Abstract
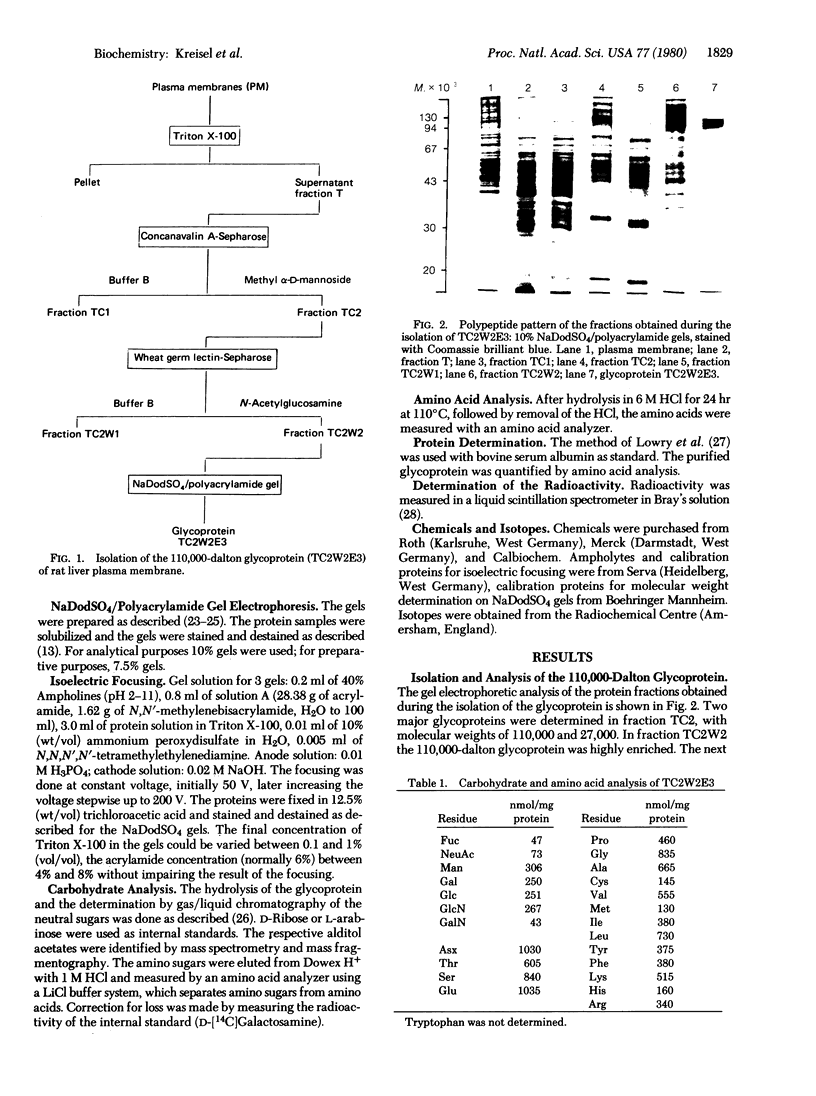
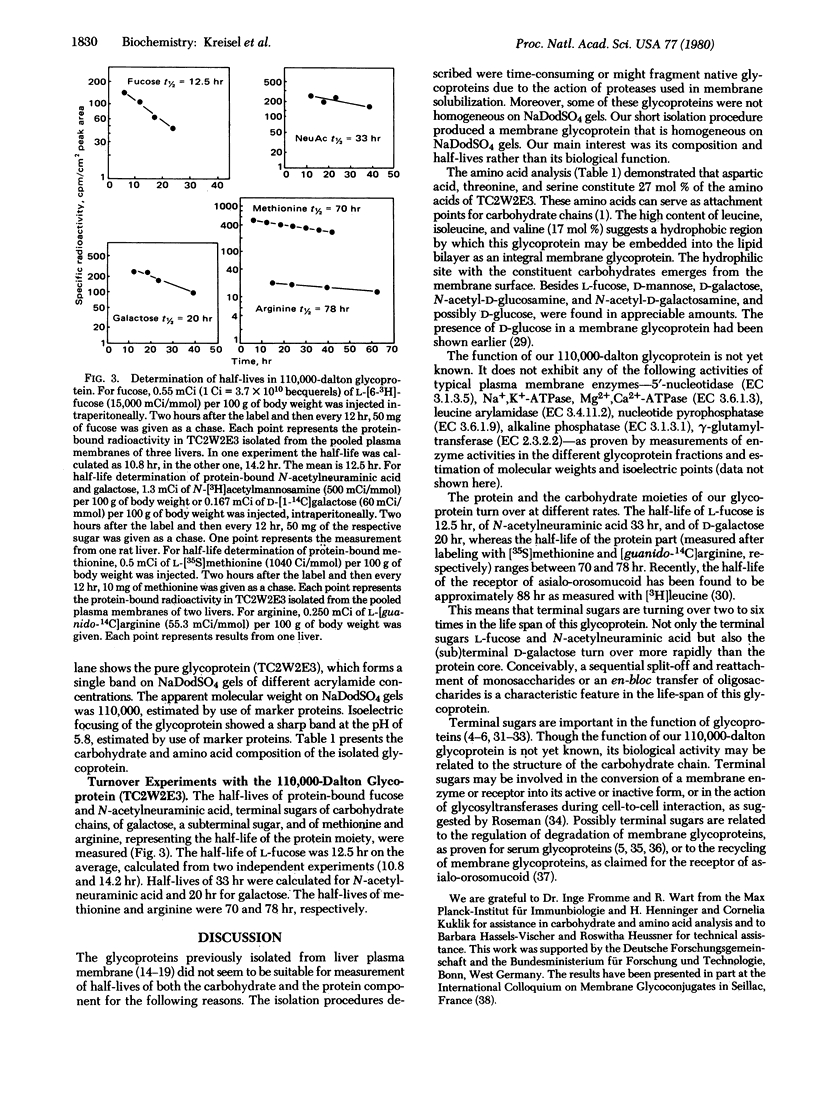
By using a four-step procedure (i, solubilization with Triton X-100; ii, affinity chromatography on concanavalin A-Sepharose; iii, affinity chromatography on wheat germ lectin-Sepharose; iv, preparative sodium dodecyl sulfate gel electrophoresis) a glycoprotein was isolated from rat liver plasma membrane. The molecular weight is 110,000 and the isoelectric point is 5.8. It contains L-fucose, N-acetylneuraminic acid, D-galactose, D-mannose, N-acetyl-D-glucosamine, N-acetyl-D-galactosamine, and considerable quantities of aspartate, threonine, serine, and leucine. In pulse-chase experiments the half-lives of methionine and arginine, representing the half-life of the protein, were determined as 70 hr and 78 hr, respectively. The half-lives of the terminal carbohydrates L-fucose and N-acetylneuraminic acid were 12.5 and 33 hr, respectively. The galactose half-life was 20 hr. From this it is concluded that terminal sugars turn over several times in the life-span of this protein molecule. This process may be operative during membrane recycling mechanisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- BROSSMER R., WALTER K. Enzymatische Abspaltung von Lactaminsäure und Inaktivierung von Choriongonadotropin-präparaten. Klin Wochenschr. 1958 Oct 1;36(19):925–925. doi: 10.1007/BF01485017. [DOI] [PubMed] [Google Scholar]
- Bachmann W., Harms E., Hassels B., Henninger H., Reuitter W. Studies on rat liver plasma membrane. Altered protein and phospholipid metabolism after injection of D-galactosamine. Biochem J. 1977 Sep 15;166(3):455–462. doi: 10.1042/bj1660455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff E., Tran-Thi T. A., Decker K. F. Nucleotide pyrophosphatase of rat liver. A comparative study on the enzymes solubilized and purified from plasma membrane and endoplasmic reticulum. Eur J Biochem. 1975 Feb 21;51(2):353–361. doi: 10.1111/j.1432-1033.1975.tb03935.x. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Siekevitz P., Palade G. E. Localization and turnover studies of membrane nicotinamide adenine dinucleotide glycohydrolase in rat liver. J Biol Chem. 1971 Jan 10;246(1):188–195. [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverde B. C., Veenkamp F. J., Homan J. D. Studies on human chorionic gonadotrophin. II. Chemical composition and its relation to biological activity. Acta Endocrinol (Copenh) 1968 Sep;59(1):105–119. [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H. Relative rates of degradation of mouse-liver surface-membrane proteins. Eur J Biochem. 1973 Jul 2;36(1):273–279. doi: 10.1111/j.1432-1033.1973.tb02910.x. [DOI] [PubMed] [Google Scholar]
- Harms E., Reutter W. Half-life of N-acetylneuraminic acid in plasma membranes of rat liver and Morris hepatoma 7777. Cancer Res. 1974 Dec;34(12):3165–3172. [PubMed] [Google Scholar]
- Jacobs S., Shechter Y., Bissell K., Cuatrecasas P. Purification and properties of insulin receptors from rat liver membranes. Biochem Biophys Res Commun. 1977 Aug 8;77(3):981–988. doi: 10.1016/s0006-291x(77)80074-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Chemical and physical properties of an hepatic membrane protein that specifically binds asialoglycoproteins. J Biol Chem. 1976 Mar 10;251(5):1296–1302. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larriba G. O-glycosidically linked fucose in high molecular weight glycoproteins, in normal and virus-transformed rat cells. FEBS Lett. 1978 Nov 1;95(1):190–193. doi: 10.1016/0014-5793(78)80081-7. [DOI] [PubMed] [Google Scholar]
- Lehnhardt W. F., Winzler R. J. Determination of neutral sugars in glycoproteins by gas-liquid chromatography. J Chromatogr. 1968 May 7;34(4):471–479. doi: 10.1016/0021-9673(68)80091-3. [DOI] [PubMed] [Google Scholar]
- Mathews R. A., Johnson T. C., Hudson J. E. Synthesis and turnover of plasma-membrane proteins and glycoproteins in a neuroblastoma cell line. Biochem J. 1976 Jan 15;154(1):57–64. doi: 10.1042/bj1540057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell A. G., Irvine R. A., Sternlieb I., Scheinberg I. H., Ashwell G. Physical and chemical studies on ceruloplasmin. V. Metabolic studies on sialic acid-free ceruloplasmin in vivo. J Biol Chem. 1968 Jan 10;243(1):155–159. [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger R. C., Anderson N. G., Snyder F. Lipid class and fatty acid composition of rat liver plasma membranes isolated by zonal centrifugation. Biochemistry. 1968 Aug;7(8):2826–2833. doi: 10.1021/bi00848a019. [DOI] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Pricer W. E., Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J Biol Chem. 1979 Feb 25;254(4):1038–1043. [PubMed] [Google Scholar]
- Tauber R., Reutter W. Protein degradation in the plasma membrane of regenerating liver and Morris hepatomas. Eur J Biochem. 1978 Feb 1;83(1):37–45. doi: 10.1111/j.1432-1033.1978.tb12065.x. [DOI] [PubMed] [Google Scholar]
- Vischer P., Reutter W. Specific alterations of fucoprotein biosynthesis in the plasma membrane of Morris hepatoma 7777. Eur J Biochem. 1978 Mar 15;84(2):363–368. doi: 10.1111/j.1432-1033.1978.tb12176.x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]