Abstract
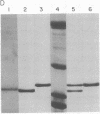
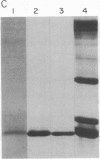
Calmodulin, a multifunctional calcium-modulated protein, has been isolated from spinach leaf tissue and from spinach leaf messenger RNA translation products. The translation protein and the spinach leaf protein have been partially characterized and compared to vertebrate calmodulins. Spinach leaf calmodulin will quantitatively activate bovine brain phosphodiesterase and will undergo a calcium-dependent shift in electrophoretic mobility similar to that of bovine brain calmodulin. In the presence of Ca2+ the spinach and brain proteins comigrate, but in the presence of chelators they do not. A polyadenylylated RNA fraction has been isolated from spinach leaf tissue and translated in a wheat germ cell-free translation system. The calmodulin synthesized in vitro has been isolated by using calcium-dependent affinity chromatography on phenothiazine-Sepharose conjugates. The translation protein comigrates with spinach calmodulin during polyacrylamide gel electrophoresis whether in the presence or the absence of Ca2+. The translation protein also undergoes a calcium-dependent mobility shift identical to that of spinach calmodulin. Amino acid analysis of the translation calmodulin indicates that it does not contain Nε-trimethyllysine, an amino acid residue that is characteristic of all calmodulins previously examined. These studies suggest that Nε-trimethyllysine is not required for the calcium-dependent interaction of calmodulin with phenothiazines and indicate the potential utility of phenothiazine-Sepharose conjugates as affinity-based adsorbents in biological and biochemical investigations.
Keywords: trimethyllsine, phenothiazine-Sepharose, calcium-modulated proteins, electrophoretic mobility
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Dedman J. R., Jackson R. L., Schreiber W. E., Means A. R. Sequence homology of the Ca2+-dependent regulator of cyclic nucleotide phosphodiesterase from rat testis with other Ca2+-binding proteins. J Biol Chem. 1978 Jan 25;253(2):343–346. [PubMed] [Google Scholar]
- Grab D. J., Berzins K., Cohen R. S., Siekevitz P. Presence of calmodulin in postsynaptic densities isolated from canine cerebral cortex. J Biol Chem. 1979 Sep 10;254(17):8690–8696. [PubMed] [Google Scholar]
- Hidaka H., Naka M., Yamaki T. Effect of novel specific myosin light chain kinase inhibitors on Ca2+-activated Mg2+-ATPase of chicken gizzard actomyosin. Biochem Biophys Res Commun. 1979 Oct 12;90(3):694–699. doi: 10.1016/0006-291x(79)91883-7. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C. Calcium-dependent affinity chromatography of calmodulin on an immobilized phenothiazine. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1048–1056. doi: 10.1016/0006-291x(79)91932-6. [DOI] [PubMed] [Google Scholar]
- Jones H. P., Matthews J. C., Cormier M. J. Isolation and characterization of Ca2+-dependent modulator protein from the marine invertebrate Renilla reniformis. Biochemistry. 1979 Jan 9;18(1):55–60. doi: 10.1021/bi00568a009. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Mechanism by which psychotropic drugs inhibit adenosine cyclic 3',5'-monophosphate phosphodiesterase of brain. Mol Pharmacol. 1976 Jul;12(4):581–589. [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Specificity of the binding of trifluoperazine to the calcium-dependent activator of phosphodiesterase and to a series of other calcium-binding proteins. Biochim Biophys Acta. 1978 May 3;540(2):197–204. doi: 10.1016/0304-4165(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Paik W. K., Kim S. Protein methylation: chemical, enzymological, and biological significance. Adv Enzymol Relat Areas Mol Biol. 1975;42:227–286. doi: 10.1002/9780470122877.ch5. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Prevention of NH2-terminal acetylation of proteins synthesized in cell-free systems. J Biol Chem. 1977 Dec 25;252(24):8781–8783. [PubMed] [Google Scholar]
- Perry S. V., Grand R. J., Nairn A. C., Vanaman T. C., Wall C. M. Calcium-binding proteins and the regulation of contractile activity. Biochem Soc Trans. 1979 Aug;7(4):619–622. doi: 10.1042/bst0070619. [DOI] [PubMed] [Google Scholar]
- Seamon K. Cation dependent conformations of brain CA2+-dependent regulator protein detected by nuclear magnetic resonance. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1256–1265. doi: 10.1016/0006-291x(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Cohen P. T., Cohen P., Nairn A. C., Perry S. V. The role of calmodulin in the structure and regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1979 Oct 15;100(2):329–337. doi: 10.1111/j.1432-1033.1979.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Srivastava A. K., Waisman D. M., Brostrom C. O., Soderling T. R. Stimulation of glycogen synthase phosphorylation by calcium-dependent regulator protein. J Biol Chem. 1979 Feb 10;254(3):583–586. [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Characterization of a calcium-modulated protein from transformed chicken fibroblasts. J Biol Chem. 1979 Oct 25;254(20):10250–10255. [PubMed] [Google Scholar]
- Walsh M., Stevens F. C., Oikawa K., Kay C. M. Circular dichroism studies of native and chemically modified Ca2+-dependent protein modulator. Can J Biochem. 1979 Mar;57(3):267–278. doi: 10.1139/o79-034. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Rapid separation and quantitation of 3',5'-cyclic nucleotides and 5'-nucleotides in phosphodiesterase reaction mixtures using high-performance liquid chromatography. J Biochem Biophys Methods. 1980 Mar;2(3):139–146. doi: 10.1016/0165-022x(80)90021-4. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]