Abstract
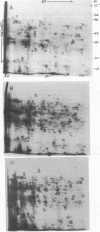
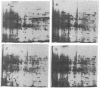
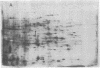
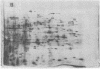
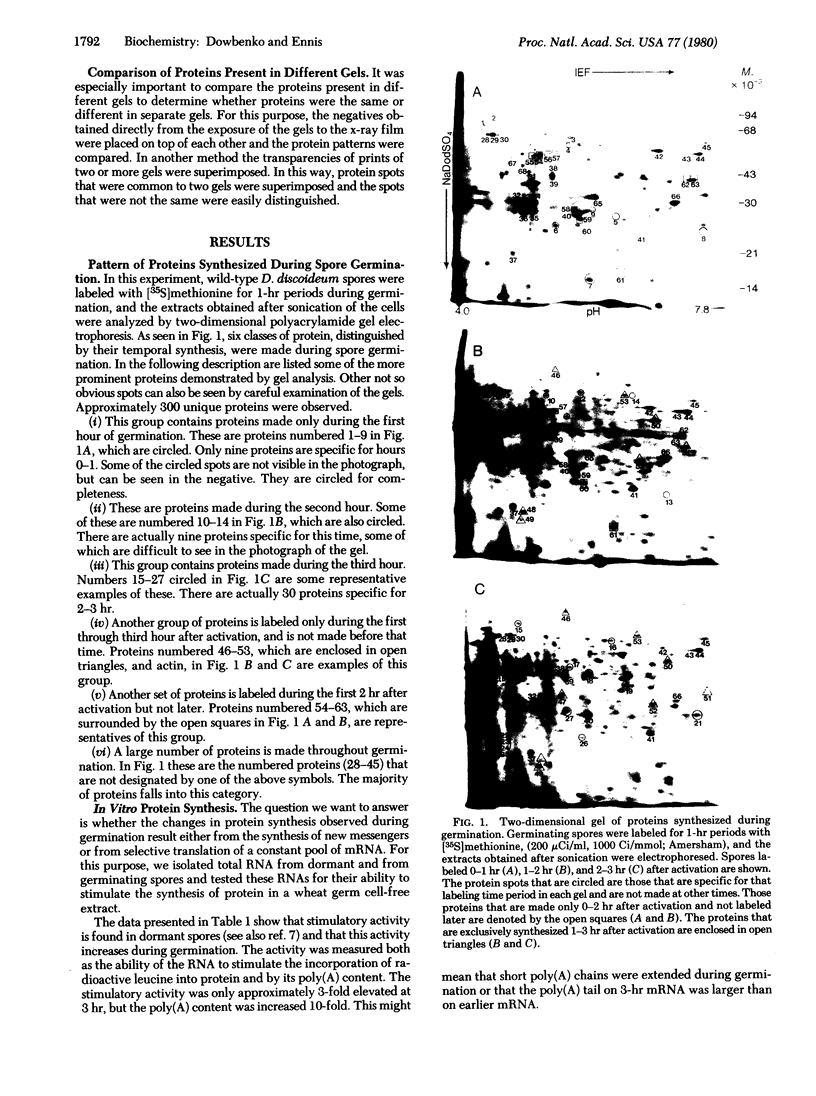
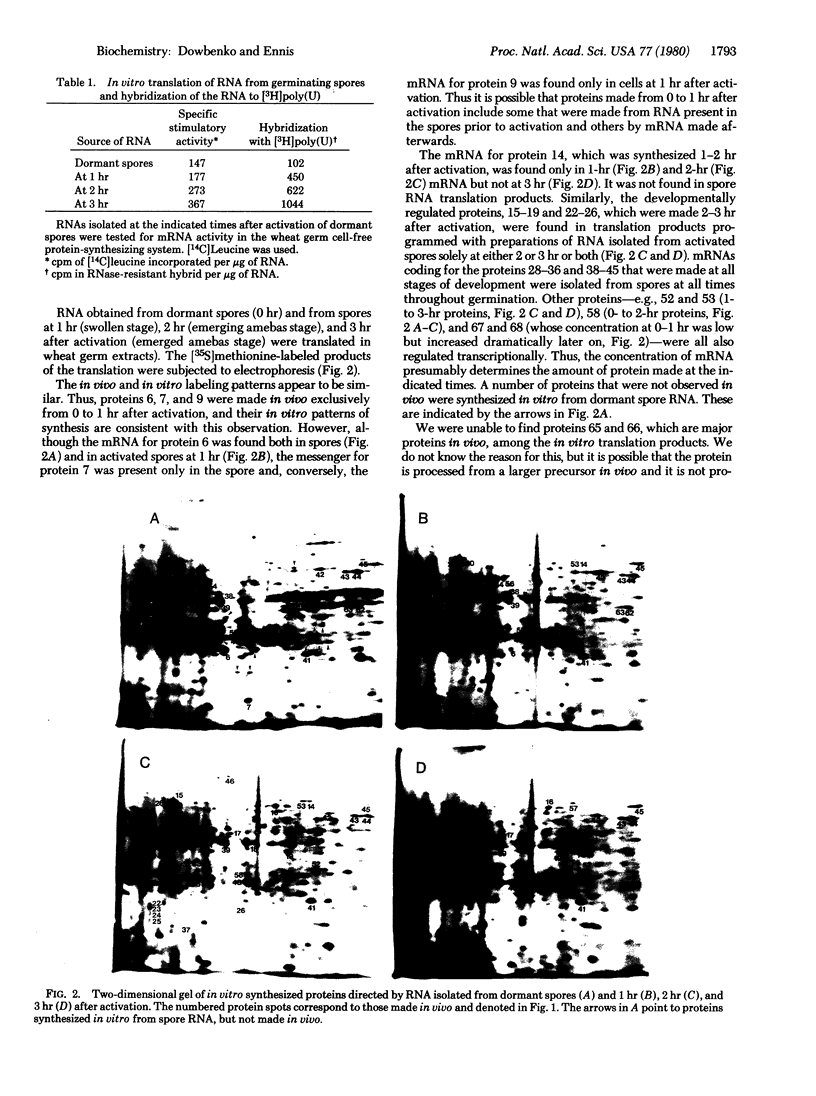
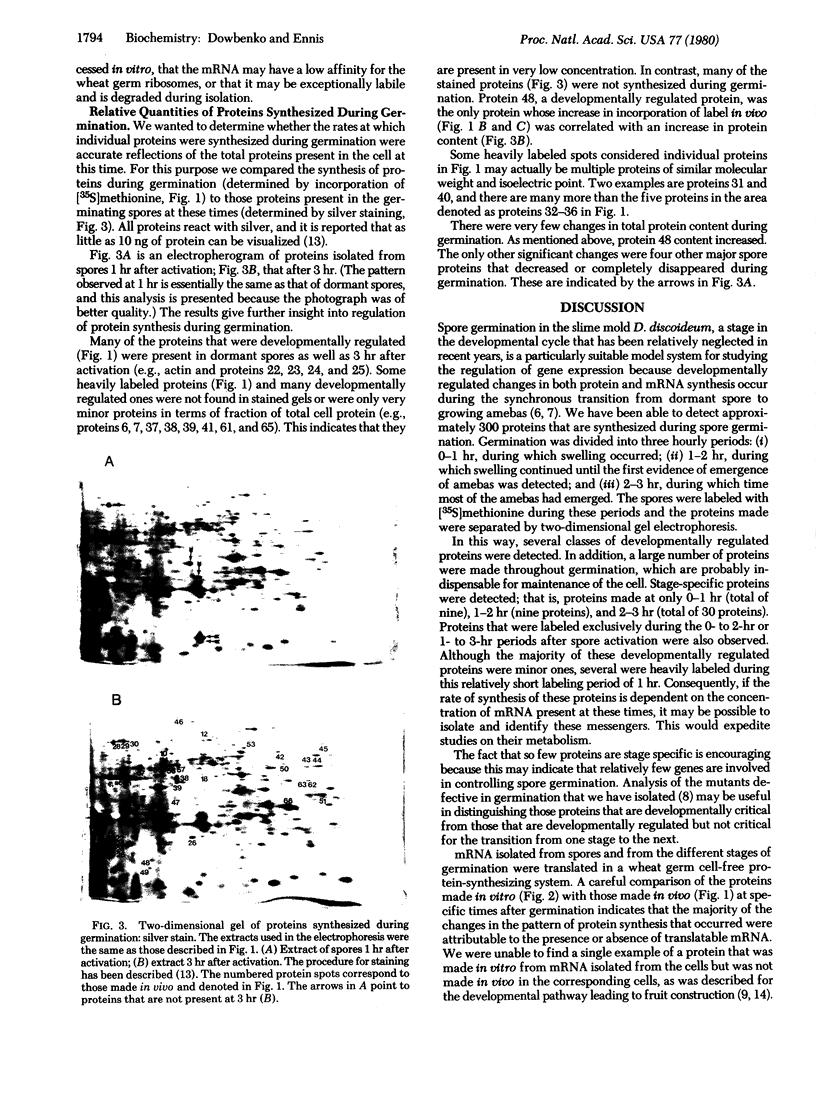
Spore germination in the slime mold Dictyostelium discoideum is a particularly suitable paradigm for studying the regulation of gene expression because developmentally regulated changes in both protein and mRNA synthesis occur during the synchronous transition from dormant spore to growing ameba. To investigate the regulation of protein synthesis during germination, we labeled activated spores with [35S]methionine at 1-hr intervals during germination, until amebas emerged (at 3 hr). The labeled proteins were resolved by two-dimensional polyacrylamide gel electrophoresis. Six classes of proteins were distinguished, depending on the time of onset and duration of their synthesis: (i) proteins made only during the first hour of germination, (ii) proteins made during the second hour, (iii) proteins made during the third hour, (iv) those synthesized only between 1 and 3 hr after activation, (v) peptides made only between 0 and 2 hr after activation, and (vi) proteins that were made throughout germination, mRNA isolated from dormant spores and from spores at different stages of germination was translated in a wheat germ cell-free protein-synthesizing system, and the proteins made in vitro were compared to those synthesized in vivo. The majority of the changes in the pattern of protein synthesis that occurred during the different stages of germination were attributable to the presence or absence of translatable mRNA. It is concluded that the synthesis of a majority of the proteins during spore germination is transcriptionally controlled.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Alton T. H., Lodish H. F. Translational control of protein synthesis during the early stages of differentiation of the slime mold Dictyostelium discoideum. Cell. 1977 Sep;12(1):301–310. doi: 10.1016/0092-8674(77)90208-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cotter D. A., Miura-Santo L. Y., Hohl H. R. Ultrastructural changes during germination of Dictyostelium discoideum spores. J Bacteriol. 1969 Nov;100(2):1020–1026. doi: 10.1128/jb.100.2.1020-1026.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A., Morin J. W., O'Connell R. W. Spore germination in Dictyostelium discoideum. II. Effects of dimethyl sulfoxide on post-activation lag as evidence for the multistate model of activation. Arch Microbiol. 1976 May 3;108(1):93–98. doi: 10.1007/BF00425097. [DOI] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Properties of germinating spores of Dictyostelium discoideum. J Bacteriol. 1968 Nov;96(5):1680–1689. doi: 10.1128/jb.96.5.1680-1689.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1966 Sep;56(3):880–887. doi: 10.1073/pnas.56.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L., Sussman M. Mutants of Dictyostelium discoideum defective in spore germination. J Bacteriol. 1975 Oct;124(1):62–64. doi: 10.1128/jb.124.1.62-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Developmental changes in RNA and protein synthesis during germination of Dictyostelium discoideum spores. Dev Biol. 1978 Nov;67(1):189–201. doi: 10.1016/0012-1606(78)90308-1. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Protein and RNA synthesis during spore germination in the cellular slime mold Dictyostelium discoideum. Biochem Biophys Res Commun. 1977 Jul 11;77(1):282–289. doi: 10.1016/s0006-291x(77)80194-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tuchman J., Alton T., Lodish H. F. Preferential synthesis of actin during early development of the slime mold Dictyostelium discoideum. Dev Biol. 1974 Sep;40(1):116–128. doi: 10.1016/0012-1606(74)90113-4. [DOI] [PubMed] [Google Scholar]