Abstract
Interindividual variation has been shown in the rates at which subjects alternate in perception during viewing of binocular rivalry and other ambiguous figures. A similar pattern of interindividual variation is evident in the rate of eye movements. The aim of this study was to determine whether individual differences in the rate of binocular rivalry predict individual differences in the rate of eye movements. First, participants reported changes in perception during contour rivalry. We found that the alternation rate during rivalry varied from 0.15 to 0.59/s between individuals. Next, participants viewed different visual displays while their eye movements were tracked. We found that the rate of saccadic eye movements varied by 1.9–4.4/s between individuals. Although the temporal characteristics of eye movements and binocular rivalry differed in their absolute rate, we found a significant positive correlation between these measures; that is, the frequency of saccadic eye movements can predict an individual's rate of perceptual alternation during rivalry. These findings suggest a potential link between the mechanisms involved in binocular rivalry and those processes involved in controlling eye movements.
Keywords: Binocular rivalry, saccadic eye movements, interindividual variation, individual differences, alternation rate, saccade rate
1. Introduction
Multistable phenomena, such as binocular rivalry, provide an opportunity to understand the process by which the visual system resolves uncertainty in the visual world (Blake and Logothetis 2002). Despite its importance, however, the mechanisms that influence changes in perception during binocular rivalry are not fully understood (Alais and Blake 2005). For example, there has been a debate for more than 100 years over whether eye movements can influence perceptual change during binocular rivalry (Helmholtz 1925; Hering 1964). Hering (1964) suggested that changes in perception could be attributable to patterns of eye movements that favoured perception of one stimulus over the other. The idea that changes in perception during rivalry can result only from changes in retinal stimulation caused by eye movements is disputed by studies showing that changes in perception can occur when rival targets are stabilised on the retina (Blake et al 1971; Wade 1975). Nevertheless, it is also clear from these studies that the rate of perceptual alternation when the retinal image is stabilised is much lower than in normal viewing (Wade 1975). Experimental support for the importance of eye movements during rivalry was provided by Breese (1899), who reported that eye movements could promote increased predominance of one stimulus over another during rivalry. Later studies by Sabrin and Kertesz (1980) showed that the rate of microsaccades is increased during rivalry compared with normal viewing and that the rate is higher at the beginning of a period of dominance and declines as the interval progresses. They also found that naturally simulated microsaccades could increase the predominance of a stimulus that was stabilized with respect to the retina (Sabrin and Kertesz 1983). These results suggest that it is the eye movements rather than the transient signals that they produce that are critical in determining perceptual dominance. However, more recent studies have shown that, although there was an increased probability of eye movements around the time that a change in perception was reported, there was no evidence that an eye movement, per se, was necessary for a perceptual transition (van Dam and van Ee 2006a). Rather, it is the transient signals generated by these movements that lead to a higher probability of a perceptual alternation (van Dam and van Ee 2006b).
This study has used an orthogonal approach to investigate the link between eye movements and binocular rivalry. Our method is based on previous studies that have shown a large interindividual variability in the rate at which spontaneous alternations occur in binocular rivalry (Aafjes et al 1966; Miller et al 2010; Pettigrew and Miller 1998; van Ee et al 2005). Studies of monozygotic and dizygotic twins have found that this interindividual variation in the rate of binocular rivalry has a strong genetic component (Miller et al 2010; Shannon et al 2011). A similar level of interindividual variation is apparent in rates of saccadic eye movements when viewing different visual displays (Andrews and Coppola 1999; Castelhano and Henderson 2008; Rayner et al 2007), with observers who have relatively high saccade rates in one viewing condition also having relatively high saccade rates in other viewing conditions. Based on these observations, our objective was to correlate individual differences in the rate of perceptual alternations during binocular rivalry with individual differences in the rate of eye movements. If similar neural processes underlie both behaviours, the prediction is that there should be a positive correlation between the rate of eye movements and the rate of perceptual change during binocular rivalry.
2. Methods
2.1. Participants
Twenty volunteers participated in this experiment. All had normal or corrected to normal vision. Written, informed consent was obtained from each participant. Each participant completed the eye-tracking phase and the binocular rivalry phase in the same session. The order in which participants completed each phase of the experiment was counterbalanced.
2.2. Binocular rivalry
Stimuli were programmed using a VSG2/5 graphics card (http://www.crsltd.com) and were presented on a Clinton Monoray monochrome monitor (mean luminance: 9.3 cd/m2) with a frame rate of 120 Hz. Gamma correction was used to ensure that the monitor was linear over the entire luminance range used in the experiments. Participants viewed the display in a darkened room at a distance of 2.28 m through Ferro-Electric Shutter Goggles (http://www.crsltd.com) that alternately occluded the two eyes at the same frequency as the frame-rate of the monitor. When the display also alternated on successive frames, each frame was seen by only one eye with no perceptible flicker.
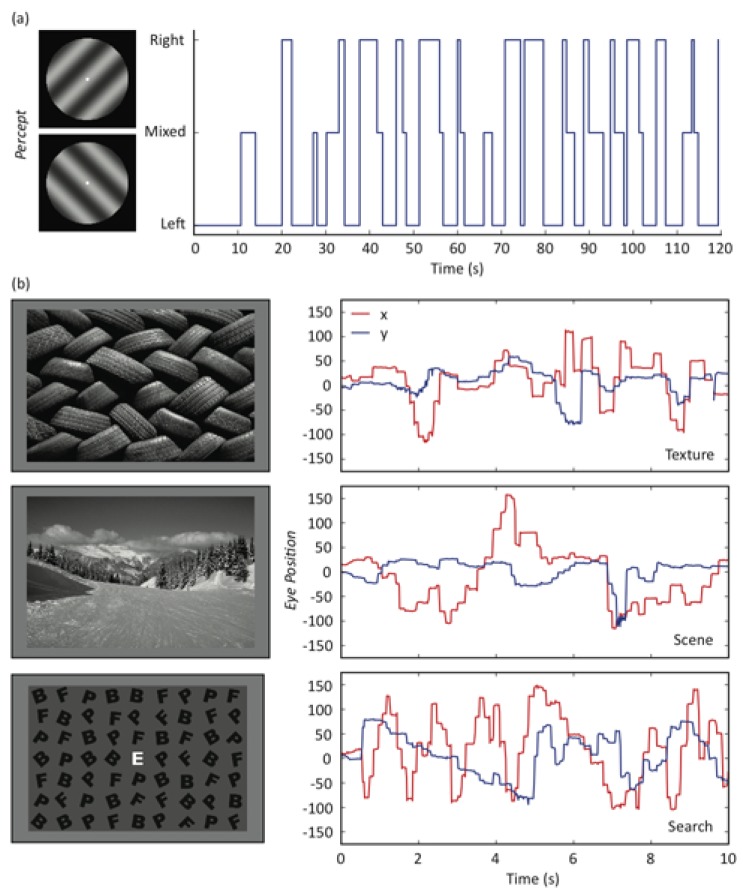
Stimuli consisted of sine-wave gratings (size: 1deg of visual angle; contrast: 50%) presented centrally on a dark background. The gratings were oriented at 45 deg (right-tilted) or 135 deg (left-tilted); had a spatial frequency of 2, 3, 4, or 5 cycles per degree; and were presented independently to the two eyes during 120-s trials. The task was to maintain central fixation and indicate changes in perception using key presses (left-tilted grating, right-tilted grating, or piecemeal rivalry). Each participant completed four trials at each spatial frequency in a pseudorandom order that was counterbalanced between participants. For each binocular rivalry trial, each change between the three perceptual states (left-tilted, right-tilted, and piecemeal rivalry) was recorded to provide a measure of alternation rate. Figure 1a shows the perceptual alternations reported by a representative participant on a single 120-s trial.
Figure 1.
(a) Time course of perceptual alternations for one representative participant over a single 120-s trial. (b) Examples of stimuli used for texture, scene and search eye tracking conditions, and sample eye movement traces from one participant. The red and blue lines represent the x and y position of the eye for each image (central fixation is at (0,0)).
2.3. Eye movements
Eye movements were recorded while participants viewed three categories of image: simple textures, scenes, and search displays. Stimuli were viewed on a Sony Trinitron GDM-F520 monitor (mean luminance: 9.3 cd/m2) at a distance of 57 cm. All images were achromatic and subtended approximately 30 × 20 deg. Textures and natural scenes were selected from photographs in the database of the software package CorelDraw. Textures included images of natural and synthetic materials; natural scenes consisted of a variety of landscapes. Participants were instructed to view the textures and scenes in a natural manner. The visual search displays consisted of 62 black letters (B, E, F, P) on a grey background. Each letter was subtended 1.4 × 1.8 deg and was rotated by up to ±45 deg. A white letter in the centre of the screen indicated the target. Subjects then had to search for the target among the other three distracter letters. Half of the search trials contained no targets. Participants were not made aware that some search arrays did not contain targets.
Participants viewed 10 images from each condition, including 10 search trials with the target present and 10 with the target absent. Eye movements were tracked using a Video Eyetracker (CRS; http://www.crsltd.com) used in conjunction with the Video Eyetracker Toolbox. Eye position was sampled at a rate of 50 Hz. Head position was stabilised with a chin and forehead rest. At the start of each block, participants completed the standard calibration procedure to check the accuracy of the eye-movement data. Prior to each image display, a centrally located fixation cross (40 pixels) was displayed for 1 s, to ensure that participants began each trial fixating the central region. Each image was displayed, and eye movements were analysed for 10 s. No responses were required to any of the images.
2.4. Eye-movement analysis
The eye-movement data were analysed off-line using custom analysis software. Saccades were selected automatically on the basis of their spatio-temporal characteristics and were verified by visual inspection. All saccades with amplitudes greater than about 0.15 deg were included. Because eye movements in the target-present search displays are likely to be influenced by the identification of the target, they were not used in the subsequent analysis. Thus, the data from three image categories were analysed: textures, scenes, and visual search (target-absent only). Figure 1b shows sample stimuli and eye-movement traces (change in horizontal and vertical eye position over time) for each of the three categories for one participant. Occasionally, there was a failure to track eye position. These periods of tracker loss were removed from the final analysis. Trials in which tracker loss accounted for more than 10% of the viewing duration were not analysed. The average saccade rate and magnitude were then calculated. The data from two participants were excluded from the visual search display analysis, as they reported during debriefing that they had misunderstood the task.
3. Results
3.1. Binocular rivalry
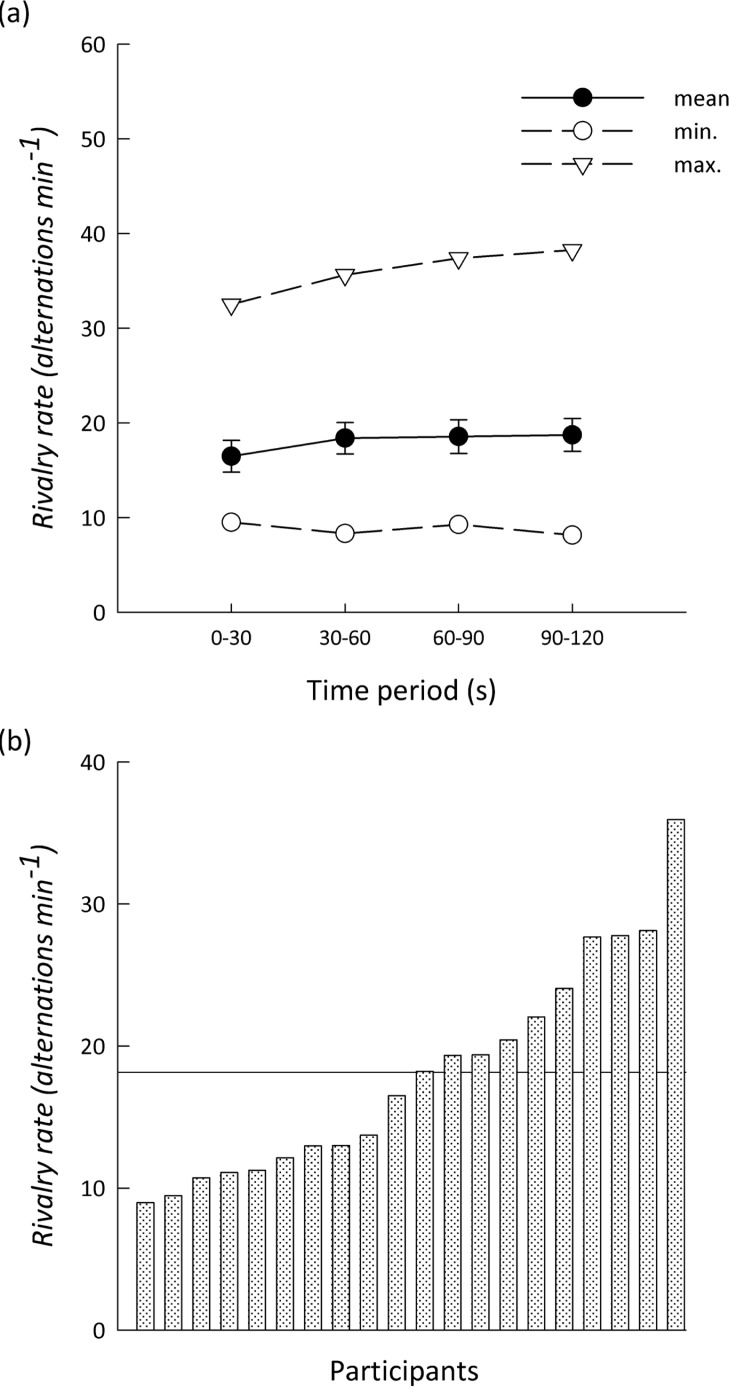
Figure 2 shows the interindividual variation in binocular rivalry rate. There was a threefold difference in the rate of perceptual change during binocular rivalry across individuals (range: 9.0 to 35.9/min; mean: 18.1/min). The average rate of perceptual alternation, across individuals, showed a small but significant increase during the 120-s trial, F(4, 76) = 5.18, p < .01. The alternation rates for the first 30-s period (mean ± SEM = 16.5 ± 1.6) were significantly lower than those for the second 30-s period (mean ± SEM = 18.4 ± 1.6), t(19) = −3.06, p < .01. No significant differences were found in the alternation rate for further successive 30-s time periods, F(2, 38) = .16, p = .75. For an individual, the alternation rate for the whole trial was strongly correlated with the rate at each 30-s time period (mean r2 = .91, p < .001).
Figure 2.
Interindividual variation in the rate of perceptual alternation during binocular rivalry. (a) Mean, minimum, and maximum alternation rates across participants for different time periods within rivalry trials. Error bars represent ±SEM. (b) Variation in overall rivalry alternation rates in different participants. The horizontal line indicates the group mean.
3.2. Eye movements
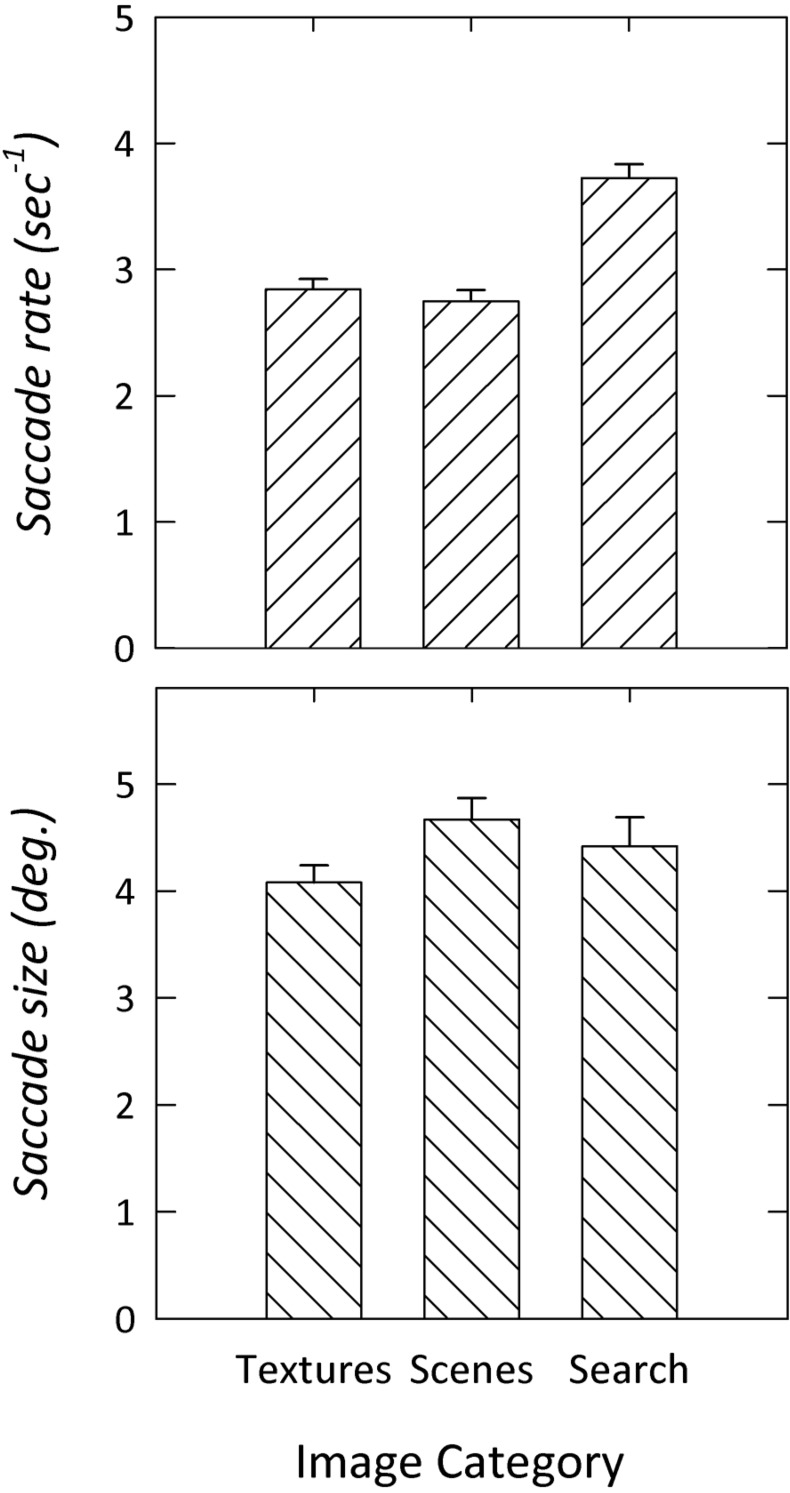
Figure 3 shows the rate and size of saccadic eye movements for each image category. The rate of saccadic eye movements varied between individuals for each category (textures: 2.1–3.9/s; scenes: 1.9–3.4/s; search: 2.9–4.4/s). Similar interindividual variation was evident in the size of eye movements (textures: 3.1–5.4 deg; scenes: 3.6–6.5 deg; search: 2.8–6.7 deg). Although individuals varied in the size and rate of eye movements, this variation was consistent across textures and scenes. For example, over 60% of the variance in the rate of a participant's saccadic eye movements when viewing textures could be explained by the pattern observed when viewing scenes (r2 = 0.62, p < .001). That is, participants who made more eye movements viewing textures also made more eye movements when viewing scenes. A similar correlation was evident in the size of the eye movements when viewing scenes and textures (r2 = 0.65, p < .001). However, there was no correlation between the rate of eye movements during a search and that observed when viewing scenes (r2 = 0.02, p = .63) or textures (r2 = 0.16, p = .09). Similarly, there was no correlation in the size of eye movements during a search and that observed when viewing scenes (r2 = 0.00, p = .95) or textures (r2 = 0.17, p = .08).
Figure 3.
Mean saccade rate and size when viewing images of textures and natural scenes, and during a visual search. Error bars represent ±SEM.
The variance in the rate and size of eye movements when viewing scenes or textures was independent. That is, the rate of eye movements when viewing scenes or textures did not predict the size of movements when viewing scenes (r2 = 0.02, p = 0.55) or textures (r2 = 0.02, p = 0.54), respectively. However, in contrast to viewing textures and scenes, the rate of saccadic eye movements during a search was positively correlated with the magnitude of the eye movements during a search (r2 = 0.40, p < .005).
3.3. Relationship between eye movements and binocular rivalry
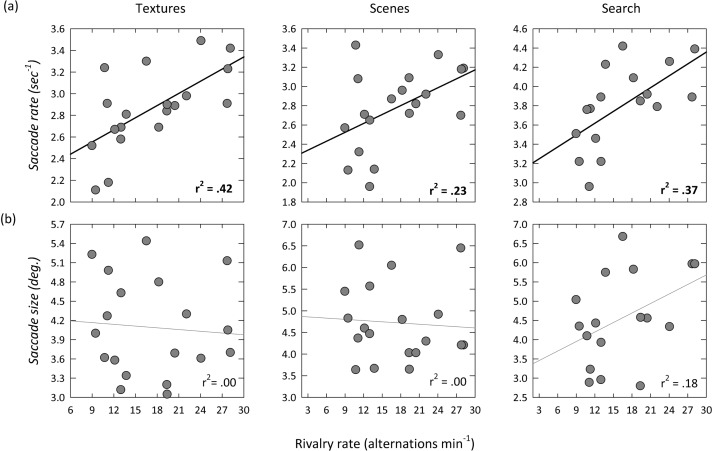
Finally, we asked whether individual variation in the spatial or temporal pattern of eye movements could predict individual variation in the rate of perceptual alternations during binocular rivalry. Figure 4 shows that there was a significant correlation between the rate of saccadic eye movements and the rate of alternation in binocular rivalry. A positive correlation was evident for the rate of saccadic eye movements when viewing textures (r2 = 0.42, p < .005) and scenes (r2 = 0.23, p < .05), and during a search (r2 = 0.37, p < .01). Thus, participants who had a high alternation rate during binocular rivalry tended to have a high frequency of eye movements in a variety of viewing conditions. In contrast to saccade rate, there was no significant correlation between the average size of saccade and the alternation rate during binocular rivalry.
Figure 4.
Scatter plots showing the correlations between rivalry rate and (a) saccade rate or (b) saccade size when viewing textures and scenes, and during a visual search task. Significant correlations are represented by black regression lines and bold r2 values.
4. Discussion
The aim of this study was to investigate the possible link between the mechanisms that control alternations in perception during binocular rivalry and the mechanisms that control eye movements. Participants viewed textures, scenes, and search displays while their eye movements were recorded. In all three natural viewing scenarios, we found a significant interindividual variation in the rate at which participants made saccades. Next, participants reported changes in perceptual dominance during binocular rivalry. Again, we found significant interindividual variation in the rate of rivalry, consistent with previous studies. Finally, we investigated whether there was any correlation between the rate of binocular rivalry and the rate of eye movements across individuals. Our results show a significant positive correlation between an individual's average alternation rate during rivalry and the frequency of saccadic eye movements across a number of viewing conditions.
To probe the link between eye movements and binocular rivalry, we have measured individual differences that occur in natural viewing. This approach is orthogonal to previous studies in this area that have measured eye movements when subjects are engaged in rivalry. Our aim was to establish an independent marker of oculomotor activity. The finding that individual variation in the temporal patterns of eye movements correlates significantly with the rate of binocular rivalry suggests a link between these behaviours. While the exact role of eye movements as a casual or contributing factor in binocular rivalry alternations remains somewhat unclear, it is interesting to note that similar brain regions are reported to be associated with perceptual transitions in bistable viewing (Kleinschmidt et al 1998; Knapen et al 2011; Lumer et al 1998; Lumer and Rees 1999; Sterzer and Kleinschmidt 2007) and with controlling eye position (Corbetta 1998). The importance of the parietal lobe in binocular rivalry has also been demonstrated in recent studies in which TMS of parietal areas has been shown to influence perceptual durations during rivalry (Carmel et al 2010; Zaretskaya et al 2010). Of particular relevance to the current study are two recent studies that have shown interindividual variation in the rate of perceptual rivalry correlates with structural variation in the parietal lobe (Kanai et al 2010, 2011). Together, these findings provide support for an overlap in the brain regions that are involved in switching perception during binocular rivalry and in initiating shifts in eye position during free viewing of the environment (Leopold and Logothetis 1999; Rees et al 2002; Sterzer et al 2009).
In conclusion, we have found a significant interindividual variation in the rate of saccadic eye movements and the rate of perceptual change during rivalry. There was a significant positive correlation between the rate of eye movements and the rate of rivalry. These results are suggestive of a link between the mechanisms involved in the control of the temporal dynamics of binocular rivalry and visuo-motor behaviour. Future studies involving clinical populations that show marked differences in the temporal dynamics of these behaviours may shed more light on the underlying neural mechanisms.
Biography
 Sarah Hancock received her undergraduate and masters degrees in Psychology from the University of Wales, Bangor, and her PhD from the University of York. This was followed by post-doctoral positions in the Nottingham Visual Neuroscience Group within the School of Psychology at the University of Nottingham. Her research interests include the neural bases of binocular rivalry and multistable perception, attention and awareness, and the mechanisms underlying the processing of curvature and contour shape in human vision.
Sarah Hancock received her undergraduate and masters degrees in Psychology from the University of Wales, Bangor, and her PhD from the University of York. This was followed by post-doctoral positions in the Nottingham Visual Neuroscience Group within the School of Psychology at the University of Nottingham. Her research interests include the neural bases of binocular rivalry and multistable perception, attention and awareness, and the mechanisms underlying the processing of curvature and contour shape in human vision.
 Tim Andrews received his degree in biology from Kingston University and his PhD from the University of London. This was followed by postdoctoral fellowships at the Department of Neurobiology at Duke University and the University Laboratory of Physiology at Oxford. He moved to the Department of Psychology in Durham in 2001 as a lecturer. In 2005, Tim moved to the Department of Psychology in York, where he is a Reader. His research interests include: the neural basis of face recognition, how information about objects is represented in the ventral visual stream, neural mechanisms underlying binocular rivalry, the neural basis of visual awareness.
Tim Andrews received his degree in biology from Kingston University and his PhD from the University of London. This was followed by postdoctoral fellowships at the Department of Neurobiology at Duke University and the University Laboratory of Physiology at Oxford. He moved to the Department of Psychology in Durham in 2001 as a lecturer. In 2005, Tim moved to the Department of Psychology in York, where he is a Reader. His research interests include: the neural basis of face recognition, how information about objects is represented in the ventral visual stream, neural mechanisms underlying binocular rivalry, the neural basis of visual awareness.
Contributor Information
Sarah Hancock, Department of Psychology, University of York, York YO10 5DD, UK; e-mail: sarah.gaskell@nottingham.ac.uk.
Lynn Gareze, Department of Psychology, University of Durham, Durham DH1 3LE, UK; e-mail: l.gareze@durham.ac.uk.
John M Findlay, Department of Psychology, University of Durham, Durham DH1 3LE, UK; e-mail: j.m.findlay@durham.ac.uk.
Timothy J Andrews, Department of Psychology, University of York, York YO10 5DD, UK; e-mail: t.andrews@psych.york.ac.uk.
References
- Aafjes M. Hueting J E. Visser P. Individual and interindividual differences in binocular retinal rivalry in man. Psychophysiology. 1966;3:18–22. doi: 10.1111/j.1469-8986.1966.tb02674.x. [DOI] [PubMed] [Google Scholar]
- Alais D. Blake R. Binocular Rivalry. Cambridge, MA: MIT; 2005. [Google Scholar]
- Andrews T J. Coppola D M. Idiosyncratic characteristics of saccadic eye movements when viewing different visual environments. Vision Research. 1999;39:2947–2953. doi: 10.1016/S0042-6989(99)00019-X. [DOI] [PubMed] [Google Scholar]
- Blake R. Logothetis N K. Visual competition. Nature Reviews Neuroscience. 2002;3:1–11. doi: 10.1038/nrn701. [DOI] [PubMed] [Google Scholar]
- Blake R R. Fox R. McIntyre C. Stochastic properties of stablized-image binocular rivalry alternations. Journal of Experimental Psychology. 1971;88:327–332. doi: 10.1037/h0030877. [DOI] [PubMed] [Google Scholar]
- Breese B B. On inhibition. Psychological Monographs. 1899;3:1–65. [Google Scholar]
- Carmel D. Walsh V. Lavie N. Rees G. Right parietal TMS shortens dominance durations in binocular rivalry. 2010. Current Biology 20 R799-R800. [DOI] [PubMed]
- Castelhano M S. Henderson J M. Stable individual differences across images in human saccadic eye movements. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale. 2008;62:1–14. doi: 10.1037/1196-1961.62.1.1. [DOI] [PubMed] [Google Scholar]
- Corbetta M. A common neural system to direct attention and the eye to visual locations. Proceedings of the National Academy of Sciences. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmholtz H. In: Helmholtz's Treatise on Physiological Optics. Southall J P C, editor. Vol. 3. New York: Optical Society of America; 1925. [Google Scholar]
- Hering K E. In: Outlines of a Theory of the Light Sense. Hurvich L M, editor; Jameson D, editor. Cambridge, MA: Harvard University Press; 1964. [Google Scholar]
- Kanai R. Bahrami B. Rees G. Human parietal cortex structure predicts individual differences in perceptual rivalry. Current Biology. 2010;20:1626–1630. doi: 10.1016/j.cub.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R. Carmel D. Bahrami B. Rees G. Structural and functional fractionation of right superior parietal cortex in bistable perception. 2011. Current Biology 21 R106-R107. [DOI] [PMC free article] [PubMed]
- Kleinschmidt A. Buchel C. Zeki S. Frackowiak R S J. Human brain activity during spontaneously reversing perception of ambiguous figures. Proceedings of the Royal Society B: Biological Sciences. 1998;265:2427–2433. doi: 10.1098/rspb.1998.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapen T. Brascamp J. Pearson J. van Ee R. Blake R. The role of frontal and parietal brain areas in bistable perception. Journal of Neuroscience. 2011;31:10293–10301. doi: 10.1523/JNEUROSCI.1727-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold D A. Logothetis N K. Multistable phenomena: changing views in perception. Trends in Cognitive Sciences. 1999;3:254–265. doi: 10.1016/S1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- Lumer E D E K J. Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- Lumer E D. Rees G. Covariation of activity in visual and prefrontal cortex associated with subjective visual perception. Proceedings of the National Academy of Sciences. 1999;96:1669–1673. doi: 10.1073/pnas.96.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. Hansell N. Ngo T. Liu G. Pettigrew J. Martin N. Wright M J. Genetic contribution to individual variation in binocular rivalry rate. Proceedings of the National Academy of Sciences. 2010;107:2664–2668. doi: 10.1073/pnas.0912149107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew J D. Miller S M. A ‘sticky’ interhemispheric switch in bipolar disorder? Proceedings of the Royal Society B: Biological Sciences. 1998;265:2141–2148. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K. Li X. Williams C C. Cave K R. Well A D. Eye movements during information processing tasks: Individual differences and cultural effects. Vision Research. 2007;47:2714–2726. doi: 10.1016/j.visres.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G. Kreiman G. Koch C. Neural correlates of consciousness in humans. Nature Reviews Neuroscience. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Sabrin H W. Kertesz A E. Microsaccadic eye movements and binocular rivalry. Perception & Psychophysics. 1980;28:150–154. doi: 10.3758/BF03204341. [DOI] [PubMed] [Google Scholar]
- Sabrin H W. Kertesz A E. The effect of imposed fixational eye movements on binocular rivalry. Perception & Psychophysics. 1983;34:155–157. doi: 10.3758/BF03211341. [DOI] [PubMed] [Google Scholar]
- Shannon R W. Patrick C J. Jiang Y. Bernat E. He S. Genes contribute to the switching dynamics of bistable perception. Journal of Vision. 2011;11:1–7. doi: 10.1167/11.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P. Kleinschmidt A. A neural basis for inference in perceptual ambiguity. Proceedings of the National Academy of Sciences. 2007;104:323–328. doi: 10.1073/pnas.0609006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P. Kleinschmidt A. Rees G. The neural bases of multistable perception. Trends in Cognitive Sciences. 2009;13:310–318. doi: 10.1016/j.tics.2009.04.006. [DOI] [PubMed] [Google Scholar]
- van Dam L C J. van Ee R. Retinal image shifts, but not eye movements per se, cause alternations in awareness during binocular rivalry. Journal of Vision. 2006a;6:1172–1179. doi: 10.1167/6.11.3. [DOI] [PubMed] [Google Scholar]
- van Dam L C J. van Ee R. The role of saccades in exerting voluntary control in perceptual and binocular rivalry. Vision Research. 2006b;46:787–799. doi: 10.1016/j.visres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- van Ee R. van Dam L C J. Brouwer G J. Voluntary control and the dynamics of perceptual bi-stability. Vision Research. 2005;45:41–55. doi: 10.1016/j.visres.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Wade N J. Binocular rivalry between single lines viewed as real images and afterimages. Perception & Psychophysics. 1975;17:571–577. doi: 10.3758/BF03203971. [DOI] [Google Scholar]
- Zaretskaya N. Thielscher A. Logothetis N K. Bartels A. Disrupting parietal function prolongs dominance durations in binocular rivalry. Current Biology. 2010;20:2106–2111. doi: 10.1016/j.cub.2010-.10.046. [DOI] [PubMed] [Google Scholar]