Abstract
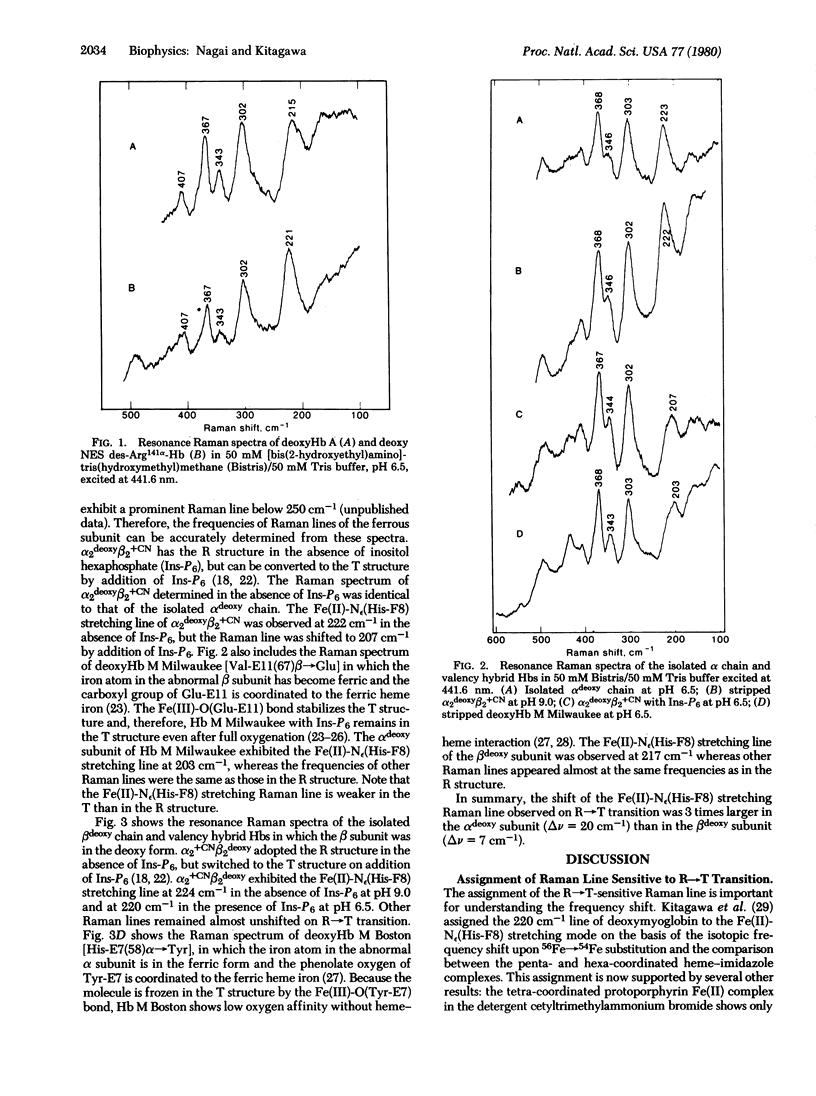
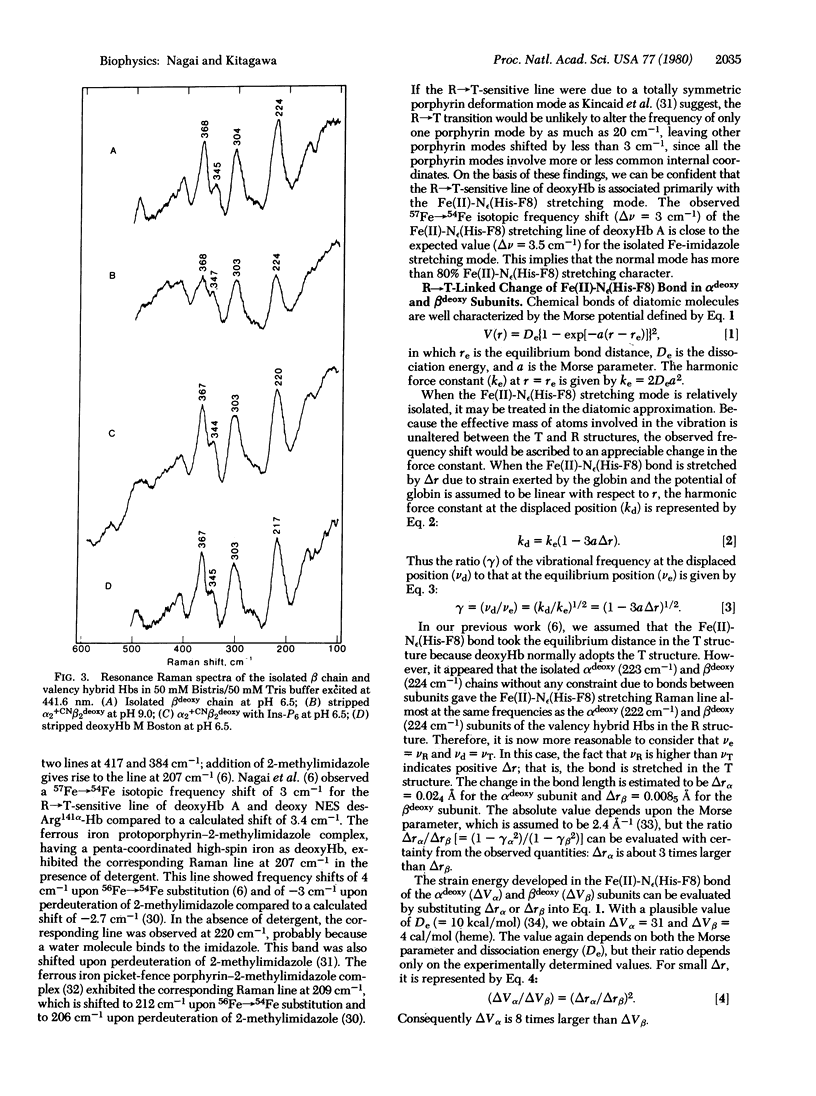
Resonance Raman spectra have been obtained of the alpha deoxy and beta deoxy subunits within valency hybrid hemoglobins both in the high-affinity (R) and low-affinity (T) structures. Upon conversion from the R to the T structure, the vibrational frequency of the Fe(II)-N epsilon(His-F8) bond changes from 223 to 207 or 203 cm-1 in the alpha deoxy subunit and from 224 to 220 or 217 cm-1 in the beta deoxy subunit. We estimate that the Fe(II)-N epsilon(His-F8) bond is stretched by the R leads to T transition 3 times more in the alpha subunit (0.024 A) than in the beta subunit (0.0085 A) and, accordingly, the strain energy developed in that bond is 8 times larger in the alpha than in the beta subunit. Hence, the oxygen affinity of the alpha and beta subunits may be regulated by different mechanisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Cassoly R., Gibson Q. H. The kinetics of ligand binding to hemoglobin valency hybrids and the effect of anions. J Biol Chem. 1972 Nov 25;247(22):7332–7341. [PubMed] [Google Scholar]
- Collman J. P., Gagne R. R., Reed C. A., Robinson W. T., Rodley G. A. Structure of an iron(II) dioxygen complex; a model for oxygen carrying hemeproteins. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1326–1329. doi: 10.1073/pnas.71.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung L. W., Minton A. P., Lindstrom T. R., Pisciotta A. V., Ho C. Proton nuclear magnetic resonance studies of hemoglobin M Milwaukee and their implications concerning the mechanism of cooperative oxygenation of hemoglobin. Biochemistry. 1977 Apr 5;16(7):1452–1462. doi: 10.1021/bi00626a033. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Imai K., Morimoto H., Watari H. Properties of hemoglobin M, Milwaukee-I variant and its unique characteristic. Biochim Biophys Acta. 1969 Nov 11;194(1):6–15. doi: 10.1016/0005-2795(69)90173-1. [DOI] [PubMed] [Google Scholar]
- Henry Y., Banerjee R. Electron paramagnetic studies of nitric oxide haemoglobin derivatives: isolated subunits and nitric oxide hybrids. J Mol Biol. 1973 Feb 5;73(4):469–482. doi: 10.1016/0022-2836(73)90094-6. [DOI] [PubMed] [Google Scholar]
- Ikeda-Saito M., Yamamoto H., Yonetani T. Studies on cobalt myoglobins and hemoglobins. Electron paramagnetic resonance investigation of iron-cobalt hybrid hemoglobins and its implication for the heme-heme interaction and for the alkaline Bohr effect. J Biol Chem. 1977 Dec 10;252(23):8639–8644. [PubMed] [Google Scholar]
- Imai K. Analyses of oxygen equilibria of native and chemically modified human adult hemoglobins on the basis of Adair's stepwise oxygenation theory and the allosteric model of Monod, Wyman, and Changeux. Biochemistry. 1973 Feb 27;12(5):798–808. doi: 10.1021/bi00729a003. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Hewitt J. A., Wootton J. F. Alteration of functional properties associated with the change in quaternary structure in unliganded haemoglobin. J Mol Biol. 1975 Apr 5;93(2):203–218. doi: 10.1016/0022-2836(75)90128-x. [DOI] [PubMed] [Google Scholar]
- Kincaid J., Stein P., Spiro T. G. Absence of heme-localized strain in T state hemoglobin: insensitivity of heme-imidazole resonance Raman frequencies to quaternary structure. Proc Natl Acad Sci U S A. 1979 Feb;76(2):549–552. doi: 10.1073/pnas.76.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T., Nagai K. Quaternary structure-induced photoreduction of haem of haemoglobin. Nature. 1979 Oct 11;281(5731):503–504. doi: 10.1038/281503a0. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Nagai K., Tsubaki M. Assignment of the Fe-Nepsilon (His F8) stretching band in the resonance Raman spectra of deoxy myoglobin. FEBS Lett. 1979 Aug 15;104(2):376–378. doi: 10.1016/0014-5793(79)80856-x. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. An infrared study of NO bonding to heme B and hemoglobin A. Evidence for inositol hexaphosphate induced cleavage of proximal histidine to iron bonds. Biochemistry. 1976 Jan 27;15(2):388–396. doi: 10.1021/bi00647a023. [DOI] [PubMed] [Google Scholar]
- Messana C., Cerdonio M., Shenkin P., Noble R. W., Fermi G., Perutz R. N., Perutz M. F. Influence of quaternary structure of the globin on thermal spin equilibria in different methemoglobin derivatives. Biochemistry. 1978 Aug 22;17(17):3652–3662. doi: 10.1021/bi00610a035. [DOI] [PubMed] [Google Scholar]
- Nagai K., Hori H., Morimoto H., Hayashi A., Taketa F. Influence of amino acid replacements in the heme pocket on the electron paramagnetic resonance spectra and absorption spectra of nitrosylhemoglobins M Iwate, M Boston, and M Milwaukee. Biochemistry. 1979 Apr 3;18(7):1304–1308. doi: 10.1021/bi00574a029. [DOI] [PubMed] [Google Scholar]
- Nagai K., Hori H., Yoshida S., Sakamoto H., Morimoto H. The effect of quaternary structure on the state of the alpha and beta subunits within nitrosyl haemoglobin. Low temperature photodissociation and the ESR spectra. Biochim Biophys Acta. 1978 Jan 25;532(1):17–28. doi: 10.1016/0005-2795(78)90443-9. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Nagai K. The effect of ferric ligands on the oxygen affinity of the ferrous subunits in valency hybrid haemoglobins. J Mol Biol. 1977 Mar 25;111(1):41–53. doi: 10.1016/s0022-2836(77)80130-7. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. High resolution nuclear magnetic resonance spectra of hemoglobin. 3. The half-ligated state and allosteric interactions. J Mol Biol. 1972 Sep 28;70(2):315–336. doi: 10.1016/0022-2836(72)90542-6. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Heidner E. J., Ladner J. E., Beetlestone J. G., Ho C., Slade E. F. Influence of globin structure on the state of the heme. 3. Changes in heme spectra accompanying allosteric transitions in methemoglobin and their implications for heme-heme interaction. Biochemistry. 1974 May 7;13(10):2187–2200. doi: 10.1021/bi00707a028. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Kilmartin J. V., Nagai K., Szabo A., Simon S. R. Influence of globin structures on the state of the heme. Ferrous low spin derivatives. Biochemistry. 1976 Jan 27;15(2):378–387. doi: 10.1021/bi00647a022. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974 May 7;13(10):2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Nature of haem-haem interaction. Nature. 1972 Jun 30;237(5357):495–499. doi: 10.1038/237495a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Pulsinelli P. D., Ranney H. M. Structure and subunit interaction of haemoglobin M Milwaukee. Nat New Biol. 1972 Jun 28;237(78):259–263. doi: 10.1038/newbio237259a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Sanders J. K., Chenery D. H., Noble R. W., Pennelly R. R., Fung L. W., Ho C., Giannini I., Pörschke D., Winkler H. Interactions between the quaternary structure of the globin and the spin state of the heme in ferric mixed spin derivatives of hemoglobin. Biochemistry. 1978 Aug 22;17(17):3640–3652. doi: 10.1021/bi00610a034. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Pulsinelli P. D., Perutz M. F., Nagel R. L. Structure of hemoglobin M Boston, a variant with a five-coordinated ferric heme. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3870–3874. doi: 10.1073/pnas.70.12.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROUGHTON F. J., OTIS A. B., LYSTER R. L. The determination of the individual equilibrium constants of the four intermediate reactions between oxygen and sheep haemoglobin. Proc R Soc Lond B Biol Sci. 1955 Aug 16;144(914):29–54. doi: 10.1098/rspb.1955.0032. [DOI] [PubMed] [Google Scholar]
- Shelnutt J. A., Rousseau D. L., Friedman J. M., Simon S. R. Protein-heme interaction in hemoglobin: evidence from Raman difference spectroscopy. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4409–4413. doi: 10.1073/pnas.76.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y. Differences in spectra of alpha and beta chains of hemoglobin between isolated state and in tetramer. J Biol Chem. 1975 Feb 25;250(4):1251–1256. [PubMed] [Google Scholar]
- Suzuki T., Hayashi A., Shimizu A., Yamamura Y. The oxygen equilibrium of hemoglobin MSaskatoon. Biochim Biophys Acta. 1966 Sep 26;127(1):280–282. doi: 10.1016/0304-4165(66)90510-1. [DOI] [PubMed] [Google Scholar]
- Tsubaki M., Nagai K. Effect of removal of a salt-bridge on the oxygen binding properties and the electronic structure of heme in cobalt-iron hybrid hemoglobin. J Biochem. 1979 Oct;86(4):1029–1035. doi: 10.1093/oxfordjournals.jbchem.a132596. [DOI] [PubMed] [Google Scholar]
- Udem L., Ranney H. M., Bunn H. F., Pisciotta A. Some observations on the properties of hemoglobin M Milwaukee-1. J Mol Biol. 1970 Mar;48(3):489–498. doi: 10.1016/0022-2836(70)90060-4. [DOI] [PubMed] [Google Scholar]


