 2012. Warming preferentially enhances microbial mineralization of humified boreal forest soil organic matter: interpretation of soil profiles along a climate transect using laboratory incubations. J. Geophys. Res. 117:G02008 doi:10.1029/2011JG001769 [Google Scholar]
- 28. Li Z, Luo Y, Teng Y. 2008. Soil and environmental microbiology research. Science Press, Beijing, China [Google Scholar]
- 29. Liechty HO, Kuuseoks E, Mroz GD. 1995. Dissolved organic-carbon in hardwood stands with differing acidic inputs and temperature regimes. J. Environ. Qual. 24:927–933 [Google Scholar]
- 30. Liikanen A, Murtoniemi T, Tanskanen H, Vaisanen T, Martikainen PJ. 2002. Effects of temperature and oxygen availability on greenhouse gas and nutrient dynamics in sediment of a eutrophic mid-boreal lake. Biogeochemistry 59:269–286 [Google Scholar]
- 31. Lipson DA, Monson RK, Schmidt SK, Weintraub MN. 2009. The trade-off between growth rate and yield in microbial communities and the consequences for under-snow soil respiration in a high elevation coniferous forest. Biogeochemistry 95:23–35 [Google Scholar]
- 32. Liu Q, et al. 2010. Initial soil responses to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China: nutrient availabilities, microbial properties and enzyme activities. Appl. Soil Ecol. 46:291–299 [Google Scholar]
- 33. Logninow W, Wisniewski W, Strony WM. 1987. Fractionation of organic carbon based on susceptibility to oxidation Polish. J. Soil Sci. 20:47–52 [Google Scholar]
- 34. Lu Z, et al. 2012. Microbial gene functions enriched in the Deepwater Horizon deep-sea oil plume. ISME J. 6:451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melillo JM, et al. 2002. Soil warming and carbon-cycle feedbacks to the climate system. Science 298:2173–2176 [DOI] [PubMed] [Google Scholar]
- 36. Messiga AJ, Ziadi N, Angers DA, Morel C, Parent LE. 2011. Tillage practices of a clay loam soil affect soil aggregation and associated C and P concentrations. Geoderma 164:225–231 [Google Scholar]
- 37. Olk DC, Gregorich EG. 2006. Overview of the symposium proceedings, “Meaningful pools in determining soil carbon and nitrogen dynamics.” Soil Sci. Soc. Am. J. 70:967–974 [Google Scholar]
- 38. Otto S, Balzer W. 1998. Release of dissolved organic carbon (DOC) from sediments of the NW European continental margin (Goban Spur) and its significance for benthic carbon cycling. Prog. Oceanogr. 42:127–144 [Google Scholar]
- 39. Reddy KR, Kadlec RH, Flaig E, Gale PM. 1999. Phosphorus retention in streams and wetlands: a review. Crit. Rev. Environ. Sci. Technol. 29:83–146 [Google Scholar]
- 40. Schindlbacher A, et al. 2011. Experimental warming effects on the microbial community of a temperate mountain forest soil. Soil Biol. Biochem. 43:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmidt IK, et al. 2004. Soil solution chemistry and element fluxes in three European heathlands and their responses to warming and drought. Ecosystems 7:638–649 [Google Scholar]
- 42. Simpson MJ, Feng XJ, Simpson AJ, Wilson KP, Williams DD. 2008. Increased cuticular carbon sequestration and lignin oxidation in response to soil warming. Nat. Geosci. 1:836–839 [Google Scholar]
- 43. Solomon S, et al. (ed). 2007. Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the IPCC Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 44. Sowerby A, Emmett BA, Williams D, Beier C, Evans CD. 2010. The response of dissolved organic carbon (DOC) and the ecosystem carbon balance to experimental drought in a temperate shrubland. Eur. J. Soil Sci. 61:697–709 [Google Scholar]
- 45. Verhoeven JTA, Arheimer B, Yin CQ, Hefting MM. 2006. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 21:96–103 [DOI] [PubMed] [Google Scholar]
- 46. Wallenstein MD, Weintraub MN. 2008. Emerging tools for measuring and modeling the in situ activity of soil extracellular enzymes. Soil Biol. Biochem. 40:2098–2106 [Google Scholar]
- 47. Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC. 1990. Measurement of soil microbial biomass C by fumigation extraction—an automated procedure. Soil Biol. Biochem. 22:1167–1169 [Google Scholar]
- 48. Wu LY, Liu X, Schadt CW, Zhou JZ. 2006. Microarray-based analysis of subnanogram quantities of microbial community DNAs by using whole-community genome amplification. Appl. Environ. Microbiol. 72:4931–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang W, et al. 2005. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob. Change Biol. 11:266–277 [Google Scholar]
- 50. Zhang ZJ, et al. 2012. The release of phosphorus from sediment into water in subtropical wetlands: a warming microcosm experiment. Hydrol. Process. 26:15–26 [Google Scholar]
- 51. Zhou JZ, et al. 2012. Microbial mediation of carbon-cycle feedbacks to climate warming. Nat. Clim. Change 2:106–110 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.