Abstract
Chagas disease (CD) is a chronic and endemic illness caused by the parasite Trypanosoma cruzi. Microvascular disturbances play an important role in the progress of the disease. The purinergic signaling system participates in regulatory functions, such as immunomodulation, neuroprotection, and thromboregulation. This study aimed to investigate the activities of purinergic system ecto-enzymes present on the platelet surface and the platelet aggregation profile from patients with indeterminate form of Chagas disease (IFCD). Thirty patients diagnosed with IFCD and 30 healthy subjects were selected. Ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase), ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP), ecto-5′-nucleotidase (E-5′-NT) and ecto-adenosine deaminase (E-ADA) activities were measured in platelets isolated from these individuals as well as the platelet aggregation. Results demonstrated an increase of 21 % in the E-NPP activity and 30 % in the E-5′-NT activity in IFCD group (P < 0.05); however, a decrease of 34 % in the E-ADA activity was determined in the same group (P < 0.001). A significant decrease of 12.7 % and 12.8 % in the platelet aggregation of IFCD group in two different concentrations of ADP (5 and 10 μM) was observed, respectively (P < 0.05). Increased E-NPP and E-5-NT activities as well as decreased E-ADA activity in platelets of patients with IFCD contributed to decrease platelet aggregation, suggesting that the purinergic system is involved in the thromboregulation process in these patients, since adenosine (the final product of ATP hydrolysis) has cardioprotective and vasodilator effects that prevent the clinical progress of the disease.
Keywords: Adenosine triphosphate, Chagas disease, Trypanosoma cruzi, Platelet aggregation, Adenosine
Introduction
Chagas disease (CD) or American trypanosomiasis, caused by hemoflagellate protozoan Trypanosoma cruzi, is a parasitic illness that infects the blood and several tissues of the host. It is a chronic and endemic disease widespread in Central and South America.
The infection is presented under two distinct clinical phases. An initial acute phase, short-term, which can be fatal from 3 % to 5 % of the cases and that progresses to a lifelong chronic phase characterized by distinct clinical forms known as indeterminate, cardiac, and digestive [1]. The most infected individuals shows the indeterminate form of the disease, which is characterized by long term (10–30 years), in which the patients have no symptoms of the disease and present normal clinical evaluation, radiological (heart, esophagus, and colon) images and conventional electrocardiogram. About 25 % to 30 % of the infected subjects develop severe cardiac and/or digestive lesions in the chronic phase of the infection [2], such as cardiac arrhythmias, congestive heart failure, thromboembolic events, megaesophagus, and megacolon [3, 4]. Microvasculature disorders play an important role in the pathogenesis of chronic cardiomyopathy [1, 5]. It has been described that the cardiac involvement constitutes the main cause of death [4, 6, 7], since heart is the most often and seriously involved organ in the chronic CD.
The protozoan invades and reproduces in a variety of host cells, such as striated muscle and endothelial cells. Endothelium dysfunction can occur during the T. cruzi infection and promoting thromboembolic disturbances. Thromboregulation is a process in which circulating blood cells and the endothelium regulate thrombus formation [8]. Platelets are important mediators in the maintenance of endothelial integrity and hemostasis [9] as well as in inflammation, which emphasize their importance in the development and treatment of vascular diseases.
The purinergic signaling system plays an important regulatory role in inflammation, cellular activation, blood flow, and vascular thrombosis by extracellular biomolecules, such as adenine nucleotides (ATP, ADP, and AMP) and their derivative nucleoside adenosine [10]. Some authors have described that as a consequence of pathogen infection, high concentration of adenine nucleotides secreted by erythrocytes, leukocytes, platelets, and endothelial cells [11] are found in the extracellular medium representing damage or stimulation of cells. These nucleotides exert their functions via three P2 purinoceptors on platelets: P2X1 (ionotropic receptor that causes rapid influx of calcium into the cytosol), P2Y1 (metabotropic receptor that mobilizes calcium from internal stores, which starts the aggregation process) and P2Y12 (receptor coupled to Gαi protein that stabilizes platelet aggregates) [12–14].
After exerting their functions, the nucleotides are hydrolyzed by a multienzymatic system in order to maintain their extracellular levels at the physiological concentration and to avoid purinergic receptors desensitization. These enzymes are found on the surfaces of virtually all mammalian cell types and include ecto-nucleotidases from distinct families such as the E-NTPDase (EC 3.6.1.5, CD39), E-NPP (EC 3.1.4.1), and E-5′-NT (EC 3.1.3.5, CD73). The ecto-nucleotidases are responsible for the hydrolysis of ATP, ADP, and AMP until the formation of adenosine, which in turn, is irreversibly converted into inosine by ADA ecto-enzyme (EC 3.5.4.4) [15]. The nucleoside adenosine inhibits platelet aggregation and acts as a potent vasodilatator, which exerts a protective function in the heart by decrease of the metabolic demands of the myocardium and by increase of coronary blood flow [10]. Its anti-aggregant effects are mediated via G-protein coupled adenosine receptors (P1 purinoceptores), specifically the A2A and A2B adenosine receptor subtypes [16, 17].
Of particular interest for this study, ADP plays a key role in the platelet activation and recruitment, establishing the physiological and pathological importance of this nucleotide in platelet function, while adenosine and high concentration of ATP inhibit ADP-induced platelet aggregation. Therefore, biochemical alterations that modify or block the action of ADP on platelets and/or favor adenosine production are important in the thromboregulation for limiting platelet aggregation and thrombous formation [18].
Taking into account the importance of ecto-enzymes activities in the maintenance of hemostasis and the presence of clinically silent lesions in the cardiac microvasculature in IFCD patients, this study aimed to investigate the participation of purinergic system in thromboregulation these patients, since alterations in the activity of these enzymes have been observed in many pathological and physiological conditions such as diabetes mellitus, multiple sclerosis, rheumatoid arthritis, uterine cervix neoplasia, ischemic heart disease, and pregnancy [19–24].
Material and methods
Chemicals
The substrates adenosine 5′-triphosphate disodium salt (ATP), adenosine 5′-diphosphate sodium salt (ADP), 5′-monophosphate sodium salt (AMP), thymidine 5′-monophosphate p-nitrophenyl ester sodium salt (p-Nph-5′-TMP), adenosine, as well as bovine serum albumin, Trizma base, HEPES and Coomassie Brilliant Blue G were obtained from Sigma-Aldrich (St. Louis, MO, USA). All the other chemicals used in this experiment were of analytical grade and of the highest purity.
Patients and samples
Thirty patients with diagnosis of CD from the Federal University of Santa Maria Hospital were selected for the study. The most of these patients (90 %) were diagnosed after a blood donation. The group of chagasic patients had an average age of 55 ± 1.5 years old, 53 % men and 47 % women, and was classified as IFCD patients by presenting absence of signs and symptoms of disease, two confirmed positive serologic reactions for CD, normal conventional electrocardiogram and normal radiological heart, esophagus and colon images according to criteria defined in the first meeting of applicated research in Chagas disease held in Minas Gerais, Brazil [25]. The control group was constituted by 30 healthy subjects with an average age of 45 ± 2.35 years old, 53 % men and 47 % women, and negative serology for CD. All participants presented normal blood pressure, were free from diabetes mellitus, alcoholism, cigarette smoking, autoimmune disease and immunodeficiency, and besides had not been submitted to any pharmacological therapy during a month before the study. Fifteen milliliters of peripheral blood was obtained from each patient and used for platelet-rich plasm (PRP) preparation and other determinations. The same procedure was carried out for the control group. The protocol was approved by the Human Ethics Committee from Federal University of Santa Maria, protocol number 23081.008343/2009-10, Brazil. All subjects gave written informed consent to participate in the study. Both general characteristics of IFCD and control groups are shown in Table 1. The study was age and gender matched.
Table 1.
General characteristics and coagulation parameters from patients with indeterminate form of Chagas disease (IFCD) and control group
| Variable | IFCD men | IFCD women | IFCD Total n = 30 | Control men | Control women | Control total n = 30 |
|---|---|---|---|---|---|---|
| Gender | 53 % | 47 % | – | 53 % | 47 % | – |
| Age (years) | 58.3 ± 1.9 | 51.2 ± 2.1 | 55.0 ± 1.5 | 47.5 ± 2.5 | 44.0 ± 2.2 | 45.9 ± 2.3 |
| Platelets (×103/μL) | 210.6 ± 13.6 | 262.9 ± 13.2 | 235.0 ± 10.5 | 210.6 ± 13.6 | 262.9 ± 13.2 | 235.0 ± 10.5 |
| PT (s) | 9.3 ± 1.7 | 10.71 ± 0.3 | 10.6 ± 0.3 | 11.2 ± 0.3 | 10.3 ± 0.2 | 10.7 ± 0.2 |
| PPT (s) | 28.4 ± 2.3 | 27.9 ± 1.2 | 28.1 ± 1.2 | 30.2 ± 1.1 | 26.1 ± 1.0 | 27.8 ± 0.9 |
Continuous variables are presented as means±SEM and the other variables are shown as percentage of individuals
PTT partial thromboplastin time, PT prothrombin time
Platelet preparation
PRP was prepared by the method of Pilla et al. [18] modified by Lunkes et al. [19]. Briefly, peripheral blood was collected in 0.129 M sodium citrate as anticoagulant and centrifuged at 160×g for 15 min. Afterwards, the PRP was centrifuged at 1,400×g for 30 min and washed twice with 3.5 mM HEPES buffer, pH 7.0, containing 142 mM NaCl, 2.5 mM KCl, and 5.5 mM glucose. The washed platelets were resuspended in HEPES isosmolar buffer and protein was adjusted to 0.4–0.6 mg/mL.
Cellular integrity
The integrity of the platelet preparation was confirmed by determining the lactate dehydrogenase (LDH) activity in intact and disrupted platelets using the kinetic method of the Labquest apparatus (Diagnostics Gold Analyzer). The procedure was repeated before and after the incubation period. The protocol was carried out according to the manufacturer's instructions. Triton X-100 (1 %, final concentration) was used to disrupt the platelet preparation. The enzymatic activity is expressed as units per liter, and one unit (1U) corresponds to 1 μmol of NADH formed per minute per liter.
Protein determination
Protein was determined by the Coomassie blue method using bovine serum albumin as standard [26].
Coagulation parameters
Total blood was collected in tubes containing 7.2 mg dipotassium EDTA to quantitative determination of peripheral blood platelets by an automated hematology analyzer (SYSMEX XT-1800i, Roche Diagnostic, USA) and in tubes containing 0.129 M sodium citrate to prothrombin time (PT) and partial thromboplastin time (PTT) measurement by an automated coagulation analyzer (SYSMEX CA-1500, Roche Diagnostic, USA). PT and PTT are expressed in seconds and quantitative determinations of platelets are expressed as quantity per microliter.
E-NTPDase and E-5′-NT activities determination
E-NTPDase enzymatic assay in platelets was carried out in a reaction medium containing 5 mM CaCl2, 120 mM NaCl, 5 mM KCl, 60 mM glucose, and 50 mM Tris–HCl buffer, pH 7.4, at a final volume of 200 μL as described by Lunkes et al. [19]. For AMP hydrolysis, E-5′-nucleotidase activity was carried out as previously described, except that 5 mM CaCl2 was replaced by 10 mM MgCl2. Twenty microliters of the isolated platelets (8–12 μg of protein) was added to the reaction mixture and pre-incubation proceeded for 10 min at 37 °C. The reaction was started by the addition of ATP or ADP at a final concentration of 1 mM, and AMP at a final concentration of 2 mM, and the time of incubation was 60 min. Both enzyme assays were stopped by the addition of 200 μL of 10 % TCA to provide a final concentration of 5 %. Subsequently, the tubes were chilled on ice for 10 min. The Pi released was measured by method of Chan et al. [27] using malachite green as the colorimetric reagent and KH2PO4 as standard. Controls were carried out to correct for non-enzymatic hydrolyses of nucleotides by adding enzyme preparation after 10 % TCA addition. All samples were run in triplicate. Enzyme-specific activities are reported as nmol Pi released/min/mg of protein.
E-NPP activity determination
The E-NPP activity from platelets was assessed using p-Nph-5′-TMP as substrate as described by Fürstenau et al. [28]. The p-Nph-5′-TMP is an artificial substrate used routinely for the in vitro assay of NPP, by hydrolysis of phosphodiester bound of this substrate, as previously outlined [29]. The reaction medium containing 50 mM Tris–HCl buffer, 120 mM NaCl, 5 mM KCl, 60 mM glucose, 5 mM CaCl2, pH 8.9 was preincubated with approximately 20 μg per tube of platelet protein for 10 min at 37 °C in a final volume of 200 μL. The enzyme reaction was started by the addition of p-Nph-5′-TMP to a final concentration of 0.5 mM. After 80 min of incubation, 200 μL NaOH 0.2 N was added to the medium to stop the reaction. The amount of p-nitrophenol released from the substrate was measured at 400 nm using a molar extinction coefficient of 18.8 × 10−3/M/cm. Controls to correct nonenzymatic substrate hydrolysis were performed by adding platelet preparations after the reaction had been stopped. All samples were performed in triplicates. Enzyme activities were expressed as nanomole of p-nitrophenol released per minute per milligram of protein (nmol p-nitrophenol released/min/mg protein). On the platelet surface, E-NPP has catalytic activity in an optimum alkaline pH between pH 8.5 and 9.0, differently from E-NTPDase, which presents catalytic activity in an optimum pH between pH 7.5 and 8 [30]. Therefore, a Tris–HCl buffering system (pH 8.9) was used to perform the E-NPP activity assay, since the hydrolysis of the nucleodylated intermediate occurs at this pH and at lower pH values, the specific activity is decreased, as previously defined by Fürstenau et al. [28].
E-ADA activity determination
E-ADA activity from platelets was determined according to Giusti and Galanti [31] based on the direct measurement of the formation of ammonia produced when adenosine deaminase acts in excess of adenosine. Briefly, 50 μL of platelets reacted with 21 mmol/L of adenosine, pH 6.5, and was incubated at 37 °C for 60 min. Afterwards, the reaction was stopped by adding a solution of 106.2 mM phenol and 167.8 mM sodium nitroprussiate and a hypochlorite solution. The amount of ammonia produced was measured at 620 nm and the results were expressed in units per milligrams of proteins (U/mg of protein). One unit (1U) of ADA is defined as the amount of enzyme required to release 1 mmol of ammonia per minute from adenosine at standard assay conditions.
Platelet aggregation
Platelet aggregation was measured by the method of Born and Cross [32] by turbidimetric measurement with a Chrono-log optical aggregometer (AGGRO/LINK® Model 810-CA software for Windows version 5.1). The PRP was obtained by centrifugation of peripheral blood for 15 min at 160×g and the preparation of platelets-poor plasm (PPP) was obtained by centrifugation of the sample by 1,400×g for 30 min. After the calibration of the aggregometer, the data concerning the assays and reagents were entered on a computer coupled to the equipment, and the test of patient was then performed. Aggregation was measured at 37 °C and expressed as the maximal percent change in light transmittance from baseline at 5 min after the addition of the agonist ADP at concentrations of 5 and 10 μM, with PPP as a reference. Results were expressed as percentage of aggregation.
Statistical analysis
Variables were expressed as mean±standard error of the mean (SEM). The data obtained were analyzed statistically by the Student's t test for independent samples. Differences were considered significant when the probability was P < 0.05.
Results
Cellular integrity
Table 2 shows the quantification of intact platelets on preparation and effect of platelet lysis on LDH activity in both groups. As demonstrated in Table 2, the measurement of LDH activity showed that approximately 4 % of the platelets of both groups were disrupted, indicating that the preparation was predominantly intact after the isolation procedure.
Table 2.
Evaluation of cellular integrity on platelet preparation
| Variable | IFCD group | Control group |
|---|---|---|
| Platelets (×107/mL) | 11.3 ± 1.6 (5) | 15.6 ± 2.2 (5) |
| LDH (intact platelets) | 228.9 ± 36.0 (5) | 174.6 ± 39.8 (5) |
| LDH (lysed platelets) | 4933.0 ± 857.0 (5) | 3609.0 ± 355.1 (5) |
Results presents as means±SEM with the number of experiments given in parenthesis
LDH activity is expressed as U/L
Coagulation parameters
The coagulation parameters of both groups are show in Table 1. IFCD and control groups showed an average of 235,000 platelets per microliter of blood (platelets/μL of blood). These data are within physiological values for humans, since adult normal values are between 150,000 and 450,000 platelets/μL of blood.
In Table 1, can also be observed that the IFCD group showed normal PT and PTT. Analysis of medical records showed that IFCD patients did not present any sign of bleeding.
E-NTPDase and E-5′-nucleotidase activities
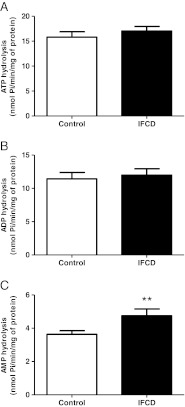
Figure 1 shows the results obtained for E-NTDase activity by ATP and ADP hydrolysis and E-5′-NT activity by AMP hydrolysis. As can be seen, neither ATP hydrolysis (Fig. 1a) nor ADP hydrolysis (Fig. 1b) was altered in the IFCD group (P > 0.05, n = 30). However, AMP hydrolysis (Fig. 1c) was 30 % increased in the same group (P < 0.01, n = 30).
Fig. 1.
E-NTPDase (ATP and ADP hydrolysis, (a and b), respectively) and E-5′-NT (AMP hydrolysis, (c)) in platelets of patients with indeterminate form of Chagas disease (IFCD). Bars represent means±SEM. The double asterisk symbol represents statistical difference from the control group (Student's t test, P < 0.01, n = 30)
E-NPP activity
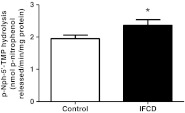
Results obtained for E-NPP activity are shown in Fig. 2. Statistical analysis showed an increase of 21 % in the E-NPP activity in the IFCD group when compared with the control group (P < 0.05, n = 30).
Fig. 2.
E-NPP activity in platelets of patients with indeterminate form of Chagas disease (IFCD). Bars represent means±SEM. The asterisk symbol represents statistical difference from the control group (Student's t test, P < 0.05, n = 30)
E-ADA activity
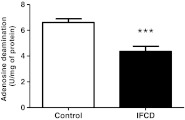
E-ADA activity in the platelets is shown in Fig. 3. Results demonstrated a decrease of 34 % in the E-ADA activity in the IFCD group when compared to the control group (P < 0.001, n = 30).
Fig. 3.
E-ADA activity in platelets of patients with indeterminate form of Chagas disease (IFCD). Bars represent means±SEM. The three-asterisk symbol represents statistical difference from the control group (Student's t test, P < 0.001, n = 30)
Platelet aggregation
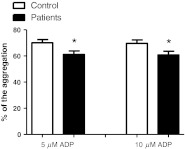
Figure 4 presents the results obtained for platelet aggregation using ADP as an agonist. As can be observed there was a decrease of 12.7 % and 12.8 % in the platelet aggregation of IFCD group in 5 and 10 μM of ADP, respectively (P < 0.05, n = 15).
Fig. 4.
Platelet aggregation profile in patients with indeterminate form of Chagas disease (IFCD) and healthy subjects (control). Platelet aggregation was evaluated by using ADP as agonist at concentrations of 5 and 10 μM. The results are expressed as percentage of aggregation. The asterisk symbol represents statistical difference from the control group (Student t test, P < 0.05, n = 15)
Discussion
CD is a chronic inflammatory illness, in which the most infected individuals are diagnosed during the latent phase, showing absence of symptoms for many years or even for the entire life. However, many studies in both animal models of chagasic myocarditis and humans presented a persistence of parasite in tissue inflammatory foci by immunohistochemistry, polymerase chain reaction (PCR), and in situ hybridization methods [33]. The presence of the parasite in tissues suggests the possibility of disease progress to a symptomatic clinical form, since the chagasic cardiomyopathy represents the major cause of morbidity and mortality of this infection and can lead to chronic congestive failure.
Several evidences obtained by human and experimental studies in both acute and chronic chagasic cardiophathy suggest that the development of myocardial abnormalities occur, at least in part, as a consequence of changes in the coronary microvasculature. Qualitatively the morphology of cardiac lesions in IFCD patients are similar to those observed in the cardiac form of the disease, however, quantitatively, lesions are much less pronounced [34, 35]. There are few pathological studies in IFCD patients, but it is known that the presence of a focal and mild carditis produces no major functional changes, which may explain the normal conventional electrocardiogram and chest X-ray and the absence of signs and symptoms in this group.
Hematological alterations are a common finding in experimental infections induced by trypanosomes. Cardoso and Brener [36] described in T. cruzi-infected mice a marked decrease of the number of thrombocytes, red blood cells and leukocytes, soon after infection. Thrombocytopenia were observed in animals inoculated with T. cruzi strain which induces chronic infection (Colombiana, CL strain), the number of platelets decreased as parasitemia ascended, and then reverted to normal values as soon as the acute infection merged into the chronic phase. In contrast, platelet counts within normal limits were observed in patients in the acute phase of CD by Jamra et al. [37]. In the present study, no change in platelet count in IFCD patients was observed. In addition, evaluation of the extrinsic and intrinsic pathway of coagulation cascade in these patients, through the prothrombin time and partial thromboplastin time assays, were within normal values, demonstrating that IFCD group has no alterations in coagulation factors.
Thrombosis and spasm of the coronary microcirculation has been implicated in the pathogenesis of the cardiomyopathy of CD. Indeed, focal myocytolytic lesions and infiltration by mononuclear cells associated with platelet aggregates in the microcirculation determinate chagasic cardiomyophathy. An excessive platelet aggregation can occur in damaged vasculature as consequence of inflammation, since mononuclear cells release potent platelet-aggregating factors (ADP; thromboxane A2 and platelet-activiting factor, PAF) contributing to the state of microvascular hypoperfusion, as evidenced in areas of myocardial damage in mice chronically infected with T. cruzi [38]. In 1990, in vitro studies [39] demonstrated that the increase in platelet adherence and aggregation accompanies T. cruzi infection and is associated to endothelial cell dysfunction. These observations can be explained by the interaction between platelets and damaged endothelium or subendothelium surfaces with consequent stimulation of the platelet activation and later thrombus formation.
As presented in this study, the IFCD group showed a decreased platelet aggregation profile. However, the activities of purinergic system ecto-enzymes were investigated to verify their possible involvement in the decrease of platelet aggregation, as hypothesized in this study.
It is known that adenine nucleotides (ATP and ADP) and adenosine are released from cells such as platelets into the extracellular medium during infection and inflammatory process and act modulating vascular response, as agonist and antagonist in the platelet aggregation, respectively. Their effects are mediated by cell surface purinergic receptors and their extracellular concentrations are regulated by ecto-enzymes of purinergic system including E-NTPDase, E-NPP, E-5′-NT and E-ADA.
Some studies have reported that vascular functions of E-NTPDase activity (thromboregulation and vascular permeability) probably correlate with high hydrolytic activity on the endothelial and vascular smooth muscle cells [40, 41] in the vasomotor responses. In contrast, in this study, no alteration was observed in the E-NTPDase activity (ATP and ADP hydrolysis) in platelets of IFCD group. Since it is well established that cells and tissues can co-express two or more ecto-enzymes for nucleotide hydrolysis, indicating they may present distinct catalytic properties and physiological functions or share common characteristics [28, 42], it was investigated the E-NPP activity in platelets of IFCD patients. As demonstrated in this study, E-NPP activity was increased in platelets of IFCD group, probably leading to a decrease in extracellular ATP levels and consequently increased AMP levels in the bloodstream as confirmed by the quantification of purines by high pressure liquid chromatography (HPLC) (data not shown). While high concentrations of extracellular ATP inhibit ADP-induced platelet aggregation by both competitive and noncompetitive mechanisms, low concentrations induce platelet aggregation [43, 44]. In this study, extracellular ADP levels was not altered (data not shown) indicating the direct hydrolysis of the extracellular ATP to AMP by E-NPP.
The sequential degradation mechanism of ATP is followed by the activity of E-5′-NT which not only terminates ATP and ADP signaling by P2 purinoceptors but also generates the intermediate adenosine. Thus, high AMP levels serve as substrate for the production of adenosine by catalytic activity of E-5′-NT. As observed in this study, the E-5′-NT activity was increased in the IFCD group, resulting in the formation of high extracellular adenosine concentration which presents anti-inflammatory properties as well as neuromodulatory and thromboregulatory effects [43, 45], besides participating as a salvage product of cellular purine metabolism.
The increased E-5′-NT activity observed in platelets of IFCD patients was also observed in heart myocytes from acutely T. cruzi-infected mice by Fretes et al. [46]. Theses authors suggested that the increased activity can be caused by hypoperfusion conditions during T. cruzi infection, leading to the production of adenosine, a cardioprotective substance. Zhai et al. [47] showed that adenosine is produced by 5′-NT activity in ischemic heart conditions, while Headrick et al. [48] demonstrated that the stimulation of β-adrenergic receptors produces adenosine by 5′-AMP hydrolysis. However, Fretes et al. [46] presented decreased density of cardiac β-adrenergic receptors, but with affinity similar to non-infected mice by T. cruzi, demonstrating that increased 5′-NT activity in heart myocytes may be independent of β-adrenergic function.
In addition to the enhancement of E-NPP and E-5′-NT activities, a decrease in E-ADA activity was observed in the platelets of IFCD group. The reduced activity of E-ADA in platelets of this group could represent an important mechanism to preserve high extracellular adenosine levels as confirmed by the quantification of purines by HPLC (data not shown), avoiding platelet aggregation by inducing vasodilation and thus increasing cardiac blood flow.
The reduction in E-ADA activity observed in platelets of IFCD patients was also observed in serum of rats experimentally infected by T. cruzi, on day 120 post-infection, associated to absence of parasitemia and clinical and hematological changes, and presence of mononuclear inflammatory infiltrate in heart tissue [49]. This enzymatic reduction would lead to increase in the extracellular adenosine levels and consequently inhibition of the pro-inflammatory effects, attenuating inflammation and tissue damage caused by parasite, since persistent presence of T. cruzi in the cardiac muscle was detected by polymerase chain reaction. Thus, this biochemical alteration was also related with the host response against the parasite, as a compensatory mechanism to preserve cells and tissues from an excessive inflammatory process.
It is known that CD vascular disorders can be caused by inflammatory process. This process may generate vascular occlusion by platelet thrombi leading to congestive heart failure. However, the organism can prevent the thrombotic process increasing the hydrolysis of extracellular ATP, ADP, and AMP. As a consequence, there is an increased production of extracellular adenosine, inducing a state of equilibrium between host and parasite by limiting endothelial dysfunction-induced platelet reactivity, and consequently vascular hipoperfusion, microspasm, and aneurysm formation, which characterize the chronic Chagas heart disease [50].
As previously mentioned, adenosine is an important signaling molecule in vasculature that has the potential to influence vasomotor responses, cardiac function, inflammatory responses, and platelet aggregation. Its anti-aggregant effects are mediated specifically via A2A and A2B adenosine receptor subtypes [16, 17]. The agonism of these receptors upregulates the production of intracellular cyclic adenosine monophosphate (cAMP), an inhibitor of platelet activation by activation of adenylyl cyclase [51, 52]. cAMP, in turn, inhibits platelet activation through the activation of protein kinase A (PKA), which phosphorylates several substrates such as the IP3 receptor, reducing the release of intracellular calcium stores [53]. Moreover, PKA inhibits signaling to the cytoskeleton and may stabilize the resting cytoskeleton by phosphorylation of cytoskeletal proteins, such as actin-binding protein and caldesmon [16]. Thus, PKA activity induced by the generation of cAMP leads to the inhibition of the reorganization of the cytoskeleton, activation of integrin, and secretion of granules [51, 53]. Intracellular signaling complexes and metabolites present prior to the binding of platelet receptors determine the rate at which platelets accumulate and participate in thrombus formation at the site of vascular injury [16, 53].
The results presented in this study support the hypothesis that catalytic activity alterations of the purinergic system ecto-enzymes may contribute to modifications in platelet aggregation in IFCD patients. In this context, these findings corroborate with the evidence that ecto-enzymes contribute to a number of process involved in normal cardiovascular function and that disorders in purinergic signaling are involved in some cardiovascular diseases.
Several studies have demonstrated that these ecto-enzymes play an important role in thromboregulation mechanisms and alterations in their activities have been verified in many pathological conditions in which platelets do not react normally. Minamino et al. [54] showed that myocardial ischemic preconditioning is associated with increasing 5′-NT activity and adenosine metabolism, suggesting a protective role for adenosine following hypoxia and reperfusion. Confirming these findings, Kitakaze et al. [55] showed that 5-NT activity increased 12–24 h after ischemic preconditioning, which limited the size of the infarct, accompanied by increased adenosine levels in coronary venous blood, and most recently, Bagatini et al. [23] presented that adenosine produced by increase in the E-NTPDase, E-NPP, and E-5′-NT activities and decrease in the E-ADA activity in platelets of patients with ischemic heart diseases contributed to decrease platelet aggregation, modulating thrombotic events that occur in these patients. In patients with rheumatoid arthritis, an increase in the E-NTPDase, E-NPP, and E-5′-NT activities was found in platelets and discussed as a physiological response to an excessive platelet aggregation which occurs during the inflammatory process. However, an increased E-ADA activity was observed and associated to accelerated atherosclerosis found in these patients, due to decrease in adenosine production [21].
Therefore, decreased E-NPP and E-5′-NT activities associated to decreased E-ADA activity in platelets of IFCD patients contributed to the observed decrease in platelet aggregation. This allows us to suggest that purinergic signaling system is involved in the thromboregulation of IFCD patients, possibly due to the production of large amounts of adenosine, a molecule with anti-aggregant properties and cardioprotective effects.
Conclusion
Altogether, the results suggest that the regulation of extracellular nucleotides levels through the modulation of the E-NPP, E-5′-NT, and E-ADA activities in the platelets of chagasic patients represents an important control of purine-mediated thrombogenic function in the cardiovascular system during IFCD, as demonstrated by decreased platelet aggregation. Thus, it was concluded that these findings were related to a physiological response of host facing chronic T. cruzi infection. The organism could be avoiding coagulation processes by increasing depletion of cell-released or ATP-produced ADP and increasing adenosine production, which exerts a cardioprotective effect promoting vasodilatation and anti-thrombotic signaling, preventing the progress of CD to cardiac form in the IFCD patients.
Acknowledgements
This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundo de Incentivo a Pesquisa (FIPE/UFSM), Brazil.
Disclosure of conflicts of interests
Author declares there are no actual or potential conflicts of interest.
References
- 1.Rossi MA, Bestetti RB. Hipótese unificada sobre a patogênese da cardiopatia chagásica crônica. Implicações terapêuticas. Arq Bras Cardiol. 1995;64:255–260. [PubMed] [Google Scholar]
- 2.Prata A. Clinical and epidemiological aspects of Chagas' disease. Lancet Infect Dis. 2001;1:92–100. doi: 10.1016/S1473-3099(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 3.Prata A. Prognóstico e complicações da doença de Chagas. Rev Goiana Med. 1959;5:87–96. [Google Scholar]
- 4.Gonçalves JG, Dias Silva VJ, Calzada Borges MC, Prata A, Correia D. Mortality indicators among chronic Chagas patients living in an endemic area. Int J Cardiol. 2010;143:235–242. doi: 10.1016/j.ijcard.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Rossi MA. Microvascular changes as a cause of chronic cardiomyopathy in Chagas' disease. Am Heart J. 1990;120:233–236. doi: 10.1016/0002-8703(90)90191-Y. [DOI] [PubMed] [Google Scholar]
- 6.Prata A. Chagas' disease. Infect Dis Clin North Am. 1994;8:61–76. [PubMed] [Google Scholar]
- 7.Rassi A, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, Rassi GG, Hasslocher-Moreno A, Sousa AS, Scanavacca MI. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355:799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 8.Marcus AJ, Broekman MJ, Drosopoulos JH, Pinsky DJ, Islam N, Maliszewsk CR. Inhibition of platelet recruitment by endothelial cell CD39/ecto-ADPase: significance for occlusive vascular disease. Ital Heart J. 2001;2:824–830. [PubMed] [Google Scholar]
- 9.Birk AV, Broekman MJ, Gladek EM, Robertson HD, Drosopoulos JH, Marcus AJ, Szeto HH. Role of extracellular ATP metabolism in regulation of platelet reactivity. J Lab Clin Med. 2002;140:166–175. doi: 10.1067/mlc.2002.126719. [DOI] [PubMed] [Google Scholar]
- 10.Ralevic V, Burnstock G. Involvement of purinergic signaling in cardiovascular diseases. Drug News Perspect. 2003;16:133–140. doi: 10.1358/dnp.2003.16.3.876886. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson B, Dwyer K, Enjyoji K, Robson SC. Ecto-nucleotidases of the cd-39/ntpdase family modulated platelet activation on thrombous formation: potential as therapeutic targets. Blood Cells Mol Dis. 2006;36:217–222. doi: 10.1016/j.bcmd.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Clifford EE, Parker K, Humphreys BD, Kertesy SB, Dubyak GR. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood. 1998;91:3172–3181. [PubMed] [Google Scholar]
- 13.Léon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/S0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 14.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature (Lond) 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedeberg Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 16.Yang D, Chen H, Koupenova M, Carroll SH, Eliades A, Freedman JE, Toselli P, Ravid K. A new role for the A2b adenosine receptor in regulating platelet function. J Thromb Haemost. 2010;8:817–827. doi: 10.1111/j.1538-7836.2010.03769.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnston-Cox HA, Yang D, Ravid K. Physiological implications of adenosine receptor-mediated platelet aggregation. J Cell Physiol. 2011;226:46–51. doi: 10.1002/jcp.22379. [DOI] [PubMed] [Google Scholar]
- 18.Pilla C, Emanuelli T, Frassetto SS, Battastini AMO, Dias RD, Sarkis JJF. ATP diphosphohydrolase activity (Apyrase, EC 3.6.1.5.) in human blood platelets. Platelets. 1996;7:225–230. doi: 10.3109/09537109609023582. [DOI] [PubMed] [Google Scholar]
- 19.Lunkes IG, Lunkes D, Stefanello F, Morch A, Morch MV, Mazzantti MC, Schetinger MCR. Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res. 2003;109:189–194. doi: 10.1016/S0049-3848(03)00178-6. [DOI] [PubMed] [Google Scholar]
- 20.Spanevello RM, Mazzanti CM, Bagatini M, Correa M, Schmatz R, Stefanello N, Thomé G, Morsch VM, Becker L, Bellé L, Oliveira L, Schetinger MR. Activities of the enzymes that hydrolyze adenine nucleotides in platelets from multiple sclerosis patients. J Neurol. 2010;257:24–30. doi: 10.1007/s00415-009-5258-4. [DOI] [PubMed] [Google Scholar]
- 21.Becker LV, Rosa CS, Souza VCG, Bagatini MD, Casali EA, Leal CA, Silva JC, Moretto MB, Pinheiro FV, Morsch VM, Schetinger MR, Leal DB. Activities of enzymes that hydrolyze adenine nucleotides in platelets from patients with rheumatoid arthritis. Clin Biochem. 2010;43:1096–1100. doi: 10.1016/j.clinbiochem.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado PA, Corrêa MC, Becker LV, Flores C, Moretto MB, Morsch V, Schetinger MRC. Ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP) and adenosine deaminase (ADA) activities in patients with uterine cervix neoplasia. Clin Biochem. 2008;41:400–406. doi: 10.1016/j.clinbiochem.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Bagatini MD, Martins CC, Battisti V, Spanevello RM, Gasparetto D, Rosa CS, Gonçalves JF, Schetinger MR, Santos RB, Morsch VM. Hydrolysis of adenine nucleotides in platelets from patients with acute myocardial infarction. Clin Biochem. 2008;41:1181–1185. doi: 10.1016/j.clinbiochem.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Leal CA, Schetinger MR, Leal DB, Bauchspiess K, Schrekker CM, Maldonado PA, Morsch VM, Silva JE. NTPDase and 5′-nucleotidase activities in platelets of human pregnants with a normal or high risk for thrombosis. Mol Cell Biochem. 2007;304:325–330. doi: 10.1007/s11010-007-9515-5. [DOI] [PubMed] [Google Scholar]
- 25.Anonymous Meeting of applied research in Chagas disease. Validity of the concept of indeterminate form. Rev Soc Bras Med Trop. 1985;18:46. [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of proteins-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Chan KM, Delfert D, Junger KD. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem. 1986;157:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 28.Fürsternau C, Trentin DS, Barreto-Chaves ML, Sarkis JJ. Ecto-nucleotide pyrophosphatase/phosphodiesterase as part of a multiple system for nucleotide hydrolysis by platelets from rats: kinetic characterization and biochemical properties. Platelets. 2006;17:84–91. doi: 10.1080/09537100500246641. [DOI] [PubMed] [Google Scholar]
- 29.Sakura H, Nagashima S, Nakashima A, Maeda M. Characterization of fetal serum 5′-nucleotide phosphodiesterase: a novel function as a platelet aggregation inhibitor in fetal circulation. Thromb Res. 1998;91:83–89. doi: 10.1016/S0049-3848(98)00073-5. [DOI] [PubMed] [Google Scholar]
- 30.Frassetto SS, Dias RD, Sarkis JJ. Characterization of an ATP diphosphohydrolase activity (APYRASE, EC 3.6.1.5) in rat blood platelets. Mol Cell Biochem. 1993;129:47–55. doi: 10.1007/BF00926575. [DOI] [PubMed] [Google Scholar]
- 31.Giusti G, Galanti B. Colorimetric Method. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; 1984. pp. 315–323. [Google Scholar]
- 32.Born GVR, Cross MJ. The aggregation of blood platelets. J Physiol. 1963;95:168–178. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Añez N, Carrasco H, Parada H, Crisante G, Rojas A, Fuenmayor C, Gonzalez N, Percoco G, Borges R, Guevara P, Ramirez JL. Myocardial parasite persistence in chronic Chagasic patients. AmJTrop Med Hyg. 1999;60:726–732. doi: 10.4269/ajtmh.1999.60.726. [DOI] [PubMed] [Google Scholar]
- 34.Lopes ER, Chapadeiro E, Andrade ZA, Almeida H, Rocha A. Pathological anatomy of hearts from asymptomatic Chagas disease patients dying in a violent manner. Mem Inst Oswald Cruz. 1981;76:189–197. doi: 10.1590/S0074-02761981000200010. [DOI] [PubMed] [Google Scholar]
- 35.Lopes ER, Rocha A, Adad SJ, Fernandes EL, Chapadeiro E. Necroscopic study of a case of chronic form of Chagas disease with electrocardiogram and x-rays of normal thorax. Special reference to the excito-conductor system of the heart. Rev Soc Bras Med Trop. 1988;21:67–70. doi: 10.1590/s0037-86821988000200007. [DOI] [PubMed] [Google Scholar]
- 36.Cardoso JE, Brener Z. Hematological changes in mice experimentally infected with Trypanosoma cruzi. Mem Inst Oswald Cruz. 1980;75:97–104. doi: 10.1590/S0074-02761980000200009. [DOI] [PubMed] [Google Scholar]
- 37.Jamra MA, Freitas JL, Amato Neto V, Silva LH, Tartari JT. Hematological aspects of the initial phases of Chagas' disease. Rev Paul Med. 1954;45:544–552. [PubMed] [Google Scholar]
- 38.Rossi MA, Gonçalves S, Ribeiro-dos-Santos R. Experimental Trypanosoma cruzi cardiomyopathy in BALB/c mice. The potential role of intravascular platelet aggregation in its genesis. Am J Pathol. 1984;114:209–216. [PMC free article] [PubMed] [Google Scholar]
- 39.Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, Factor SM, Hatcher VB, Bilezikian JP, Baum SG, Wittner M. Enhanced platelet adherence and aggregation in Chagas' disease: a potential pathogenic mechanism for cardiomyopathy. AmJTrop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- 40.Gordon EL, Pearson JD, Dickinson ES, Moreau D, Slakey LL. The hydrolysis of extracellular adenine nucleotides by arterial smooth muscle cells. Regulation of adenosine production at the cell surface. J Biol Chem. 1989;264:18986–18995. [PubMed] [Google Scholar]
- 41.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orléans-Juste P, Marceau F, Thorin E, Sévigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heine P, Braun N, Heilbronn A, Zimmermann H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after heterologous expression in CHO cells. Eur J Biochem. 1999;262:102–107. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 43.Soslau G, Youngprapakorn D. A possible dual physiological role of extracellular ATP in the modulation of platelet aggregation. Biochim Biophys Acta. 1997;1355:131–140. doi: 10.1016/S0167-4889(96)00123-1. [DOI] [PubMed] [Google Scholar]
- 44.Park HS, Hourani SM. Differential effects of adenine nucleotide analogues on shape change and aggregation induced by adenosine 5-diphosphate (ADP) in human platelet. Br J Pharmacol. 1999;127:1359–1366. doi: 10.1038/sj.bjp.0702690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borowiec A, Lechward K, Tkacz-Stachowska K, Skladanowski AC. Adenosine as a metabolic regulator of tissue function: production of adenosine by cytoplasmic 5′-nucleotidases. Acta Biochim Pol. 2006;53:269–278. [PubMed] [Google Scholar]
- 46.Fretes RE, Paglini P, Fernández AR, Enders J, Fabro SP. Trypanosoma cruzi: increased 5′-nucleotidase activity associated with dysfunction of adrenergic receptors in acutely infected albino Swiss mice. J Parasitol. 1999;85:970–972. doi: 10.2307/3285840. [DOI] [PubMed] [Google Scholar]
- 47.Zhai X, Zhou X, Ashraf M. Interaction of singlet oxygen with 5′-nucleotidase in rat hearts. J Mol Cell Cardiol. 1995;27:2453–2464. doi: 10.1006/jmcc.1995.0233. [DOI] [PubMed] [Google Scholar]
- 48.Headrick JP, Emerson CS, Berr SS, Berne RM, Matherne GP. Interstitial adenosine and cellular metabolism during beta-adrenergic stimulation of the in situ rabbit heart. Cardiovasc Res. 1996;31:699–710. [PubMed] [Google Scholar]
- 49.Silva AS, Pimentel VC, Fiorenza AM, França RT, Tonin AA, Jaques JA, Leal CA, Silva CB, Morsch V, Sschetinger MR, Lopes ST, Monteiro SG. Activity of cholinesterases and adenosine deaminase in blood and serum of rats experimentally infected with Trypanosoma cruzi. Ann Trop Med Parasitol. 2011;105:385–391. doi: 10.1179/1364859411Y.0000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M. Chagas' disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul S, Feoktistov I, Hollister AS, Robertson D, Biaggioni I. Adenosine inhibits the rise in intracellular calcium and platelet aggregation produced by thrombin: evidence that both effects are coupled to adenylate cyclase. Mol Pharmacol. 1990;37:870–875. [PubMed] [Google Scholar]
- 52.Linden MD, Barnard MR, Frelinger AL, Michelson AD, Przyklenk K. Effect of adenosine A2 receptor stimulation on platelet activation-aggregation: differences between canine and human models. Thromb Res. 2008;121:689–698. doi: 10.1016/j.thromres.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sim DS, Merrill-Skoloff G, Furie BC, Furie B, Flaumenhaft R. Initial accumulation of platelets during arterial thrombus formation in vivo is inhibited by elevation of basal cAMP levels. Blood. 2004;103:2127–2134. doi: 10.1182/blood-2003-04-1133. [DOI] [PubMed] [Google Scholar]
- 54.Minamino T, Kitakaze M, Morioka T, Node K, Komamura K, Takeda H, Inoue M, Hori M, Kamada T. Cardioprotection due to preconditioning correlates with increased ecto-5′-nucleotidase activity. Am J Physiol. 1996;270:H238–H244. doi: 10.1152/ajpheart.1996.270.1.H238. [DOI] [PubMed] [Google Scholar]
- 55.Kitakaze M, Minamino T, Node K, Komamura K, Hori M. Activation of ecto-5′-nucleotidase and cardioprotection by ischemic preconditioning. Basic Res Cardiol. 1996;91(1):23–26. doi: 10.1007/BF00795358. [DOI] [PubMed] [Google Scholar]