Abstract
Barley (Hordeum vulgare L.) plants were grown at different photon flux densities ranging from 100 to 1800 μmol m−2 s−1 in air and/or in atmospheres with reduced levels of O2 and CO2. Low O2 and CO2 partial pressures allowed plants to grow under high photosystem II (PSII) excitation pressure, estimated in vivo by chlorophyll fluorescence measurements, at moderate photon flux densities. The xanthophyll-cycle pigments, the early light-inducible proteins, and their mRNA accumulated with increasing PSII excitation pressure irrespective of the way high excitation pressure was obtained (high-light irradiance or decreased CO2 and O2 availability). These findings indicate that the reduction state of electron transport chain components could be involved in light sensing for the regulation of nuclear-encoded chloroplast gene expression. In contrast, no correlation was found between the reduction state of PSII and various indicators of the PSII light-harvesting system, such as the chlorophyll a-to-b ratio, the abundance of the major pigment-protein complex of PSII (LHCII), the mRNA level of LHCII, the light-saturation curve of O2 evolution, and the induced chlorophyll-fluorescence rise. We conclude that the chlorophyll antenna size of PSII is not governed by the redox state of PSII in higher plants and, consequently, regulation of early light-inducible protein synthesis is different from that of LHCII.
Photosynthetic organisms adapt to changes in irradiance by altering and optimizing the abundance of specific components in the photosynthetic apparatus. For instance, acclimation to high irradiances typically induces a decrease in the abundance of LHCII (Melis et al., 1985; Anderson et al., 1995) and a concomitant accumulation of ELIPs (Adamska et al., 1992; Pötter and Kloppstech, 1993). Reduction of growth irradiance elicits the opposite responses. An increased amount of LHCII is an adaptation to shade, since it enhances the efficiency of light harvesting by the photosystems. ELIPs and their algal homolog, the cbr protein, have strong homologies with LHCII (Grimm et al., 1989). They are supposed to bind chlorophylls and carotenoids (Levy et al., 1993; Green and Kühlbrandt, 1995) and to have a photoprotective function as minor pigment antennae with a reduced light-harvesting function and enhanced energy dissipation capacity (Levy et al., 1993; Krol et al., 1995; Montané et al., 1997). Alternatively, ELIPs may be involved in the repair of the PSII reaction centers and/or the protein-pigment complexes in photoinhibitory light (Adamska, 1997). Strong light stress is also known to stimulate carotenoid synthesis (Jones and Porter, 1986). Particularly, high-light-grown leaves are selectively enriched in carotenoids involved in the xanthophyll cycle, namely violaxanthin, zeaxanthin, and antheraxanthin (Demmig-Adams and Adams, 1992). The photoprotective function of xanthophyll is well accepted, although the exact molecular bases of this protection are still unclear (Demmig-Adams and Adams, 1992; Horton et al., 1996; Havaux, 1998).
Light-intensity-dependent changes in pigment-protein complexes are primarily controlled at the level of the LHCII gene transcription (Silverthorne and Tobin, 1984; Escoubas et al., 1995). However, little is known about the system that senses differences in light intensity and converts that signal to changes in gene expression. It seems unlikely that phytochrome and the blue-light receptor(s) are directly involved in the light acclimation of photosynthesis (Anderson et al., 1995; Walters and Horton, 1995; Durnford and Falkowski, 1997); they are much more sensitive to spectral quality or photoperiod than to PFD. Recent experimental data have been obtained in algae that support the notion that LHCII (Lhcb1) gene transcription (compare with Paulsen [1995] for a comparison of different nomenclatures for Chl a/b complexes) is coupled to irradiance through the redox status of some photosynthetic electron carriers, possibly the PQ pool (Allen et al., 1995; Huner et al., 1996).
Using electron transport inhibitors, Escoubas et al. (1995) observed repression or induction of LHCII gene transcription by maintaining the PQ pool reduced or oxidized, respectively. Similarly, growing algae at low temperature simultaneously increases the excitation pressure on PSII (i.e. the reduction level of its electron acceptors) and mimics high-light acclimation (Maxwell et al., 1994, 1995). However, it is unlikely that PSII alone is the primary redox sensor within chloroplasts, because no strict quantitative relationship between PSII excitation pressure and pigment content, Chl a/b, and LHCII apoprotein abundance was found in algae grown at different temperatures (Savitch et al., 1996). Possible alternative redox sensors include thioredoxin (Danon and Mayfield, 1994), the Cyt b6/f complex (Pearson et al., 1993), or soluble photosynthetic metabolites such as ATP and NADPH (Melis et al., 1985). Recently, it has been suggested that chlorophyll precursors could also be plastidic factors involved in the light induction of nuclear genes (Kropat et al., 1997).
In higher-plant chloroplasts the relation between PSII excitation pressure and the level of carotenoid and light-harvesting pigment complexes is elusive. Gray et al. (1996) reported that, in cereals, increasing the PSII excitation pressure by decreasing the growth temperature resulted in minimal adjustment to LHC polypeptides of PSII, Chl a/b, or xanthophylls. Petracek et al. (1997) found that accumulation of LHCII mRNA in tobacco seedlings was unaffected by DCMU treatment. Montané et al. (1997) showed in barley that low light at 5°C causes a strong decrease in LHCII mRNA but not in polypeptides. They also observed an increase in ELIP mRNA and protein, which took place during the first hours after the transfer to low light in the cold, as it usually occurs in strong light at 25°C. Growth of pea plants in the presence of sublethal concentrations of a PSII inhibitor (SAN 9785), which blocks electron transport at a site close or similar to that of DCMU, leads to a noticeable increase in the PSII chlorophyll antenna size (Joshi et al., 1995), suggesting a role for the reduction state of the PQ pool in the regulation of LHCII gene expression. Bilger et al. (1995) found an apparent link between xanthophyll-cycle pigment content and the photosynthetic capacity in potato but not in tobacco.
The lack of conclusive evidence on the redox control of photosynthetic acclimation of higher plants to the light environment has prompted us to re-examine the pigment content and organization in leaves adapted to various PSII excitation pressures in long-term experiments. In this study PSII excitation pressure was modulated by manipulating the CO2 and O2 concentrations of the atmosphere in which plants were grown. By this means, different PSII excitation pressures can be obtained under conditions of constant PFD and temperature. The results presented here indicate that both the xanthophyll pigments and the ELIPs respond positively to the excitation pressure on PSII, whereas the LHCII abundance was correlated with the light irradiance but not with the reduction of PSII.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Barley (Hordeum vulgare L.) plants were grown for 2 weeks in a phytotron under controlled conditions of temperature (23°C/20°C, day/night), light (100 or 350 μmol photons m−2 s−1, 12 h d−1), and air humidity (70%). Plants were then transferred to closed growth chambers (700 L in volume) in which the gas composition of the atmosphere was strictly controlled (Fabreguettes et al., 1994). Plants were adapted for 1, 3, 7, or 8 d in various atmospheres at various PFDs. Air humidity, photoperiod, and temperature remained unchanged.
Pigment Determination
Pigments were extracted from leaf discs in methanol. After centrifugation and filtration, the samples were analyzed by reversed-phase HPLC as described previously (Havaux and Tardy, 1996).
Chlorophyll Fluorescence
Chlorophyll fluorescence was measured with a PAM-2000 modulated fluorometer (Walz, Effeltrich). The initial level of chlorophyll fluorescence, Fo, was measured with a dim-red light modulated at 600 Hz after applying a 2-s pulse of far-red light. The maximal level, Fm, of chlorophyll fluorescence was determined with a 800-ms pulse of intense white light (4000 μmol m−2 s−1). The maximal quantum yield of PSII photochemistry was determined by (Fm − Fo)/Fm. When leaves were adapted to white light, the relative variable fluorescence, V, was determined from Fm, Fo, and the steady-state level, F: (F − Fo)/(Fm − Fo) (Havaux et al., 1991). V is identical to 1 − qp, where qp is the so-called photochemical quenching coefficient.
Leaves were infiltrated with 50 μm DCMU for 30 min in darkness. Chlorophyll fluorescence was induced by a red- light beam of PFD 40 μmol m−2 s−1. The half-time of the induced fluorescence rise was used as an indicator of the functional chlorophyll antenna size of PSII (Malkin et al., 1981).
Photosynthetic O2 Evolution
Leaf discs 1 cm in diameter were placed in the hermetically closed cell of a laboratory-constructed photoacoustic spectrometer previously described (Havaux and Tardy, 1996). The samples were illuminated with white light modulated at 19 Hz (50 μmol photons m−2 s−1). The photochemistry was saturated with a strong background light of PFD = 4500 μmol photons m−2 s−1. Photosynthetic O2 evolution was measured and separated from the photothermal signal as described by Poulet et al. (1983). The quantum yield of O2 evolution, Φ, in relative values was determined as the ratio between the amplitude of the O2-evolution-related photoacoustic signal and the amplitude of the photothermal signal (Poulet et al., 1983). Photoacoustic measurements of Φ were performed at a low CO2 concentration, presumably at the CO2 compensation point. The light-saturation curve of photosynthesis was determined by measuring the gradual decrease in the quantum yield while progressively increasing the PFD of a continuous white light. E of O2 evolution was measured with a modulated blue-green light (25 μmol m−2 s−1) obtained with a combination of BG18 and BG38 filters (Schott, Mainz, Germany), and with a far-red background light (730 nm, 18 W m−2 approximately 110 μmol m−2 s−1). E was measured in state 1 after 15 min of adaptation to the far-red light as the ratio of the O2 evolution in the presence of far-red light to the O2 signal in the absence of far-red light (Canaani and Malkin, 1984).
Isolation of mRNA and Northern Hybridization
Leaf samples were harvested every 3 h during the diurnal phase at the indicated days after transfer of the plants into different atmospheres. Isolation and northern hybridization with LHCII and ELIP cDNA probes were performed as described in detail elsewhere (Montané et al., 1997). Two exposure times with Bio Max films (Kodak) were systematically done to test for the linearity of the signals. The blots were quantified with a scanner (Gel Doc 1000, Bio-Rad) and the corresponding software. DNA probes were cDNA inserts of cloned barley genes. The small ELIP was HV90 (Grimm et al., 1989). The LHC clone was a gift from Dr. K. Gausing (Aarhus University, Aarhus, Denmark).
Analysis by SDS-PAGE and Immunoblotting
Leaf samples were harvested the same way as for mRNA analysis. The procedures of protein extraction, electrophoresis, and immunoblotting with rabbit anti-barley small ELIP or anti-barley LHCIIb antibodies and quantification were as previously described by Montané et al. (1997). Protein determination was according to the method of Lowry et al. (1951). Ten micrograms was loaded per slot for ELIP determination and a dilution of 1:300 was used for LHCII determination. The separated proteins were blotted onto Westran membranes (Schleicher & Schuell) blocked with 5% low-fat milk powder (Ülzenia, Ülzen, Germany) in PBS 0.1%, Tween 20. The antibody from rabbit was applied in fresh 5% milk in a 1:2,000 dilution overnight at 4°C, the next morning for 1 h at 20°C, and then washed six times with PBS 0.1% and Tween 20. The goat secondary antibody (anti-rabbit) conjugated with alkaline phosphatase (A0418, Sigma) was applied at a 1:20,000 dilution for 2 h at 20°C. Determinations of phosphatase were as described previously (Montané et al., 1997). Dilutions of 1:300 and 1:600 of the protein extracts showed linearity of the signals. Quantification was done the same way as for mRNA analysis.
RESULTS
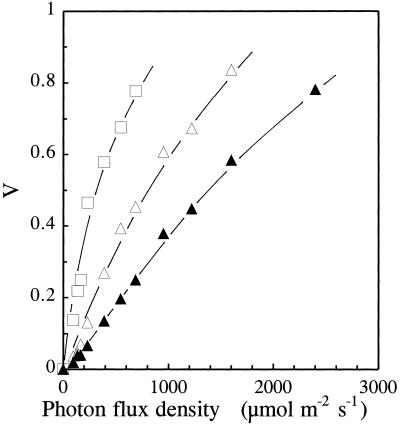
V (1 − qP) is nonlinearily related to the fraction of closed PSII centers, i.e. the fraction of reaction centers with their primary quinone electron acceptor, QA, in the reduced state ([QA−]/[QA]total) (Havaux et al., 1991). Thus, V can be used as a semiquantitative indicator of relative changes in the reduction state of PSII reaction centers in plants exposed to changing environmental conditions. V was measured in barley leaves adapted to different PFDs of white light. Figure 1 shows that V increases with increasing PFD, as expected. In air a V value of 0.5 was obtained at a PFD of approximately 1500 μmol photons m−2 s−1. When the availability of the final electron acceptors (O2 and CO2) was reduced, PSII excitation pressure strongly increased. For instance, V was 0.5 at 800 or 300 μmol photons m−2 s−1 in an atmosphere containing 70 μg mL−1 CO2 and 10% O2 or 20 μg mL−1 CO2 and 2% O2, respectively. High PSII excitation pressures could then be obtained at low or moderate PFDs by decreasing the partial pressure of CO2 and O2 in the gas environment.
Figure 1.
PSII excitation pressure, as measured by V, in barley leaves exposed to different PFDs in different atmospheres (▴, air; ▵, 70 μg mL−1 CO2 and 10% O2; □, 20 μg mL−1 CO2 and 2% O2).
Pigments
Based on the data shown in Figure 1, we grew barley plants under different PSII excitation pressures created by manipulating the light and gas environment. After 8 d of growth in the different environments listed in Table I, the photosynthetic pigments were analyzed. When the PFD was increased in air from 250 to 1800 μmol photons m−2 s−1, V increased from 0.08 to 0.6 and Chl a/b of the plants that were exposed to this initial excitation pressure increased from 2.50 to 2.73. This latter modification suggests a reduction of the Chl-b-containing light-harvesting antennae relative to the reaction centers of PSII. Concomitantly, the pool size of the pigments of the xanthophyll cycle increased from 15.1 ng mm−2 to 35.3 ng mm−2.
Table I.
Effects of various growth conditions leading to different PSII excitation pressures (as measured by V) on the chlorophyll (Chl) and carotenoid content, Chl a/b, and the xanthophyll-cycle pigments pool (A+Z+V) in barley leaves
| Growth Condition | V | Total Chl | Chl a/b | Total Carotenoid | A+V+Z |
|---|---|---|---|---|---|
| μmol photons m−2 s−1 | ng mm−2 | ng mm−2 | |||
| Air | |||||
| 250 | 0.08 | 327.9 ± 56.6 | 2.50 ± 0.11 | 69.1 ± 12.6 | 15.1 ± 2.6 |
| 400 | 0.13 | 386.9 ± 13.4 | 2.41 ± 0.03 | 85.9 ± 4.9 | 18.8 ± 0.4 |
| 750 | 0.25 | 372.5 ± 3.1 | 2.62 ± 0.07 | 76.3 ± 3.2 | 18.5 ± 0.6 |
| 1300 | 0.47 | 326.7 ± 42.7 | 2.76 ± 0.03 | 74.3 ± 4.5 | 21.8 ± 0.8 |
| 1500 | 0.52 | 339.0 ± 47.2 | 2.72 ± 0.24 | 84.1 ± 13.2 | 24.9 ± 2.7 |
| 1800 | 0.60 | 395.1 ± 17.1 | 2.73 ± 0.05 | 110.3 ± 5.9 | 35.3 ± 4.6 |
| 70 ppm CO2, 10% O2 | |||||
| 750 | 0.48 | 264.3 ± 8.7 | 2.34 ± 0.05 | 68.3 ± 0.6 | 18.5 ± 0.1 |
Plants were grown for 8 d under the different gas and light conditions. Data are means ± sd of 3 to 10 separate experiments.
Growth at high PFD affected preferentially the xanthophyll-cycle carotenoids, since the ratio between the sum of A+V+Z and the total carotenoid content rose from 0.22 at 250 μmol photons m−2 s−1 to 0.32 at 1800 μmol photons m−2 s−1. A high PSII excitation pressure (V = 0.48) was also obtained in moderate light (750 μmol photons m−2 s−1) when air was replaced by a mixture of 70 μg mL−1 CO2 and 10% O2 in nitrogen. Instead of increasing, Chl a/b decreased to 2.34 (versus 2.62 in air at the same PFD).
On a leaf-area basis, the xanthophyll-cycle carotenoids did not change significantly. However, leaves grown in an atmosphere impoverished in CO2 and O2 were much thinner than leaves grown in air, so comparison of pigment contents on a leaf-area basis is meaningless. The leaf-specific weight was 0.152 mg mm−2 in the former plants and 0.164 and 0.204 mg mm−2 in plants cultivated in air at 400 μmol m−2 s−1 and 1500 μmol m−2 s−1, respectively. This problem was overcome by normalizing the A+Z+V content to the total carotenoid content or to the total chlorophyll content.
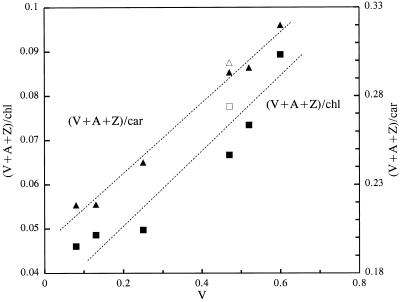
The use of normalized values shows that leaves grown under a high PSII excitation pressure in moderate light are enriched in xanthophyll-cycle carotenoids: the ratio of A+Z+V to carotenoids was 0.27 and the ratio of A+Z+V to chlorophylls was 0.070. These values are very close to the values found in leaves grown at a similar excitation pressure at high PFD (1500 μmol m−2 s−1) in air (0.29 and 0.073, respectively), whereas the corresponding ratios in leaves grown in air at low PFD were substantially lower (0.22 and 0.046). This is seen more clearly in Figure 2, which shows the A+Z+V content normalized to the chlorophyll and carotenoid content as a function of V in various PFDs and gas conditions. The xanthophyll-cycle pigment content was correlated with the PSII excitation pressure. When the CO2 and O2 partial pressures were further reduced to very low levels (e.g. 20 μg mL−1 CO2 and 2% O2), plant growth was dramatically inhibited and very limited adjustment of the photosynthetic pigments took place (data not shown).
Figure 2.
Xanthophyll-cycle carotenoids pool (V+A+Z) normalized to the total chlorophyll content or the total carotenoid content ([V+A+Z]/chl, ▴; [V+A+Z]/car, ▪) as a function of the PSII excitation pressure (V) obtained by varying the PFD in air (closed symbols) or by decreasing the CO2 and O2 content of the atmosphere (70 μg mL−1 CO2 and 10% O2, 750 μmol m−2 s−1, open symbols). The data were calculated from the data of Table I.
PSII Chlorophyll Antenna Size
Table II shows some chlorophyll fluorescence characteristics of plants grown at low or high excitation pressure in high- or low-intensity light. The maximal quantum yield of PSII photochemistry was similar in plants grown in strong light in air and in plants grown in moderate light under conditions of low CO2 and O2 concentrations. In contrast, the induction of chlorophyll fluorescence in the presence of DCMU was much faster in the latter plants (half-time of the fluorescence rise approximately 11 ms) compared with the former ones (21 ms) or to plants grown at low PSII excitation pressure (air and low PFD, 16 ms). This indicates an increase in the functional PSII chlorophyll antenna size in plants grown at reduced CO2 and O2 concentrations, thus confirming the Chl a/b data.
Table II.
Effects of various growth conditions leading to different PSII excitation pressures (as indicated by V) on the maximal quantum yield of PSII photochemistry [(Fm − Fo)/Fm], the half time (t1/2) of the chlorophyll fluorescence rise in the presence of DCMU, and the E of O2 evolution in barley leaves
| Growth Condition | V | (Fm − Fo)/Fm | t1/2 | E |
|---|---|---|---|---|
| μmol photons m−2 s−1 | ms | |||
| Air | ||||
| 400 | 0.13 | 0.78 ± 0.01 | 16.2 ± 1.7 | 1.26 ± 0.02 |
| 1500 | 0.52 | 0.75 ± 0.01 | 21.1 ± 1.4 | 1.24 ± 0.03 |
| 70 ppm CO2, 10% O2 | ||||
| 750 | 0.47 | 0.75 ± 0.02 | 10.7 ± 2.1 | 1.29 ± 0.03 |
Plants were grown for 8 d under the different gas and light conditions. Data are means ± sd of three separate experiments.
A decreased Chl a/b can also result from a reduction of the PSII-to-PSI ratio. This latter possibility can be excluded in our experiments from the measurements of E shown in Table II. E is a measure of the α/β ratio, where α and β are the photochemical potential (quantum yield × light absorption) of PSII and PSI, respectively (Canaani and Malkin, 1984). Growth of plants under high excitation pressure on PSII (in low light) did not decrease E. In reality, E tended to increase, thus indicating a trend toward a PSII/PSI activity imbalance in favor of PSII, as expected from the larger PSII antennae.
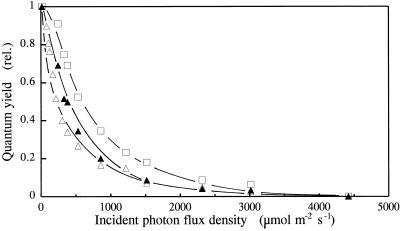
Figure 3 shows the light saturation curve of photosynthetic O2 evolution in the different types of plants. O2 evolution was measured with the photoacoustic technique at the CO2 compensation point. Φ decreased much more rapidly with increasing PFD in leaves grown in low CO2 and O2. The PFD corresponding to a 50% decrease in Φ was 200 and 600 μmol m−2 s−1 for plants grown under high PSII excitation pressure at moderate and high PFD, respectively. These results are consistent with larger antennae of the photosystems in the former plants. When O2 evolution was measured with a Clark-type O2 electrode under CO2 saturation conditions, qualitatively similar results were obtained (data not shown).
Figure 3.
Plots of the photoacoustically monitored Φ in barley leaves grown under different PSII excitation pressures induced by different light and gas environments versus the PFD of the incident light. ▵, Air and 400 μmol photons m−2 s−1; □, air and 1500 μmol photons m−2 s−1; ▵, 70 μg mL−1 CO2 and 10% O2 at 750 μmol photons m−2 s−1. Plants were grown for 8 d in the different light and gas environments.
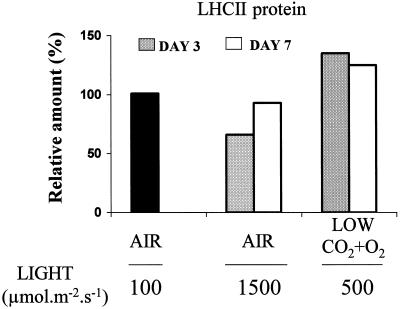
LHCII was quantified by western-blot analysis. Figure 4 shows that the LHCII abundance substantially decreased during growth in air at 1500 μmol m−2 s−1, whereas the LHCII level significantly increased when barley plants were transferred to low CO2 and O2 in moderate light (500 μmol m−2 s−1). Those changes were more marked after 3 d of exposure to the high PSII excitation pressure than after 7 d. One can suggest that, in the long term, other adaptive changes in the photosynthetic system of leaves exposed to bright light could result in a reduced need for small pigment antennae.
Figure 4.
Changes in LHCII abundance after transfer of barley plants from air and low light (100 μmol m−2 s−1) to air plus strong light (1500 μmol m−2 s−1) or to low CO2 and O2 (50 μg mL−1 CO2 and 3% O2) in moderate light (500 μmol m−2 s−1). The results are expressed relative to the LHCII level before the transfer to the new growth regimes. Lanes on the gels were loaded with equal protein content and were probed with anti-LHCIIb antibodies. The LHCII abundance was measured after 3 and 7 d of growth under the new conditions. Data are mean values of three experiments with sd being lower than 15% for all values.
Table II and Figures 3 and 4 clearly show that the PSII chlorophyll antenna size is not under the direct control of the PSII excitation pressure in barley leaves.
Gene Expression
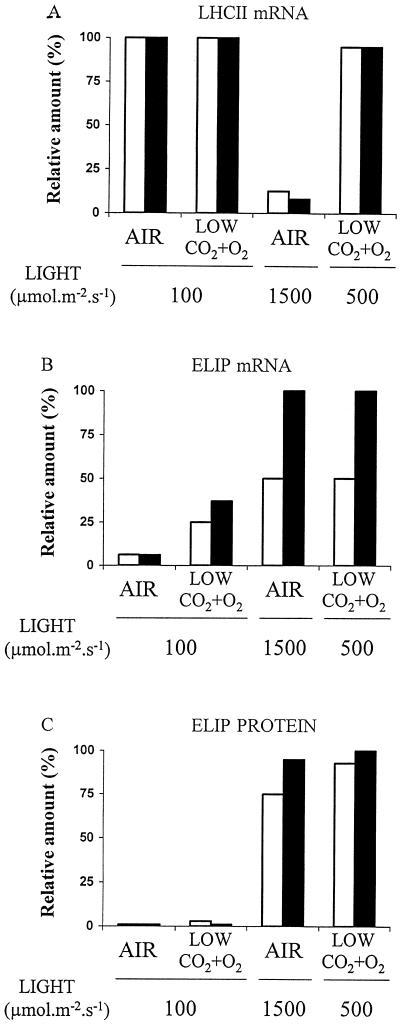
Because of the oscillation of the mRNA of ELIP and LHCII genes (Kloppstech, 1985; Tavladorakis et al., 1989), samples were taken every 3 h during the photoperiod to get the peak value. Figure 5 A shows the level of LHCII mRNA in 2-week-old barley leaves grown for 1 or 3 d under low or high PSII excitation pressure. Transfer of barley plants from low light (100 μmol photons m−2 s−1) to strong light (1500 μmol m−2 s−1) in air dramatically reduced the mRNA level to approximately 10% of the control level. This reduction is a well-known response to strong light stress.
Figure 5.
Maximal level of LHCII mRNA and levels of ELIP mRNA and protein in barley leaves grown for 1 (white bars) or 3 (black bars) d under low or high PSII excitation pressure in air or in 50 μg mL−1 CO2 plus 3% O2. Maximum quantum yield for PSII photochemistry (Fm − Fo)/Fm: 0.79 ± 0.01 after 3 d in air at 100 μmol photons m−2 s−1; 0.81 ± 0.01 after 3 d at 100 μmol photons m−2 s−1 in low CO2 and O2; 0.69 ± 0.02 after 3 d in air at 1500 μmol photons m−2 s−1; and 0.74 ± 0.02 after 3 d at 500 μmol photons m−2 s−1 in low CO2 and O2. Results are expressed relative to the highest value. Data are mean values of three experiments, with sd being always less than 15%.
It is interesting that decreasing the CO2 and O2 concentration to 50 μg mL−1 and 3%, respectively, did not induce a reduction of the mRNA abundance, although the PSII excitation pressure (V approximately 0.45) was similar to that obtained at high light in air. Thus, the same excitation pressure on PSII resulted in strikingly different effects on LHCII transcripts, depending on the way high-excitation pressure was obtained. Figure 5B shows that, in contrast to the LHCII mRNA, the level of ELIP mRNA, which until now was known only as a response to cold and strong light stress, was closely correlated with the PSII excitation pressure but not with the PFD. A similar observation was made when the abundance of ELIP was analyzed (Fig. 5C). Virtually no ELIP was detected in barley leaves grown under low PSII excitation pressure, whereas ELIPs accumulated in leaves submitted to conditions of high excitation pressure on PSII. However, at reduced CO2 and O2 partial pressures, ELIP mRNA accumulated at low and moderate PFDs (100 and 500 μmol m−2 s−1, respectively), whereas the polypeptides accumulated only at moderate PFD. These findings suggest that the redox-sensing mechanism acts at the level of ELIP transcription and that another mechanism dependent on the irradiance level is required for ELIP accumulation.
DISCUSSION
Our data support the hypothesis that the chloroplast redox poise controls the transcription of nuclear-encoded genes in photosynthetic organisms (Allen, 1993; Allen et al., 1995; Huner et al., 1996). The abundance of xanthophyll-cycle carotenoids and of ELIPs in barley leaves was closely related to the reduction state of PSII, with high V values being associated with the appearance of ELIPs and the accumulation of xanthophylls (Figs. 2 and 5). These changes took place independently of whether the PSII electron acceptor QA was reduced by increasing the light irradiance or by decreasing the availability of the final electron acceptors in moderate light. Disconnection between ELIP synthesis and light irradiance was previously observed in leaves exposed to cold and light stresses (Adamska and Kloppstech, 1994; Montané et al., 1997). For instance, in barley leaves chilled at 5°C, high levels of ELIP mRNA were detected at low light, which did not induce any ELIP transcript at 25°C (Montané et al., 1997). Low temperature noticeably increases excitation pressure on PSII (Havaux, 1987), so this cold-induced accumulation of ELIPs can be interpreted, in the light of our results, in terms of redox control of gene expression.
Induction of ELIPs was previously shown to be unrelated to the formation of active O2 species (Adamska et al., 1993) or to photooxidative damage of the thylakoid membranes (Montané et al., 1997). Moreover, in this study accumulation of ELIPs and xanthophyll carotenoids occurred in the absence of appreciable photoinhibition of PSII (Table II; legend of Fig. 5). Our results are also compatible with the finding that ELIP transcription is specifically induced by PSII light (blue-green light) but not by PSI light (red plus far-red light) (Adamska et al., 1992). Exposure of leaves to strong PSII light is expected to over-reduce the intersystem electron transport chain, whereas PSI light oxidizes it. Our data also show that the excitation pressure on PSII appears to be involved in regulation at the level of gene transcription (Fig. 5). Effective accumulation of stress proteins and “stress pigments” presumably requires another mechanism to act translationally or posttranslationally and depends on PFD (Adamska et al., 1992; Debel et al., 1994).
In algae cold treatment in low light was also reported to bring about accumulation of xanthophyll-cycle pigments (Maxwell et al., 1995). However, when cereals were transferred to low light at chilling temperature, very little change in the xanthophyll content of the leaves was found in response to steady-state reduction of QA (Gray et al., 1996). Possibly, this lack of photosynthetic adaptation in cold-treated higher plants could result from the strongly reduced growth rate at low temperature, which could perturb photosynthetic acclimation to high PSII excitation pressure conditions. However, Adams et al. (1994) found seasonal changes in the xanthophyll-cycle pool size in different plant species, with a much larger pool present in the leaves examined during the winter. The originality of our work is that different PSII excitation pressures were achieved at constant and ambient temperature and in the absence of any photosynthetic inhibitors, thus avoiding side effects of chilling stress and unspecific effects of chemical treatments, allowing long-term experiments and significant growth of the plants. It was previously reported that, after exposure for 7 d to different PFDs, the pool size of the xanthophyll-cycle pigments was systematically higher in leaves of genetically manipulated potato plants, which expressed an antisense mRNA coding for a key enzyme of the Calvin cycle compared with wild-type leaves (Bilger et al., 1995). As the drastic inhibition of the Calvin cycle activity in those transformed plants is expected to cause a steady-state reduction of photosynthetic electron carriers, this result can be seen as another illustration of xanthophyll biosynthesis regulation dependent on the reduction state of the electron transport chain. The apparent coordination of the accumulations of ELIPs and xanthophyll-cycle carotenoids found in the present study, with both phenomena responding apparently to similar light switches, is consistent with the suggestion that ELIP or its algal homolog and the xanthophyll cycle might be interrelated in their biological function (Adamska et al., 1992, 1993; Levy et al., 1993; Krol et al., 1995).
Although the amplitude of variable chlorophyll fluorescence is directly related to the reduction state of QA (Havaux et al., 1991), it is not possible to identify from our study the photosynthetic electron carrier(s) involved in regulation of gene expression. Since PQ reoxidation is the slowest step in the intersystem electron transport chain (Haehnel, 1984), a high reduction level of QA and a high V value are generally accompanied by a reduction of the PQ pool and can also be associated with reduction of components of the Cyt b6/f complex. In this context, it is worth mentioning that strongly reduced levels of Cyt b6/f complex in transgenic tobacco markedly increase the excitation pressure on PSII without significantly affecting the carotenoid content of the leaves (Hurry et al., 1996). In fact, Cyt b6/f is known to play a key role in the short-term response to light via the state-transition phenomenon (Wollman and Lemaire, 1988; Gal et al., 1987). The state transitions are a mechanism by which chloroplasts adjust the light distribution between PSII and PSI via reversible phosphorylation of LHCII (Williams and Allen, 1987). It is believed that the LHCII-kinase is directly associated with the Cyt b6/f complex and that the activation of the kinase system is mediated by the redox state of a component of the Cyt b6/f complex (Gal et al., 1990). As transcriptional regulation of nuclear-encoded genes by the chloroplast redox status has been suggested to act through a signal transduction pathway that is initiated by the action of a chloroplast protein kinase (Allen, 1993; Escoubas et al., 1995), the Cyt b6/f complex is a plausible candidate as the primary redox sensor, as previously hypothesized by Pearson et al. (1993). In the cyanobacterium Synechocystis, adjustment of the stochiometry between PSI and PSII to light quality and intensity was well correlated with the redox steady state of the Cyt b6/f complex but not with the state of the PQ pool (Murakami and Fujita, 1991).
ELIP and LHCII are usually known to respond to light stress in a reciprocal manner (Pötter and Kloppstech, 1993). This behavior was confirmed in the present study when barley leaves were exposed to strong light stress (Figs. 4 and 5). However, the main result of the present work is that LHCII does not respond to the same light-sensing mechanism as carotenoids and ELIPs. The PSII light-harvesting pigment antenna size, probed by biochemical and functional measurements (Tables I and II; Figs. 3–5), clearly decreased with increasing light irradiance but not with increasing PSII excitation pressure. In fact, high PSII excitation pressure in low light brought about an increase rather than a decrease in the PSII antenna size, mimicking shade adaptation. A similar, though less-pronounced, phenomenon was reported in transgenic tobacco with reduced levels of Cyt b6/f complex (Price et al., 1995). In the trangenic tobacco, high PSII excitation pressure in low light was accompanied by a substantially reduced Chl a/b compared with the wild type, suggesting reduced amounts of LHCII in the former plants. The fact that ELIP was detected when antennae size increased, remained constant, or decreased shows that ELIP accumulation and LHCII disappearance are not strictly correlated. In this context, one should remember that during greening, ELIP accumulation always precedes the LHCII accumulation in chloroplast biogenesis when low amounts of chlorophyll are present (Meyer and Kloppstech, 1984; Beator and Kloppstech, 1994). Although this phenomenon could be related to a different coupling to the circadian oscillator (Tavladorakis et al., 1989), it is in favor of a distinct signal for LHCII and ELIP regulation. We conclude from our data that the excitation pressure on PSII and the redox state of the intersystem electron transport chain are unlikely to be the primary redox sensors controlling LHCII gene expression in barley leaves. Consequently, gene regulation during photoacclimation of photosynthesis seems to differ in higher plants and in green algae. The physiological and biochemical reasons for this difference deserve to be studied in the future.
ACKNOWLEDGMENTS
We are grateful to Dr. M. Péan and the members of the C23A unit (Département d'Ecophysiologie Végétale et de Microbiologie, Commissariat à l'Energie Atomique/Cadarache) for their help in growing plants in controlled atmospheres.
Abbreviations:
- A+Z+V
xanthophyll-cycle pigment complex, composed of antheraxanthin, zeaxanthin, and violaxanthin
- Chl a/b
chlorophyll a-to-b ratio
- E
Emerson enhancement
- ELIP
early light-inducible protein
- LHCII
major light-harvesting Chl a/b-protein complex of PSII
- PFD
photon flux density
- PQ
plastoquinone
- V
relative variable chlorophyll fluorescence
LITERATURE CITED
- Adams WW, III, Demmig-Adams B, Verhoeven AS, Barker DH. ‘Photoinhibition’ during winter stress: involvement of sustained xanthophyll cycle-dependent energy dissipation. Aust J Plant Physiol. 1994;22:261–276. [Google Scholar]
- Adamska I. ELIPs: light-induced stress proteins. Physiol Plant. 1997;100:794–805. [Google Scholar]
- Adamska I, Kloppstech K. Low temperature increases the abundance of early light-inducible transcript under light stress conditions. J Biol Chem. 1994;269:30221–30226. [PubMed] [Google Scholar]
- Adamska I, Kloppstech K, Ohad I. The effect of free radical enhancers and scavengers on accumulation of early light-inducible protein during light stress. Z Naturforsch. 1993;48c:391–396. [Google Scholar]
- Adamska I, Ohad I, Kloppstech K. Synthesis of the early light-inducible protein is controlled by blue light and related to light stress. Proc Natl Acad Sci USA. 1992;89:2610–2613. doi: 10.1073/pnas.89.7.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JF. Redox control of gene expression and the function of chloroplast genomes: an hypothesis. Photosynth Res. 1993;36:95–102. doi: 10.1007/BF00016274. [DOI] [PubMed] [Google Scholar]
- Allen JF, Alexciev K, Hakansson G. Regulation by redox signalling. Curr Biol. 1995;5:869–872. doi: 10.1016/s0960-9822(95)00176-x. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Chow WS, Park Y-I. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- Beator J, Kloppstech K. The circadian oscillator coordinates the synthesis of apoproteins and their pigments during chloroplast development. Plant Physiol. 1994;103:191–196. doi: 10.1104/pp.103.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Fisahn J, Brummet W, Kossmann J, Willmizer L. Violaxanthin cycle pigment contents in potato and tobacco plants with genetically reduced photosynthetic capacity. Plant Physiol. 1995;108:1479–1486. doi: 10.1104/pp.108.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani O, Malkin S. Distribution of light excitation in an intact leaf between the two photosystems of photosynthesis. Biochim Biophys Acta. 1984;766:513–524. [Google Scholar]
- Danon A, Mayfield SP. Light-regulated translation of chloroplast messenger RNAs through redox potential. Science. 1994;266:1717–1719. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- Debel K, Knack G, Kloppstech K. Accumulation of plastid HSP 23 of Chnenopodium rubrum is controlled post-translationally by light. Plant J. 1994;6:79–85. [Google Scholar]
- Demmig-Adams B, Adams WW., III Photoprotection and other responses of plants to high light stress. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]
- Durnford DG, Falkowski PG. Chloroplast redox regulation of nuclear gene transcription during photoacclimation. Photosynth Res. 1997;53:229–241. [Google Scholar]
- Escoubas J-M, Lomas M, LaRoche J, Falkowski PG. Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabreguettes V, Gibiat F, Pintena J, Vidal D, André M (1994) The C23A system: a tool for global control of plant environment and exchange measurements. In Proceedings of the 24th International Conference on Environmental Systems, 941544. Society of Automotive Engineers, Warrendale, PA
- Gal A, Shahak Y, Schuster G, Ohad I. Specific loss of LHCII phosphorylation in the Lemna mutant 1073 lacking the cytochrome b6/f complex. FEBS Lett. 1987;221:205–210. [Google Scholar]
- Gal A, Hauska G, Herrmann R, Ohad I. Interaction between light harvesting chlorophyll-a/b protein (LHCII) kinase and cytochrome b6/f complex. J Biol Chem. 1990;265:19742–19749. [PubMed] [Google Scholar]
- Gray GR, Savitch LV, Ivanov AG, Huner NPA. Photosystem II excitation pressure and development of resistance to photoinhibition. Plant Physiol. 1996;110:61–71. doi: 10.1104/pp.110.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BR, Kühlbrandt W. Sequence conservation of light harvesting and stress-response proteins in relation to the three dimensional molecular structure of LHCII. Photosynth Res. 1995;44:139–148. doi: 10.1007/BF00018304. [DOI] [PubMed] [Google Scholar]
- Grimm B, Kruse E, Kloppstech K. Transiently expressed early light-inducible proteins share transmembrane domains with light-harvesting chlorophyll binding proteins. Plant Mol Biol. 1989;13:583–593. doi: 10.1007/BF00027318. [DOI] [PubMed] [Google Scholar]
- Haehnel W. Photosynthetic electron transport in higher plants. Annu Rev Plant Physiol. 1984;35:659–693. [Google Scholar]
- Havaux M. Effects of chilling on the redox state of the primary electron acceptor QA of photosystem II in chilling-sensitive and resistant plant species. Plant Physiol Biochem. 1987;25:735–743. [Google Scholar]
- Havaux M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998;3:147–151. [Google Scholar]
- Havaux M, Strasser RJ, Greppin H. A theoretical and experimental analysis of the qP and qN coefficients of chlorophyll fluorescence quenching and their relation with photochemical and nonphotochemical events. Photosynth Res. 1991;27:41–55. doi: 10.1007/BF00029975. [DOI] [PubMed] [Google Scholar]
- Havaux M, Tardy F. Temperature-dependent adjustment of the thermal stability of photosystem II in vivo: possible involvement of xanthophyll-cycle pigments. Planta. 1996;198:324–333. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Huner NPA, Maxwell DP, Gray GR, Savitch LV, Krol M, Ivanov AG, Falk S. Sensing environmental temperature change through imbalances between energy supply and energy consumption: redox state of photosystem II. Physiol Plant. 1996;98:358–364. [Google Scholar]
- Hurry V, Anderson JM, Badger MR, Price GD. Reduced levels of cytochrome b6/f in transgenic tobacco increases the excitation pressure on photosystem II without increasing sensitivity to photoinhibition in vivo. Photosynth Res. 1996;50:159–169. doi: 10.1007/BF00014886. [DOI] [PubMed] [Google Scholar]
- Jones BL, Porter JW. Biosynthesis of carotenes in higher plants. CRC Crit Rev Plant Sci. 1986;3:295–324. [Google Scholar]
- Joshi MK, Mohanty P, Van Rensen JJS, Bose S. In situ prolonged partial inhibition of photosystem II in pea plants leads to an increase in the unit size and number of photosystem II units. Plant Sci. 1995;106:19–30. [Google Scholar]
- Kloppstech K. Diurnal and circadian rhythmicity in the expression of light-induced plant nuclear mRNAs. Planta. 1985;165:502–506. doi: 10.1007/BF00398095. [DOI] [PubMed] [Google Scholar]
- Krol M, Spangfort MD, Huner NPA, Oquist G, Gustafsson P, Jansson S. Chlorophyll a/b-binding proteins, pigment conversion, and early light-induced proteins in chlorophyll b-less barley mutant. Plant Physiol. 1995;107:873–883. doi: 10.1104/pp.107.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J, Oster U, Rüdiger W, Beck CF. Chlorophyll precursors are signals of chloroplast origin involved in light induction of nuclear heat-shock genes. Proc Natl Acad Sci USA. 1997;94:14168–14172. doi: 10.1073/pnas.94.25.14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H, Tal T, Shaish A, Zamir A. Cbr, an algal homolog of plant early light-induced proteins, is a putative zeaxanthin binding protein. J Biol Chem. 1993;268:20892–20896. [PubMed] [Google Scholar]
- Lowry DH, Rosebrought NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Malkin S, Armond PA, Mooney HA, Fork DC. Photosystem II photosynthetic unit size from fluorescence induction in leaves. Plant Physiol. 1981;67:570–579. doi: 10.1104/pp.67.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Falk S, Huner NPA. Photosystem II excitation pressure and development of resistance to photoinhibition. I. Light-harvesting complex II abundance and zeaxanthin content in Chlorella vulgaris. Plant Physiol. 1995;107:687–694. doi: 10.1104/pp.107.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell DP, Falk S, Trick CG, Huner NPA. Growth at low temperature mimics high-light acclimation in Chlorella vulgaris. Plant Physiol. 1994;105:535–543. doi: 10.1104/pp.105.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A, Manodori A, Glick RE, Ghirardi ML, McCauley SW, Neale PJ. The mechanism of photosynthetic membrane adaptation to environmental stress conditions: a hypothesis on the role of electron-transport capacity and of ATP/NADPH pool in the regulation of thylakoid membrane organization and function. Physiol Vég. 1985;23:757–765. [Google Scholar]
- Meyer G, Kloppstech K. A rapidly light-induced chloroplast protein with a high turnover coded for by pea nuclear DNA. Eur J Biochem. 1984;138:201–207. doi: 10.1111/j.1432-1033.1984.tb07900.x. [DOI] [PubMed] [Google Scholar]
- Montané M-H, Dreyer S, Triantaphylidès C, Kloppstech K. Early light-inducible proteins during long-term acclimation of barley to photooxidative stress caused by light and cold: high level of accumulation by posttranscriptional regulation. Planta. 1997;202:293–302. [Google Scholar]
- Murakami A, Fujita Y. Regulation of photosystem stoichiometry in the photosynthetic system of the cyanophyte Synechocystis PCC 6714 in response to light-intensity. Plant Cell Physiol. 1991;32:223–230. [Google Scholar]
- Paulsen H. Chlorophyll a/b-binding proteins. Photochem Photobiol. 1995;62:367–382. [Google Scholar]
- Pearson CK, Wilson SB, Schaffer R, Ross AW. NAD turnover and utilisation of metabolites for RNA synthesis in a reaction sensing the redox state of the cytochrome b6f complex in isolated chloroplasts. Eur J Biochem. 1993;218:397–404. doi: 10.1111/j.1432-1033.1993.tb18389.x. [DOI] [PubMed] [Google Scholar]
- Petracek ME, Dickey LF, Huber SC, Thompson WF. Light-regulated changes in abundance and polyribosome association of ferredoxin mRNA are dependent on photosynthesis. Plant Cell. 1997;9:2291–2300. doi: 10.1105/tpc.9.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pötter E, Kloppstech K. Effects of light stress on the expression of early light-inducible proteins in barley. Eur J Biochem. 1993;214:779–786. doi: 10.1111/j.1432-1033.1993.tb17980.x. [DOI] [PubMed] [Google Scholar]
- Poulet P, Cahen D, Malkin S. Photoacoustic detection of photosynthetic oxygen evolution from leaves: quantitative analysis by phase and amplitude measurements. Biochim Biophys Acta. 1983;724:433–446. [Google Scholar]
- Price GD, Yu J-W, von Caemmerer S, Evans JR, Chow WS, Anderson JM, Hurry V, Badger MR. Chloroplast cytochrome b6/f and ATP synthase complexes in tobacco: transformation with antisense RNA against nuclear-encoded transcripts for the Rieske FeS and ATPd polypeptides. Aust J Plant Physiol. 1995;22:285–297. [Google Scholar]
- Savitch LV, Maxwell DP, Huner NPA. Photosystem II excitation pressure and photosynthetic carbon metabolism in Chlorella vulgaris. Plant Physiol. 1996;111:127–136. doi: 10.1104/pp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverthorne J, Tobin EM. Demonstration of transcriptional regulation of specific genes by phytochrome action. Proc Natl Acad Sci USA. 1984;81:1112–1116. doi: 10.1073/pnas.81.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavladorakis P, Kloppstech K, Argyroudi-Akoyunoglou J. Circadian rhythm in the expression of the mRNA coding for the light-harvesting complex of photosystem II apoprotein: phytochrome control. Plant Physiol. 1989;90:665–672. doi: 10.1104/pp.90.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG, Horton P. Acclimation of Arabidopsis thaliana to the light environment: regulation of chloroplast composition. Planta. 1995;197:475–481. doi: 10.1007/BF00196669. [DOI] [PubMed] [Google Scholar]
- Williams WP, Allen JF. State 1/state 2 changes in higher plants and algae. Photosynth Res. 1987;13:19–45. doi: 10.1007/BF00032263. [DOI] [PubMed] [Google Scholar]
- Wollman F-A, Lemaire C. Studies on kinase-controlled state transitions in photosystem II and b6f mutants from Chlamydomonas reinhardtii which lack quinone-binding proteins. Biochim Biophys Acta. 1988;933:85–94. [Google Scholar]