Abstract
Multidrug-resistant Acinetobacter baumannii poses a tremendous challenge to traditional antibiotic therapy. Due to the crucial role of iron in bacterial physiology and pathogenicity, we investigated iron metabolism as a possible target for anti-A. baumannii chemotherapy using gallium as an iron mimetic. Due to chemical similarity, gallium competes with iron for binding to several redox enzymes, thereby interfering with a number of essential biological reactions. We found that Ga(NO3)3, the active component of an FDA-approved drug (Ganite), inhibits the growth of a collection of 58 A. baumannii strains in both chemically defined medium and human serum, at concentrations ranging from 2 to 80 μM and from 4 to 64 μM, respectively. Ga(NO3)3 delayed the entry of A. baumannii into the exponential phase and drastically reduced bacterial growth rates. Ga(NO3)3 activity was strongly dependent on iron availability in the culture medium, though the mechanism of growth inhibition was independent of dysregulation of gene expression controlled by the ferric uptake regulator Fur. Ga(NO3)3 also protected Galleria mellonella larvae from lethal A. baumannii infection, with survival rates of ≥75%. At therapeutic concentrations for humans (28 μM plasma levels), Ga(NO3)3 inhibited the growth in human serum of 76% of the multidrug-resistant A. baumannii isolates tested by ≥90%, raising expectations on the therapeutic potential of gallium for the treatment of A. baumannii bloodstream infections. Ga(NO3)3 also showed strong synergism with colistin, suggesting that a colistin-gallium combination holds promise as a last-resort therapy for infections caused by pan-resistant A. baumannii.
INTRODUCTION
The threat to public health posed by multidrug-resistant (MDR) bacteria has become a pressing problem on a global scale. In this scenario, the Gram-negative bacterium Acinetobacter baumannii provides a paradigmatic example of the impressively fast acquisition and accumulation of antibiotic resistance (32, 45, 61). A. baumannii is a versatile pathogen implicated in a wide range of hospital infections, particularly among critically ill patients in intensive care units (ICUs), and certain worldwide epidemic lineages are responsible for large citywide and even nationwide outbreaks (19, 45, 61).
During the 1970s, A. baumannii infections could be treated successfully with gentamicin, minocycline, nalidixic acid, ampicillin, or carbenicillin, but high proportions of strains rapidly became resistant to most antimicrobials, including carbapenems (7, 32, 45). Colistin (CST) and tigecycline retain activity against most A. baumannii isolates (49), though infections sustained by resistant strains have already been documented in several countries (12, 15, 36), and such infections are untreatable with any commercially available antibiotic.
The paucity of effective drugs for the treatment of MDR infections has highlighted the overwhelming need for research and development programs aimed at identifying new therapeutic options. The siderophore-mimetic BAL30072 is a new monosulfactam containing a dihydropyridone siderophore-like moiety which is believed to accelerate its flux across the Gram-negative cell envelope (27). Gallium [Ga(III)] is an iron-mimetic metal that exerts a significant antimicrobial activity against a number of bacteria, also including the reference A. baumannii strain ATCC 17978 (5, 13, 18, 23, 31, 39, 40, 41). Ga(III), in the form of citrate-buffered Ga(NO3)3 solution (Ganite; Genta), is an FDA-approved drug for intravenous (i.v.) treatment of hypercalcemia associated with malignancy (14). Ga(III) activity is due to its chemical resemblance to Fe(III). Both metals show similarities in nuclear radius and coordination chemistry so that Ga(III) can efficiently compete with Fe(III) for binding to iron-containing enzymes, as well as to transferrin, lactoferrin, and microbial iron chelators (siderophores). However, Ga(III) cannot be physiologically reduced, and when it replaces Fe(III) in redox enzymes, a number of essential biological reactions are inhibited, including those responsible for DNA and protein synthesis and energy production (8). Given the competitive nature of Ga(III)-dependent inhibition of bacterial metabolism, the antibacterial activity of Ga(III) is reversed by Fe(III), influenced by ligand complexation, and strongly reduced in iron-rich media (5, 6, 18, 31, 50).
Invading pathogens are faced with an extreme iron limitation during infection as a means of host defense and must gain access to Fe(III) retained by chelating proteins such as transferrin and lactoferrin (64). To accomplish this essential function, microorganisms have evolved different strategies, including the expression of high-affinity, siderophore-mediated iron uptake systems (11). Production of siderophores is stimulated under iron-limiting conditions [Fe(III) ≤ 1 to 5 μM] and repressed when sufficient iron is available. The Fur (ferric uptake regulator) repressor protein acts as the master regulator of iron homeostasis in bacteria. In cells containing sufficient iron levels, the Fur-Fe(II) complex blocks transcription arising from Fur-controlled promoters, which in turn are transcribed under conditions of iron starvation (33). Iron acquisition from the human host appears to be crucial for A. baumannii pathogenicity, as inferred by the presence in this species of multiple iron-uptake systems (3, 24) and the reduced lethality of mutants impaired in acinetobactin siderophore synthesis (25). The importance of iron in host-pathogen interactions led us to investigate the effect of Ga(III) on A. baumannii growth, iron regulation, and virulence. Here we demonstrate that Ga(NO3)3 exerts potent growth inhibition of clinical A. baumannii strains in both chemically defined medium and human serum. While the inhibitory mechanism of Ga(NO3)3 appears to involve interference with iron metabolism, Ga(NO3)3 did not affect iron-dependent regulation of gene expression mediated by the Fur protein. Ga(NO3)3 also caused a dramatic reduction of A. baumannii lethality in the Galleria mellonella insect model of infection and showed significant in vitro synergism with CST.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The 58 A. baumannii strains used in this study are listed in Table S1 in the supplemental material. The collection includes the following strains: type strain ATCC 19606T, representative strains for the international clonal lineages (ICL) I, II, and III, RUH 875, RUH 134, and RUH 5875, respectively (38, 59), strains AYE (ICL I), ACICU (ICL II), and ATCC 17978, for which the annotated genome sequence is available (28, 53, 58), CST-resistant clinical isolate 50C (15, 16), and 50 genotypically diverse, previously described clinical isolates collected as part of the European Union-funded Antibiotic Resistance, Prevention and Control (ARPAC) project (3, 35). All strains, except ATCC 19606T, ATCC 17978, RUH 875, and RUH 134, showed an MDR phenotype (16, 28, 57, 58).
Culture media were as follows: M9 minimal medium (51) supplemented with 20 mM sodium succinate as the carbon source (hereby called M9) and, when indicated, 100 μM 2,2′-dipyridyl (DIP) to reduce iron availability (M9-DIP), 1% and 10% tryptic soy broth (TSB; Neogen Co., Lansing, MI) (31), iron-depleted Bacto Casamino Acids (Becton, Dickinson, Sparks, MD) medium (DCAA) (60); BBL Mueller-Hinton broth (MH; Becton, Dickinson). When needed, media were supplemented with FeCl3 or FeSO4 at appropriate concentrations to increase iron availability.
Human serum collected from 50 healthy donors was pooled, filtered, and inactivated (30 min, 56°C) as described previously (2). Serum chemistry was as follows: total iron, 0.89 μg/ml; ferritin, 0.17 μg/ml; transferrin, 2.53 mg/ml; and total iron binding capacity, 3.16 μg/ml (28% transferrin saturation, equivalent to 46.7 μM unsaturated iron binding sites). To achieve complete transferrin saturation, an excess of FeCl3 (200 μM) was added to human serum when indicated.
Isolates were grown overnight at 37°C in the above media and then diluted to an optical density at 600 nm (OD600) of 0.01 in the same medium with or without FeCl3. For growth in human serum, inocula were obtained upon dilution to the OD600 of 0.01 of an overnight culture at 37°C in M9. Two hundred microliters of bacterial suspensions were dispensed in 96-well microtiter plates, and growth (OD600) was periodically monitored for up to 48 h in a Wallac 1420 Victor3 V multilabel plate reader (Perkin Elmer). Large-scale cultures were performed in flasks containing 25 ml of medium, and growth (OD600) was monitored spectrophotometrically for up to 48 h. All cultures were incubated at 37°C with vigorous shaking (200 rpm). Each strain was tested at least twice in independent experiments.
Assays for siderophore production.
The total iron-chelating activity in culture supernatants was determined by the chromoazurol S (CAS) liquid assay (52). The assay was preliminarily performed on 24- and 48-h large-scale cultures of strain ATCC 17978 and then extended to the whole collection in microtiter scale. For this purpose, 24- and 48-h cultures were centrifuged for 10 min at 3,000 × g in a microtiter plate centrifuge (Eppendorf model no. 5810r), and 100 μl of supernatant was mixed with an equal volume of the CAS solution. Iron-chelating activity was expressed as siderophore units (U) normalized to the cell density (OD600) of the bacterial culture. Siderophore units are defined as [(OD630 reference − OD630 sample)/OD630 reference] × 100 (42).
Generation of the Fur-regulated basA::lacZ promoter fusion and β-galactosidase activity assay.
The 596-bp DNA fragment encompassing the Fur box within the basA (A1S_2391) promoter region was obtained by PCR amplification with primers PbasA_FW (CGGAATTCGCCATATTCTTGTTTCGAT) and PbasA_RV (TTATGCTGAGGTAGGGACTCTAGACG) and cloned at the EcoRI-XbaI restriction sites (underlined) of the pMP220 broad-host-range promoter probe plasmid upstream of the reporter lacZ gene (54) to yield pMP220::PbasA. The Fur titration assay (FURTA) (55) was used to confirm the presence of a functional Fur box in the promoter region of the A. baumannii ATCC 17978 basA gene. Briefly, the 596-bp DNA fragment encompassing the basA promoter was cloned in pBluescript II SK vector (Stratagene) to yield pBS::PbasA and introduced in Escherichia coli H1717 to assess the FURTA phenotype as described previously (55).
The pMP220::PbasA transcriptional fusion was introduced in A. baumannii strain ATCC 17978 by electroporation, and transformants were selected on 10-μg/ml tetracycline plates. Plasmid pMP220::PbasA was used to probe the intracellular iron pool of bacteria, as inferred by Fur and iron regulation of the PbasA fusion in different growth media and in the presence or absence of Ga(NO3)3 or FeSO4. For the latter purpose, A. baumannii ATCC 17978(pMP220::PbasA) was grown at 37°C for up to 48 h in M9-DIP with or without FeSO4 (1.25, 2.5, 5, or 10 μM) and/or Ga(NO3)3 (6.25 or 12.5 μM).
The β-galactosidase (LacZ) activity was determined spectrophotometrically on toluene/SDS-permeabilized cells using o-nitrophenyl-β-d-galactopyranoside as the substrate, normalized to the OD600 of the bacterial culture, and expressed in Miller units (37). Results are the means of triplicate experiments.
Ga(NO3)3 susceptibility testing and iron-gallium competition assays.
Ga(NO3)3 activity was tested in 96-well microtiter plates. Briefly, A. baumannii isolates were grown overnight at 37°C in M9, diluted in M9-DIP to a final OD600 of 0.01, and 200 μl of the bacterial suspensions were dispensed in 96-well microtiter plates containing increasing Ga(NO3)3 concentrations (0 to 128 μM). Inhibitory concentrations (ICs) of Ga(NO3)3 in human serum were determined using a protocol similar to that described for M9-DIP, except that bacteria were diluted in inactivated pooled human serum supplemented with increasing Ga(NO3)3 concentrations (0 to 128 μM). For Fe(III)-Ga(III) competition experiments, the same procedure was performed, except that FeCl3 was added in a range of 0.4 to 50.0 μM. Microtiter plates were incubated for up to 48 h at 37°C and growth (OD600) was measured in a Wallac 1420 Victor3 V multilabel plate reader. For each strain, the Ga(NO3)3 concentrations that inhibited growth by 50% and 90% (IC50 and IC90, respectively) in M9-DIP and human serum were calculated at 24 and/or 48 h using GraphPad Prism software (version 5.0; GraphPad Software, San Diego, CA). The effect of Fe(III) on Ga(III) activity was expressed as a percentage relative to bacterial growth at 48 h in M9-DIP without Fe(III) and Ga(III).
For large-scale cultures, a subset of representative A. baumannii isolates with IC values comparable to the median values of the bacterial collection (see Tables S2 and S3 in the supplemental material), including ACICU, A376, A399, and A451, were grown overnight at 37°C in M9, diluted to an OD600 of 0.01 in flasks containing 25 ml of M9-DIP and different Ga(NO3)3 concentrations (0, 4, 16, 64, and 128 μM), and grown at 37°C for up to 48 h.
G. mellonella killing assays and in vivo Ga(III) treatments.
The A. baumannii virulence assay in G. mellonella was performed as described previously (2), with few alterations. In order to establish the A. baumannii inoculum required to kill G. mellonella (580 mg ± 60 mg weight), 10 caterpillars were injected with 10 μl of three serial 10-fold dilutions in saline solution of bacteria grown in M9 to an OD600 of 1.0 (late exponential phase, predicted to contain ∼1 × 109 CFU/ml). A panel of seven strains showing different levels of Ga(NO3)3 resistance in vitro (see Tables S2 and S3 in the supplemental material), including ATCC 19606T, ATCC 17978, RUH 5875, AYE, ACICU, 50C, and A371, was used. Bacterial colony counts on TSB agar plates were used to estimate the number of viable cells in each inoculum. Ten larvae that received no injection and 10 larvae injected with 10 μl of sterile saline solution were used as negative controls for each experiment. Two independent experiments were performed for each strain. Larvae were incubated at 37°C in petri dishes and monitored for up to 96 h. Survival curves were plotted using the Kaplan-Meier estimator and expressed in percentages.
In preliminary toxicology assays, G. mellonella caterpillars were injected with 10 μl of Ga(NO3)3 (0 to 3 M corresponding to 0 to 52 mmol/kg of body weight) or sodium nitrate (NaNO3) (0 to 6 M corresponding to 0 to 102 mmol/kg) solutions. At least 10 larvae were inoculated per condition, incubated at 37°C in petri dishes, and monitored daily for up to 96 h. Caterpillars were considered dead when they did not react to gentle prodding (2).
The Ga(NO3)3 dosage for treatment of A. baumannii infections in G. mellonella (1.2 mmol/kg) was extrapolated from the human therapeutic dose (∼47 μM) by allometric scaling based on body surface area (BSA), as recommended by the FDA. Normalization to BSA is preferable to simple weight conversion since it takes into consideration metabolic rates and corrects for problems of overdosing (48). Human and larval BSAs were thus calculated using the formula described by DuBois and DuBois (20), and dose translation was performed using the formula described by Reagan-Shaw et al. (48).
The effect of Ga(NO3)3 on A. baumannii-infected caterpillars was assessed by injecting the right proleg of 16 G. mellonella caterpillars with a lethal dose of each A. baumannii strain, followed by injection into the left proleg with 1.2 mmol/kg of Ga(NO3)3 after 15 min. Ten larvae that received 10 μl of sterile saline solution in place of Ga(NO3)3 were used as a Ga(III)-untreated control, and 10 larvae injected twice with 10 μl of sterile saline solution were used as negative controls for Ga(NO3)3 protection experiments.
Checkerboard assay for Ga(III)-CST interactions.
The interaction between Ga(III) and CST (purchased from Sigma-Aldrich as sulfate salt) was determined in MH broth and M9-DIP, using the checkerboard assay as described previously (46). A panel of nine A. baumannii strains showing levels of Ga(NO3)3 resistance representative of the range observed for the whole bacterial collection (see Tables S2 and S3 in the supplemental material) was selected, including the reference strains ATCC 19606T, ATCC 17978, RUH 875, RUH 134, RUH 5875, AYE, and ACICU and the clinical isolates A371 and 50C, the latter one being CST resistant (15, 16). The MIC of CST was determined in 96-well microtiter plates as described previously (46). The criteria proposed by Gales et al. (26) were used for interpretation of CST susceptibility. For the checkerboard assay, microtiter plates were inoculated with each A. baumannii isolate to yield ∼5 × 105 CFU/ml in a 100-μl final volume of MH broth or, alternatively, OD600 of 0.01 in a 200-μl final volume of M9-DIP and incubated at 37°C. Two independent experiments were performed per isolate. Results were recorded after 18 h for MH plates and after 48 h for M9-DIP plates. The CST concentration range tested was from 0.0625 to 256 μg/ml. The MIC was defined as the lowest drug concentration that completely inhibited the growth of the organism, as detected by visual inspection. The fractional inhibitory concentration index (FICI) was calculated for each combination using the following formula: FICI = FICGa(III) + FICCST, where FICGa(III) represents the MIC of Ga(III) in combination/the MIC of Ga(III) alone and FICCST represents the MIC of CST in combination/the MIC of CST alone. The FICI was interpreted as follows: synergy, FICI ≤ 0.5; indifference, 0.5 < FICI ≤ 4; and antagonism, FICI > 4 (46).
Statistical analysis.
All statistical analyses, including inhibition and survival curve analyses, were performed using GraphPad Prism software. Since a large variation in growth yields and response to Ga(III) was observed among strains, results were reported in boxplots showing medians rather than means.
RESULTS
Assessment of growth media for Ga(NO3)3 susceptibility testing.
The antibacterial properties of gallium are attributable to its ability to substitute for iron in bacterial metabolism, and a number of previous reports have demonstrated that an iron surplus abrogates gallium activity (18, 31, 43). Moreover, invading pathogens are generally faced with an extreme iron limitation during growth in vivo as the result of the host innate defense against infection (64). To mimic the iron-poor environment of the host and define suitable conditions for Ga(III) activity testing, a preliminary investigation of siderophore production and intracellular iron levels was performed in the A. baumannii prototype strain ATCC 17978, grown in a set of chemically defined (M9, M9-DIP, and DCAA) and undefined (1% TSB, 10% TSB, and MH broth) media. As a trend, growth of ATCC 17978 was poor in DCAA and 1% TSB, moderate in 10% TSB and M9 with or without DIP, and abundant in MH broth (Fig. 1A). Addition of 100 μM FeCl3 strongly increased growth yields in DCAA and, to a lesser extent, in M9 and M9-DIP, suggesting that iron is a limiting nutrient in these media (Fig. 1A). Accordingly, high siderophore production was observed in M9 and M9-DIP but not in 1% TSB, 10% TSB, and MH broth (Fig. 1B). Siderophore production was moderate in DCAA, plausibly due to the very poor growth (Fig. 1A and B). Expression of the Fur-Fe(II)-controlled basA::lacZ transcriptional fusion was then measured in A. baumannii ATCC 17978 carrying plasmid pMP220::PbasA. The presence of a functional Fur box in the promoter region of the A. baumannii ATCC 17978 basA gene was confirmed through the FURTA (see Fig. S1 in the supplemental material). Since the Fur repressor protein acts as an iron sensor, the activity of the Fur-controlled basA promoter provides an indirect estimate of the intracellular iron levels of bacteria grown in the different media. Consistent with siderophore production, iron repressible β-galactosidase expression was high in M9 and M9-DIP but low or null in DCAA, 1% TSB, 10% TSB, and MH broth (Fig. 1C). Based on the above results, it was concluded that M9-DIP could be used as an easily reproducible, chemically defined, iron-deplete medium for Ga(III) susceptibility testing. Addition of 100 μM DIP to M9 would ensure an iron-deficient medium in the event that iron traces were present as contaminants of the M9 basal salt solution.
Fig 1.
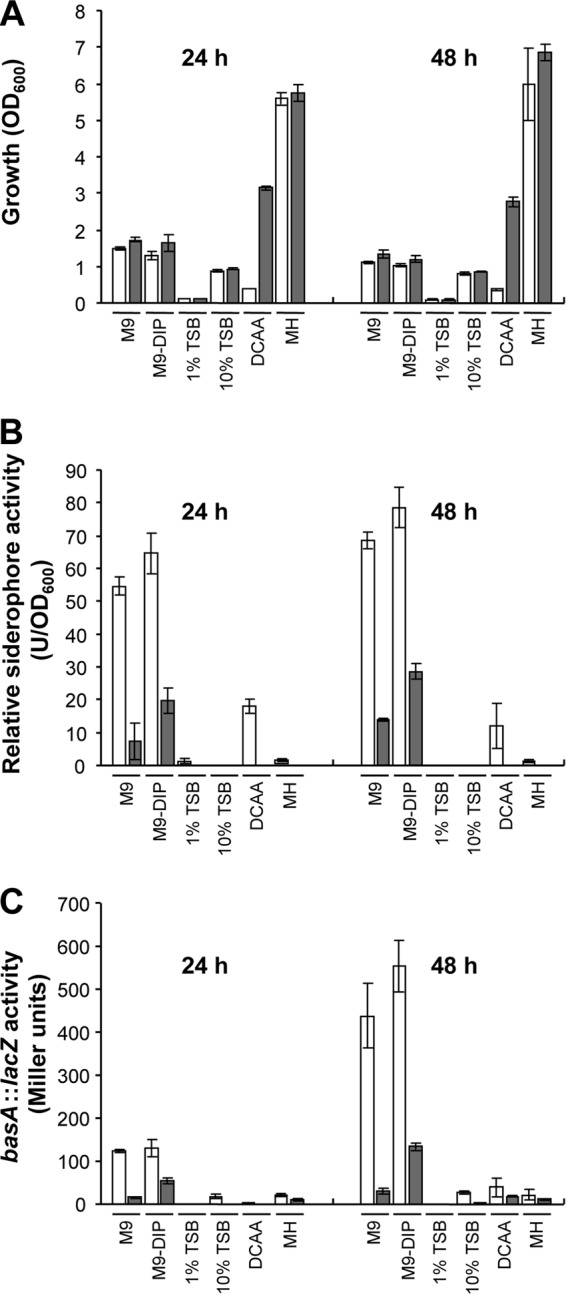
A. baumannii ATCC 17978 growth (A), siderophore production (B), and activity of the basA::lacZ iron-regulated fusion (C). Bacteria were grown in different media for 24 or 48 h, in the absence (white bars) or presence (gray bars) of 100 μM FeCl3. Data are the means (± standard deviations [SD]) of triplicate experiments.
Growth and siderophore production was then evaluated for the whole collection of 58 A. baumannii strains in M9-DIP using a microtiter plate assay. All strains showed sufficient growth and produced detectable siderophore levels in M9-DIP (Fig. 2; see also Table S4 in the supplemental material). As expected, addition of 100 μM FeCl3 to M9-DIP increased growth yields and abrogated siderophore production (Fig. 2; see also Table S4).
Fig 2.
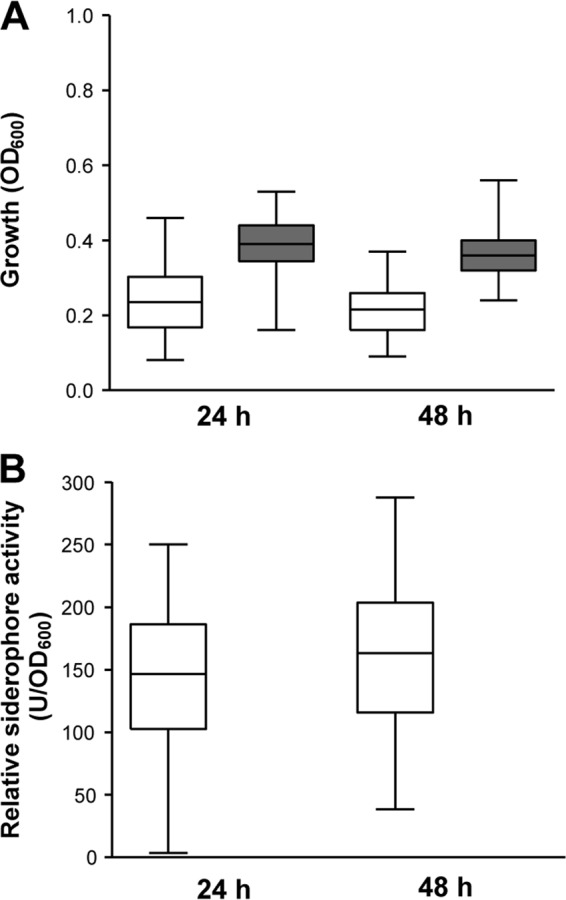
Growth (A) and siderophore production (B) in a collection of 58 A. baumannii strains grown for 24 and 48 h in M9-DIP. Boxes represent medians and second and third interquartiles; whiskers represent range of 58 strains. White boxes, M9-DIP without added iron; gray boxes, M9-DIP supplemented with 100 μM FeCl3. For each strain, the mean value of two independent microtiter plate assays was considered.
Ga(III) inhibits A. baumannii growth in a chemically defined medium.
The effect of Ga(III) on A. baumannii growth in M9-DIP was determined for all strains in a microtiter plate assay and expressed as IC50 and IC90 after 24 and 48 h growth (see Table S2 in the supplemental material). Ga(III) inhibited A. baumannii growth in a dose- and strain-dependent manner. In general, the IC values were lower at 24 than 48 h (Fig. 3; see also Table S2). At 48 h, the IC50 and IC90 values varied in the ranges of 1 to 28 μM and 2 to 80 μM Ga(NO3)3, respectively, with median values of 8.5 ± 5.0 and 23.8 ± 12.0 μM Ga(NO3)3, respectively (Fig. 3). Growth inhibition was also assessed in flask cultures using a representative subset of strains, namely, ACICU, A376, A399, and A451, endowed with Ga(III) susceptibility levels close to the median values determined for the whole collection. The IC50s of Ga(NO3)3 did not differ substantially between strains grown in flasks or in microtiter plates, while IC90 values were 1.02- to 1.74-fold higher for strains grown in flasks (see Fig. S2 in the supplemental material; also data not shown), suggesting that Ga(III) activity is slightly influenced by culture scale and conditions.
Fig 3.
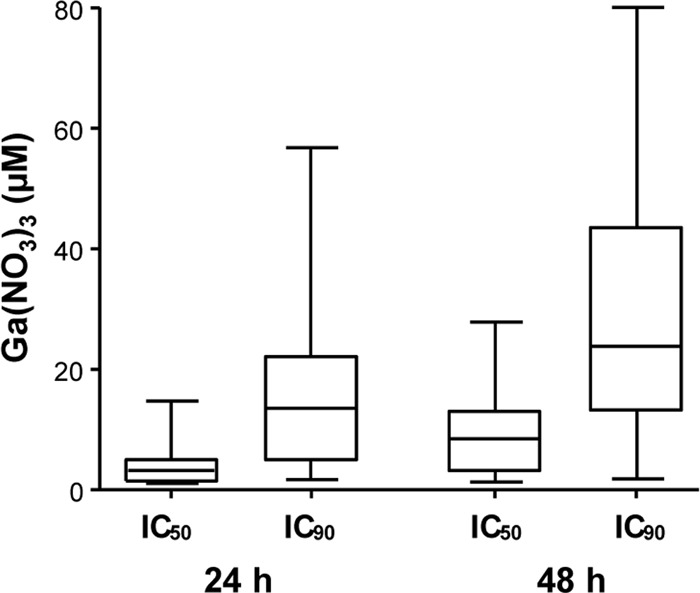
Inhibitory activity of Ga(NO3)3 on a collection of 58 A. baumannii strains grown in chemically defined medium. Plots show the Ga(NO3)3 concentrations (μM) required to inhibit the growth of each isolate by 50% (IC50) and 90% (IC90) at 24 and 48 h in M9-DIP. Boxes represent medians and second and third interquartiles; whiskers represent range of 58 strains. For each strain, the mean value of two independent microtiter plate assays was considered.
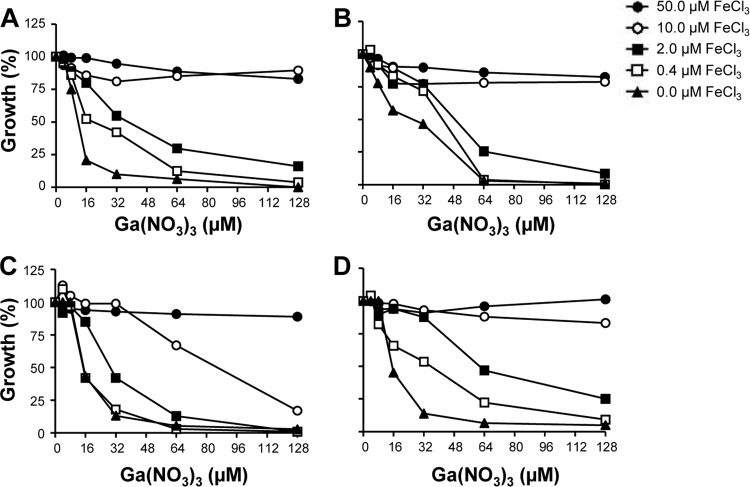
The anti-A. baumannii activity of Ga(III) is reversed by Fe(III).
Since Ga(III) exerts its inhibiting activity by interfering with bacterial iron metabolism, the effect of Fe(III) on Ga(III)-dependent growth inhibition was investigated using the subset of A. baumannii strains described above. As expected, Ga(III)-dependent growth inhibition was reversed with increasing Fe(III) concentrations (Fig. 4). For all strains tested, Fe(III) concentrations from 0.4 to 2 μM were sufficient to double the IC50s, while 2 μM Fe(III) caused a 2- to 4-fold increase of the IC90 values after 48 h (Fig. 4). The addition of 2 μM Fe(III) was sufficient to increase Ga(NO3)3 IC50 and IC90 values from 13 and 36 μM to 29 and 128 μM, respectively, as exemplified by A. baumannii strain ACICU (Fig. 4). In general, addition of 50 μM Fe(III) completely abrogated the activity of 128 μM Ga(NO3)3 (Fig. 4), indicating that an Fe(III)/Ga(III) molar ratio of ∼1:2.5 is sufficient to reverse the effect of Ga(III).
Fig 4.
Effect of iron on the inhibitory activity of Ga(NO3)3. A. baumannii strains ACICU (A), A376 (B), A399 (C), and A451 (D) were grown for 48 h in M9-DIP supplemented with increasing FeCl3 (0 to 50 μM) and Ga(NO3)3 (0 to 128 μM) concentrations. Growth is expressed as percentage relative to growth (OD600) of control cultures in M9-DIP.
Effect of Ga(III) on iron-regulated gene expression and siderophore production.
Since Ga(III) cannot be reduced by the cell, it could not substitute for Fe(II) in Fur binding and consequent repression of Fur-controlled promoters. To verify this hypothesis, the effect of Ga(III) on iron homeostasis was investigated in the prototype isolate A. baumannii ATCC 17978 by using the Fur-controlled basA promoter (see above; see also Fig. S1 in the supplemental material) as a probe for the intracellular iron level. FeSO4 was used in place of FeCl3 since Fe(II) is more soluble and readily available for Fur binding. Addition of up to 2.5 μM FeSO4 resulted in a dose-dependent reduction of PbasA::lacZ expression, while complete repression was observed at >5 μM FeSO4 (Fig. 5). In contrast, addition of 6.25 and 12.5 μM Ga(NO3)3 did not influence the level of β-galactosidase activity (Fig. 5). The expression profile of basA::lacZ in the presence of both Ga(NO3)3 and FeSO4 was comparable to that observed with FeSO4 alone (Fig. 5). These results suggest that Ga(III) does not interfere with iron homeostasis and Fur-dependent iron regulation in A. baumannii. To confirm this conclusion, the effect of Ga(III) on siderophore production, which is a typical response to iron starvation, was investigated. Siderophore production by the subset of A. baumannii strains described above was measured after 48 h growth in M9-DIP supplemented with subinhibitory Ga(NO3)3 concentrations (0, 2, 4, and 8 μM). Overall, addition of Ga(NO3)3 up to 8 μM did not repress siderophore production (see Fig. S3 in the supplemental material), further supporting the hypothesis that Ga(III) does not interfere with the bacterial response to iron starvation. Higher Ga(NO3)3 concentrations could not be tested due to strong inhibition of bacterial growth.
Fig 5.
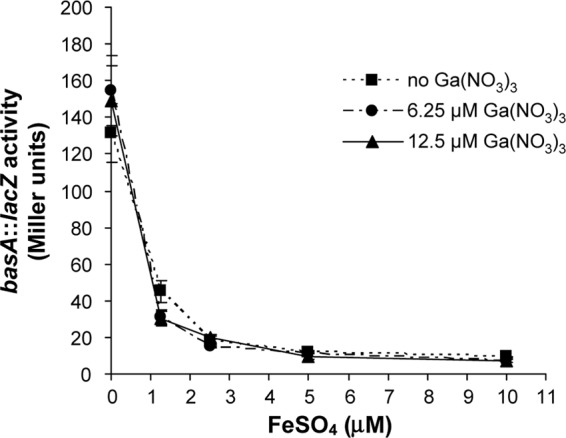
Effect of Ga(NO3)3 on the activity of the Fur-Fe(II)-regulated basA::lacZ transcriptional fusion. A. baumannii ATCC 17978 carrying plasmid pMP220::PbasA was grown in M9-DIP supplemented or not supplemented with increasing FeSO4 (1.25, 2.5, 5, or 10 μM) and/or Ga(NO3)3 (6.25 or 12.5 μM) concentrations. LacZ activity was measured at 48 h and expressed in Miller units (37). Data are the means (±SD) of triplicate experiments.
Ga(III) inhibits A. baumannii growth in human serum.
The spread of bacteria into the bloodstream is a frequent complication of primary A. baumannii infection. Therefore, the inhibitory activity of Ga(III) in complement-inactivated human serum was investigated. Initially, the growth of strain ACICU in serum with or without FeCl3 was monitored for 56 h using both flask and microtiter plate conditions. Irrespective of the culture mode, A. baumannii ACICU growth yields in serum without exogenously added iron were always lower than in iron-replete serum (see Fig. S4 in the supplemental material), confirming that (apo-)transferrin confers to human serum the characteristics of an iron-deplete medium. Since growth yields in microtiter plates were lower than in flasks and the stationary phase was delayed (see Fig. S4), Ga(NO3)3 inhibitory concentrations for the whole collection could only be calculated at 48 h. At this time point, the IC50 and IC90 values of Ga(NO3)3 varied in the range of 2 to 35 μM and 4 to 64 μM, respectively, with median values of 6.0 ± 1.7 and 14.5 ± 0.7 μM, respectively (Fig. 6; see also Table S3 in the supplemental material). Growth of strain ATCC 19606T was very poor in serum, so the inhibitory effect of Ga(III) could not be assessed even after 48 h of growth (see Table S3). The Ga(III) inhibitory effect in serum was completely abrogated by the addition of 200 μM FeCl3 (data not shown), i.e., an excess of Fe(III) over the transferrin Fe(III) binding capacity (∼47 μM).
Fig 6.
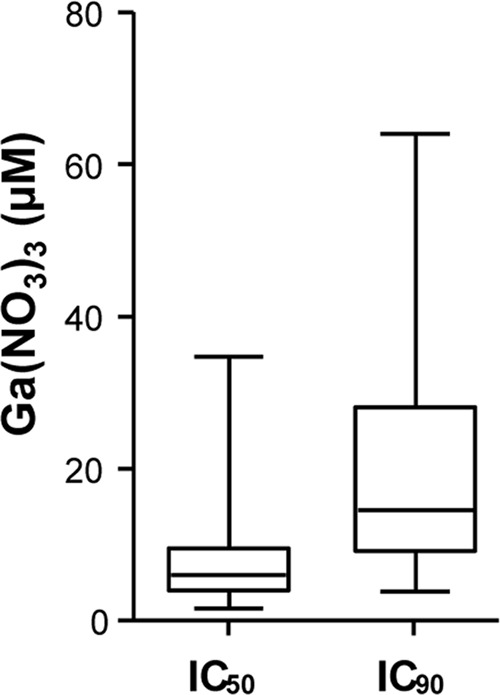
Inhibitory activity of Ga(NO3)3 on a collection of 57 A. baumannii strains grown in human serum. Strains were grown for 48 h in serum supplemented with increasing Ga(NO3)3 concentrations (0 to 128 μM). Plots show the Ga(NO3)3 concentrations (μM) required to inhibit the growth of each isolate by 50% (IC50) and 90% (IC90) at 48 h. Boxes represent medians and second and third interquartiles; whiskers represent range of 57 strains. For each strain, the mean value of two independent microtiter plate assays was considered.
No statistically significant difference in Ga(III) susceptibility between A. baumannii sequence groups (Welch's t test, P > 0.05) and no correlation between IC values of Ga(III) in M9-DIP and human serum (Pearson correlation coefficient, P > 0.05) were observed.
Ga(III) promotes the survival of G. mellonella larvae infected with A. baumannii.
The promising results obtained in vitro prompted an investigation of the ability of Ga(III) to inhibit A. baumannii pathogenicity in vivo. To this aim, an infection model based on the larvae of the greater wax moth G. mellonella was used. This model has previously been used successfully in the study of A. baumannii pathogenesis and therapeutics (2, 44).
Ga(NO3)3 toxicity to G. mellonella was initially tested by injecting larvae with 10 μl of water solutions containing Ga(NO3)3 up to 3 M, i.e., the maximum experimental solubility of Ga(NO3)3 in water, and by monitoring the survival of larvae for 96 h. At this time point, killing was only observed for larvae injected with >4.2-mmol/kg Ga(NO3)3, with lethal doses 50% (LD50) and 90% (LD90) corresponding to 10.0 and 17.8 mmol/kg of Ga(NO3)3, respectively (see Fig. S5A in the supplemental material). At high Ga(NO3)3 concentrations (>LD90), nearly all larvae died within 24 h (see Fig. S5A). Injecting G. mellonella with NaNO3 as a control for the effect of nitrate resulted, at 96 h, in an LD50 and an LD90 of 25.4 and 51.7 mmol/kg, respectively (see Fig. S5B). These values are comparable to those observed for Ga(NO3)3, so it can be argued that the observed mortality of larvae can primarily be attributed to nitrate toxicity. However, the G. mellonella equivalent of the therapeutic Ga(NO3)3 dosage used in humans is 1.2 mmol/kg (see Materials and Methods) (14, 48), corresponding to ∼1/10 of the LD50 predetermined for Ga(III) and nitrate in G. mellonella. At this Ga(NO3)3 dosage, no larvae were killed at any time (see Fig. S5).
A. baumannii lethal doses for G. mellonella were then calculated for strains ATCC 19606T, ATCC 17978, RUH 5875, AYE, ACICU, 50C, and A371. At 96 h postinfection, all strains but ATCC 19606T showed LD50s between 1 × 105 and 2 × 106 CFU/larva and LD90s between 3 × 105 and 8 × 106 CFU/larva (see Table S5 in the supplemental material). The LD50 of ATCC 19606T for G. mellonella was very high (∼ 4 × 107 CFU/larva) (see Table S5), typical of nonpathogenic species (2, 4). For this reason, and considering the extreme susceptibility of ATCC 19606T to Ga(III) in vitro, this strain was excluded from subsequent Ga(III) protection experiments.
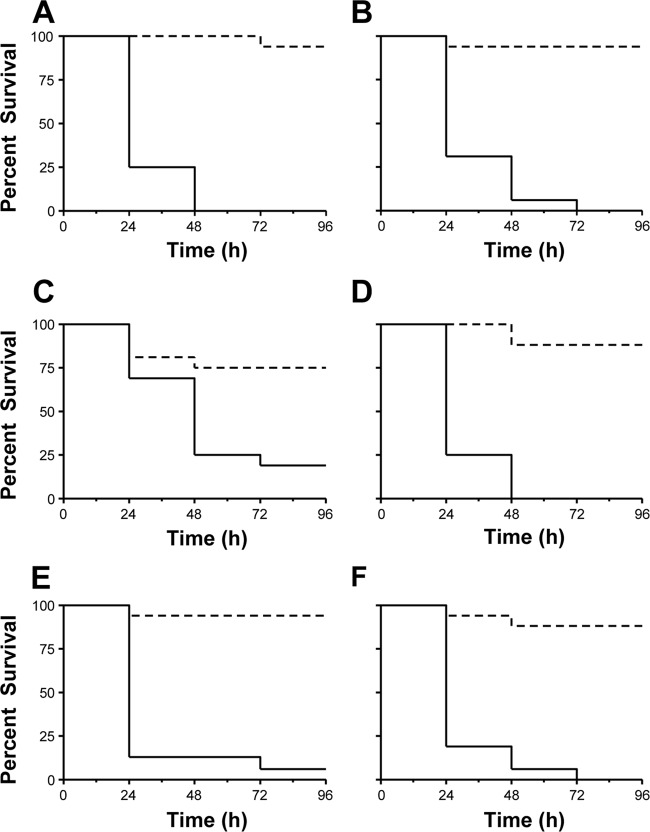
To test Ga(III) efficacy in vivo, a lethal dose of A. baumannii (∼106 to ∼107 CFU/larva, depending on the strain) (Fig. 7) was administered to G. mellonella larvae, followed by the administration of 1.2 mmol/kg of Ga(NO3)3 to mimic the therapeutic dose used in humans (see Materials and Methods for details). Administration of Ga(NO3)3 protected G. mellonella from A. baumannii-mediated killing, with survival rates of ≥75% for all strains at 96 h postinoculation (Fig. 7). Conversely, rapid killing and <20% survival rates were observed for Ga(III)-untreated larvae (Fig. 7).
Fig 7.
Effect of Ga(NO3)3 on the viability of G. mellonella caterpillars infected with a lethal dose of the following A. baumannii strains: ATCC 17978 (∼ 5 × 106 CFU/larva) (A), RUH 5875 (∼ 1 × 107 CFU/larva) (B), AYE (∼ 5 × 106 CFU/larva) (C), ACICU (∼ 7 × 106 CFU/larva) (D), 50C (∼ 1 × 106 CFU/larva) (E), and A371 (∼ 6 × 105 CFU/larva) (F). Solid lines, Ga(III)-untreated larvae; dotted lines, Ga(III)-treated larvae.
Ga(III) interacts synergistically with CST.
Synergistic effects of the Ga(III)-CST combination were determined using a panel of nine A. baumannii strains endowed with different levels of susceptibility to Ga(NO3)3 and CST. Checkerboard assays in MH broth only allowed inhibition by CST to be determined, since Ga(NO3)3 concentrations as high as 128 μM had no apparent effect on bacterial growth, likely due to the high iron concentration in this medium (Fig. 1). However, MIC values of CST were substantially lower in the presence of Ga(NO3)3. Strains ATCC 19606T, ACICU, A371, and 50C showed a 4-fold reduction of the CST MIC in the presence of 2 μM Ga(NO3)3, while strains ATCC 17978, AYE, ACICU, RUH 875, RUH 134, and RUH 5875 showed an 8-fold reduction of the CST MIC in a Ga(NO3)3 range of 2 to 16 μM (Table 1). Notably, the synergistic effect of the tested combinations was also evident for the CST-resistant isolate 50C, even if Ga(NO3)3 did not lower the CST MIC below the susceptibility threshold (Table 1). Strong synergistic effects were evident in M9-DIP, with FICI values ranging from 0.13 to 0.50 (Table 1). Overall, synergism resulted in a 4- to 32-fold reduction in the MIC of Ga(NO3)3 and a 2- to 16-fold reduction in the MIC of CST for all but one of the strains tested (Table 1). Noteworthy, the CST-resistant isolate 50C showed an impressive synergism, with a 128-fold reduction of the CST MIC in the presence of 8 μM Ga(NO3)3 (Table 1).
Table 1.
Checkerboard assay for CST-Ga(III) interactions in MH broth and M9-DIP with a selected panel of A. baumannii strainsa
| Strain | MH broth |
M9-DIP |
||||||
|---|---|---|---|---|---|---|---|---|
| Effect (FICI value) of combinationb | MIC |
Effect (FICI value) of combination | MIC |
|||||
| CST (μg/ml)c | Ga(NO3)3 (μM) | CST (μg/ml)-Ga(NO3)3 (μM) | CST (μg/ml) | Ga(NO3)3 (μM) | CST (μg/ml)-Ga(NO3)3 (μM) | |||
| ATCC 19606T | Sy (≤0.26) | 0.5 | ≥256 | 0.125–2 | Sy (0.31) | 2 | 8 | 0.125–2 |
| ATCC 17978 | Sy (≤0.13) | 1 | ≥256 | 0.125–2 | Sy (0.28) | 4 | 64 | 1–2 |
| RUH 875 | Sy (≤0.19) | 1 | ≥256 | 0.125–16 | Sy (0.25) | 4 | 64 | 0.5–8 |
| RUH 134 | Sy (≤0.16) | 2 | ≥256 | 0.25–8 | Sy (0.16) | 4 | 64 | 0.5–2 |
| RUH 5875 | Sy (≤0.14) | 1 | ≥256 | 0.125–4 | Sy (0.50) | 4 | 16 | 1–4 |
| AYE | Sy (≤0.14) | 1 | ≥256 | 0.125–4 | Sy (0.25) | 4 | 16 | 0.5–2 |
| ACICU | Sy (≤0.26) | 1 | ≥256 | 0.25–2 | Sy (0.37) | 8 | 32 | 1–4 |
| 50C | Sy (≤0.26) | 16 | ≥256 | 4–2 | Sy (0.13) | 256 | 64 | 2–8 |
| A371 | Sy (≤0.26) | 1 | ≥256 | 0.25–2 | Sy (0.25) | 4 | 64 | 0.5–8 |
MICs for drug(s) alone and in effective synergistic combination, measured after 18 h growth in MH or 48 h growth in M9-DIP.
In order to calculate the FICI, the MIC of Ga(NO3)3 in MH broth was assumed to be ≥256 μM for all strains.
Breakpoint criteria for CST in MH broth were susceptible, ≤2 mg/liter, and resistant, ≥4 mg/liter (26).
DISCUSSION
To face the challenge of progressively expanding resistance in A. baumannii, novel microbial targets must be identified and alternative therapeutic approaches developed. Here, we exploited iron metabolism as a possible target for antibacterial chemotherapy. A. baumannii possesses several iron uptake systems (3, 24), and almost all of these are encoded by genes belonging to the core genome of nosocomial strains (29). Acinetobactin, the most extensively studied A. baumannii siderophore, has the ability to withhold iron from transferrin and lactoferrin (65) and appears to be crucial for A. baumannii to establish infection and kill both G. mellonella and mice (25). These features reflect an exceptional adaptability to growth in iron-limiting environments and highlight the essential role of iron metabolism in A. baumannii pathogenicity. For this reason, a variety of chelating agents have recently been tested for suppression of A. baumannii growth (18, 56).
In the present study, the iron-mimetic metal gallium was used to interfere with A. baumannii iron metabolism. The results demonstrated that Ga(III), the active component of an FDA-approved drug, exerts a strong anti-A. baumannii activity both in vitro and in vivo. We showed that Ga(III) suppresses the growth of genotypically diverse MDR A. baumannii strains, representative of the major sequence groups encountered worldwide (see Table S1 in the supplemental material). The inhibitory activity of Ga(III) was counteracted by iron, confirming that the mechanism of action of Ga(III) in A. baumannii involves the disruption of iron metabolism. Consistent with previous findings (18), the anti-A. baumannii activity of Ga(III) in vitro was strongly dependent on iron availability, being higher in iron-poor, chemically defined media and lower, or even absent, in iron-rich, complex media (Table 1; also data not shown). At present, neither standard protocols nor reference media for Ga(III) susceptibility testing have been defined. MH broth, the standard medium for antibiotic susceptibility testing, appears inappropriate for assessment of Ga(III) activity due to the high iron content (Fig. 1) (5). By comparing bacterial growth and expression of iron-uptake genes in a variety of growth media (Fig. 1), we selected an iron-depleted minimal salt medium (M9-DIP) in which Ga(III) activity could be determined in microtiter plate assays (Fig. 3). M9-DIP is an easily reproducible, chemically defined medium that can be recommended for future interlaboratory assessment of the in vitro antibacterial activity of Ga(III). In this medium, Ga(III) inhibited A. baumannii growth in a dose-, strain-, and time-dependent manner (Fig. 2 and 3; see also Fig. S2 and Table S2 in the supplemental material).
To gain insight into the mechanism of action of Ga(III), the effect of Ga(NO3)3 on iron-regulated gene expression and siderophore production was then investigated. No effect of Ga(III) on Fur-Fe(II)-dependent regulation of gene expression in A. baumannii was observed (Fig. 5), and accordingly, siderophore production by A. baumannii was not repressed in the presence of subinhibitory concentrations of Ga(NO3)3 (see Fig. S3 in the supplemental material). This is consistent with the notion that Ga(III) cannot be reduced under physiological conditions and hence cannot act as cofactor in the repression of Fur-regulated genes, including those encoding iron uptake functions. We therefore hypothesize that Ga(III) exerts its negative effect on A. baumannii growth by interfering with iron-containing essential enzymes, including those participating in the electron transport chain, resulting in a deleterious effect for A. baumannii because of its obligate aerobic metabolism.
Since the two most severe types of A. baumannii infection (meningitis and bloodstream infections) involve systemic dissemination of the organism via the circulatory system, the activity of Ga(III) was also assessed in human serum. Evidence has recently been provided that expression of iron acquisition systems in A. baumannii is induced during growth in human serum (30), due to the presence of transferrin. Thus, growth inhibition assays in human serum provide a more realistic assessment of Ga(NO3)3 therapeutic potential when supplied in vivo. The IC90 values of Ga(NO3)3 calculated at 48 h in human serum for the collection of A. baumannii strains were in the range of 4 to 64 μM, which was similar to the range determined in M9-DIP (2 to 80 μM). Overall, the in vitro activity of Ga(III) against A. baumannii appears to be comparable to or higher than that reported for other Gram-negative pathogens endowed with high resistance to antibiotics, such as Pseudomonas aeruginosa and the Burkholderia cepacia complex (31, 43), although data comparison between different studies can only be speculative because of the medium-dependent variability of Ga(III) activity. Moreover, the IC90 for the reference strain ATCC 17978 in human serum (3.8 μM) was very similar to that recently determined for this strain in RPMI 1640 supplemented with 10% calf serum (3.1 μM) (18). It is worth noting that 90% of isolates showed IC90 values of <47 μM, corresponding to the transferrin iron binding capacity of the serum sample used. Below this concentration, Ga(III) is expected to be completely bound by transferrin due to the high stability constant of the transferrin-Ga(III)2 complex (∼1020 M−1) (1). Since Ga(III) must enter the cell to exert its activity, it can be hypothesized that the antibacterial effect of Ga(III) in serum is attributable to gallium removal from transferrin and subsequent acquisition by the bacterial cell via either a siderophore-dependent mechanism or proteolytic cleavage of transferrin. Of note, Ga(III) transport as a siderophore complex has been reported in some bacterial species (9, 10, 22, 63), and extracellular proteolytic activity has previously been documented in A. baumannii (2).
Not only was Ga(III) effective in inhibiting bacterial growth in vitro, but it also provided a powerful protection against A. baumannii infection in vivo. Experiments in the G. mellonella model demonstrated that Ga(III) is also active in preventing lethal infection by different A. baumannii strains (Fig. 7). G. mellonella hemolymph contains both iron transport and storage proteins (transferrin and ferritin, respectively), and iron has been shown to be essential to an early antimicrobial response of the insect, where hemocytes limit iron availability to invading pathogens (21). Considering that iron concentration in G. mellonella hemolymph is ca. 1.7 μg/ml, i.e., the upper normal range of human serum iron (ca. 0.5 to 1.8 μg/ml), the protective effect of Ga(III) in this insect model could somehow mirror its activity in vertebrate models. In fact, a protective effect of Ga(III) has previously been documented in two mouse models of infection, in which local or systemic administration of different gallium formulations significantly reduced A. baumannii growth (17, 18).
The currently recommended dosing regimens for the treatment of cancer-related hypercalcemia (200 to 300 mg/m2 BSA, i.v.) ensure a peak serum concentration of Ga(NO3)3 of ca. 28 μM (8, 14). This concentration is higher than the observed IC50 and IC90 values of Ga(NO3)3 in human serum for about 98% and 76% of the MDR A. baumannii isolates tested in this study, respectively, suggesting that Ga(NO3)3 therapy could be effective in reducing the ability of A. baumannii to cause bacteremia and spread in the human host.
CST is currently used as a last-line antibiotic for otherwise untreatable MDR A. baumannii infections, often in combination with other drugs (34). The present study demonstrated that Ga(NO3)3, besides being active per se, also synergizes with CST in vitro against both CST-sensitive and -resistant A. baumannii isolates (Table 1). CST acts as a cationic polypeptide that perturbs the outer membrane through interaction with lipopolysaccharide (LPS) molecules (34). It can therefore be hypothesized that CST facilitates Ga(III) diffusion into A. baumannii cells, thereby promoting growth inhibition. In addition, interference of Ga(III) with A. baumannii iron metabolism impairs essential cellular functions, which could potentiate the CST effect. It was observed that CST MICs were higher in M9-DIP than in MH broth (Table 1). This effect can be ascribed to a high content of magnesium and calcium ions (1 and 0.2 mM, respectively), which stabilize the outer membrane and, thus, antagonize CST activity at the bacterial cell surface (47), consequently increasing CST MICs in M9-DIP. In conclusion, the CST-Ga(III) combination could represent a promising therapeutic option against pan-resistant A. baumannii, since it would provide the dual benefit of (i) broadening the range of activity of Ga(III) by making it also effective against strains moderately resistant to therapeutic Ga(III) serum concentrations and (ii) reducing the CST dosages required to treat A. baumannii infections, ultimately mitigating the adverse effects of CST therapy.
Though Ga(NO3)3 therapy could potentially be used as a last resort for treating otherwise untreatable A. baumannii infections, the nephrotoxicity of Ga(NO3)3 should be taken into account, especially in patients who are at risk for renal insufficiency (8). Nevertheless, nephrotoxicity only occurs at extremely high Ga(III) serum concentrations (>200 μM), much higher than those required to treat hypercalcemia and suppress the growth of most A. baumannii strains in serum (up to 28 μM) (8) (Fig. 6; see Table S3 in the supplemental material). At these serum concentrations of Ga(III), serum creatinine remains in the normal concentration range and there are no adverse reactions (62). Clinical studies are therefore needed to confirm the promising potential of Ga(III) as an antibacterial agent.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to K. J. Towner (Department of Clinical Microbiology, Nottingham University Hospitals, Nottingham, United Kingdom) for critical reading of the manuscript and for providing strains collected as part of the European Union ARPAC project and to P. Nordmann (Hôpital de Bicêtre, le Kremlin-Bicêtre, France) for kindly providing the AYE strain.
Luísa C. S. Antunes was supported by a Ph.D. fellowship from the Portuguese Fundação para a Ciência e a Tecnologia (FCT) (grant SFRH/BD/43420/2008).
Footnotes
Published ahead of print 10 September 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Aisen P, Leibman A, Zweier J. 1978. Stoichiometric and site characteristics of the binding of iron to human transferrin. J. Biol. Chem. 253:1930–1937 [PubMed] [Google Scholar]
- 2. Antunes LC, Imperi F, Carattoli A, Visca P. 2011. Deciphering the multifactorial nature of Acinetobacter baumannii pathogenicity. PLoS One 6:e22674 doi:10.1371/journal.pone.0022674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antunes LC, Imperi F, Towner KJ, Visca P. 2011. Genome-assisted identification of putative iron-utilization genes in Acinetobacter baumannii and their distribution among a genotypically diverse collection of clinical isolates. Res. Microbiol. 162:279–284 [DOI] [PubMed] [Google Scholar]
- 4. Aperis G, et al. 2007. Galleria mellonella as a model host to study infection by the Francisella tularensis live vaccine strain. Microbes Infect. 9:729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baldoni D, Steinhuber A, Zimmerli W, Trampuz A. 2010. In vitro activity of gallium maltolate against Staphylococci in logarithmic, stationary, and biofilm growth phases: comparison of conventional and calorimetric susceptibility testing methods. Antimicrob. Agents Chemother. 54:157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banin E, et al. 2008. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. U. S. A. 105:16761–16766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bergogne-Bérézin E, Towner KJ. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bernstein LR. 1998. Mechanisms of therapeutic activity for gallium. Pharmacol. Rev. 50:665–682 [PubMed] [Google Scholar]
- 9. Braud A, Hannauer M, Mislin GL, Schalk IJ. 2009. The Pseudomonas aeruginosa pyochelin-iron uptake pathway and its metal specificity. J. Bacteriol. 191:3517–3525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braud A, Hoegy F, Jezequel K, Lebeau T, Schalk IJ. 2009. New insights into the metal specificity of the Pseudomonas aeruginosa pyoverdine-iron uptake pathway. Environ. Microbiol. 11:1079–1091 [DOI] [PubMed] [Google Scholar]
- 11. Braun V, Hantke K. 2011. Recent insights into iron import by bacteria. Curr. Opin. Chem. Biol. 15:328–334 [DOI] [PubMed] [Google Scholar]
- 12. Capone A, D'Arezzo S, Visca P, Petrosillo N; GRAB 2008. In vitro activity of tigecycline against multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 62:422–423 [DOI] [PubMed] [Google Scholar]
- 13. Coleman M, et al. 2010. In vitro antimicrobial activity of gallium maltolate against virulent Rhodococcus equi. Vet. Microbiol. 146:175–178 [DOI] [PubMed] [Google Scholar]
- 14. Collery P, Keppler B, Madoulet C, Desoize B. 2002. Gallium in cancer treatment. Crit. Rev. Oncol. Hematol. 42:283–296 [DOI] [PubMed] [Google Scholar]
- 15. D'Andrea MM, et al. 2009. Characterization of pABVA01, a plasmid encoding the OXA-24 carbapenemase from Italian isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 53:3528–3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. D'Arezzo S, et al. 2009. Epidemic multidrug-resistant Acinetobacter baumannii related to European clonal types I and II in Rome (Italy). Clin. Microbiol. Infect. 15:347–357 [DOI] [PubMed] [Google Scholar]
- 17. DeLeon K, et al. 2009. Gallium maltolate treatment eradicates Pseudomonas aeruginosa infection in thermally injured mice. Antimicrob. Agents Chemother. 53:1331–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Léséleuc L, Harris G, Kuolee R, Chen W. 23 July 2012. In vitro and in vivo biological activity of iron chelators and gallium nitrate against Acinetobacter baumannii. Antimicrob. Agents Chemother. doi:10.1128/AAC.00778-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 20. DuBois D, DuBois EF. 1916. Clinical calorimetry: formula to estimate approximate surface area if height and weight be known. Arch. Int. Med. 17:863–871 [Google Scholar]
- 21. Dunphy GB, Niven DF, Chadwick JS. 2002. Iron contributes to the antibacterial functions of the haemolymph of Galleria mellonella. J. Insect Physiol. 48:903–914 [DOI] [PubMed] [Google Scholar]
- 22. Emery T. 1986. Exchange of iron by gallium in siderophores. Biochemistry 25:4629–4633 [DOI] [PubMed] [Google Scholar]
- 23. Fecteau ME, et al. 2011. Antimicrobial activity of gallium nitrate against Mycobacterium avium subsp. paratuberculosis in neonatal calves. J. Vet. Intern. Med. 25:1152–1155 [DOI] [PubMed] [Google Scholar]
- 24. Funahashi T, et al. 2012. Identification and characterization of an outer membrane receptor gene in Acinetobacter baumannii required for utilization of desferricoprogen, rhodotorulic acid, and desferrioxamine B as xenosiderophores. Biol. Pharm. Bull. 35:753–760 [DOI] [PubMed] [Google Scholar]
- 25. Gaddy JA, et al. 2012. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect. Immun. 80:1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gales AC, Reis AO, Jones RN. 2001. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 39:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higgins PG, Stefanik D, Page MG, Hackel M, Seifert H. 2012. In vitro activity of the siderophore monosulfactam BAL30072 against meropenem-non-susceptible Acinetobacter baumannii. J. Antimicrob. Chemother. 67:1167–1169 [DOI] [PubMed] [Google Scholar]
- 28. Iacono M, et al. 2008. Whole-genome pyrosequencing of an epidemic multidrug-resistant Acinetobacter baumannii strain belonging to the European clone II group. Antimicrob. Agents Chemother. 52:2616–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Imperi F, et al. 2011. The genomics of Acinetobacter baumannii: insights into genome plasticity, antimicrobial resistance and pathogenicity. IUBMB Life 63:1068–1074 [DOI] [PubMed] [Google Scholar]
- 30. Jacobs AC, et al. 2012. Characterization of the Acinetobacter baumannii growth phase-dependent and serum responsive transcriptomes. FEMS Immunol. Med. Microbiol. 64:403–412 [DOI] [PubMed] [Google Scholar]
- 31. Kaneko Y, Thoendel M, Olakanmi O, Britigan BE, Singh PK. 2007. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Invest. 117:877–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kempf M, Rolain JM. 2012. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int. J. Antimicrob. Agents 39:105–114 [DOI] [PubMed] [Google Scholar]
- 33. Lee JW, Helmann JD. 2007. Functional specialization within the Fur family of metalloregulators. Biometals 20:485–499 [DOI] [PubMed] [Google Scholar]
- 34. Li J, et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601 [DOI] [PubMed] [Google Scholar]
- 35. MacKenzie FM, et al. 2005. Report of the Consensus Conference on Antibiotic Resistance; Prevention and Control (ARPAC). Clin. Microbiol. Infect. 11:938–954 [DOI] [PubMed] [Google Scholar]
- 36. Michalopoulos A, Falagas ME. 2010. Treatment of Acinetobacter infections. Expert Opin. Pharmacother. 11:779–788 [DOI] [PubMed] [Google Scholar]
- 37. Miller JH. 1972. Experiments in molecular genetics, p 252–255 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 38. Nemec A, Dijkshoorn L, van der Reijden TJ. 2004. Long-term predominance of two pan-European clones among multi-resistant Acinetobacter baumannii strains in the Czech Republic. J. Med. Microbiol. 53:147–153 [DOI] [PubMed] [Google Scholar]
- 39. Nerren JR, et al. 2011. Evaluation of the effect of gallium maltolate on fecal Salmonella shedding in cattle. J. Food Prot. 74:524–530 [DOI] [PubMed] [Google Scholar]
- 40. Olakanmi O, Britigan BE, Schlesinger LS. 2000. Gallium disrupts iron metabolism of mycobacteria residing within human macrophages. Infect. Immun. 68:5619–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Olakanmi O, et al. 2010. Gallium disrupts iron uptake by intracellular and extracellular Francisella strains and exhibits therapeutic efficacy in a murine pulmonary infection model. Antimicrob. Agents Chemother. 54:244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Payne SM. 1994. Detection, isolation, and characterization of siderophores. Methods Enzymol. 235:329–344 [DOI] [PubMed] [Google Scholar]
- 43. Peeters E, Nelis HJ, Coenye T. 2008. Resistance of planktonic and biofilm-grown Burkholderia cepacia complex isolates to the transition metal gallium. J. Antimicrob. Chemother. 61:1062–1065 [DOI] [PubMed] [Google Scholar]
- 44. Peleg AY, et al. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. 53:2605–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Perez F, et al. 2007. Global challenge of multidrug-resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Petersen PJ, Labthavikul P, Jones CH, Bradford PA. 2006. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J. Antimicrob. Chemother. 57:573–576 [DOI] [PubMed] [Google Scholar]
- 47. Raetz CR, Reynolds CM, Trent MS, Bishop RE. 2007. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76:295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reagan-Shaw S, Nihal M, Ahmad N. 2008. Dose translation from animal to human studies revisited. FASEB J. 22:659–661 [DOI] [PubMed] [Google Scholar]
- 49. Roca I, Espinal P, Vila-Farrés X, Vila J. 2012. The Acinetobacter baumannii oxymoron: commensal hospital dweller turned pan-drug-resistant menace. Front. Microbiol. 3:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rzhepishevska O, et al. 2011. The antibacterial activity of Ga3+ is influenced by ligand complexation as well as the bacterial carbon source. Antimicrob. Agents Chemother. 55:5568–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sambrook J, Fitsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, p 2344 Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 52. Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47–56 [DOI] [PubMed] [Google Scholar]
- 53. Smith MG, et al. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1J1. Plant Mol. Biol. 9:27–39 [DOI] [PubMed] [Google Scholar]
- 55. Stojiljkovic I, Baumler AJ, Hantke K. 1994. Fur regulon in Gram negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a Fur titration assay. J. Mol. Biol. 236:531–544 [DOI] [PubMed] [Google Scholar]
- 56. Thompson MG, Corey BW, Si Y, Craft DW, Zurawski DV. 30 July 2012. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. doi:10.1128/AAC.01197-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Towner KJ, et al. 2011. Distribution of intrinsic plasmid replicase genes and their association with carbapenem-hydrolyzing class D beta-lactamase genes in European clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:2154–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vallenet D, et al. 2008. Comparative analysis of Acinetobacters: three genomes for three lifestyles. PLoS One 3:e1805 doi:10.1371/journal.pone.0001805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van Dessel H, et al. 2004. Identification of a new geographically widespread multiresistant Acinetobacter baumannii clone from European hospitals. Res. Microbiol. 2:105–112 [DOI] [PubMed] [Google Scholar]
- 60. Visca P, Ciervo A, Sanfilippo V, Orsi N. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J. Gen. Microbiol. 139:1995–2001 [DOI] [PubMed] [Google Scholar]
- 61. Visca P, Seifert H, Towner KJ. 2011. Acinetobacter infection-an emerging threat to human health. IUBMB Life 63:1048–1054 [DOI] [PubMed] [Google Scholar]
- 62. Warrell RP, Jr, Skelos A, Alcock NW, Bockman RS. 1986. Gallium nitrate for acute treatment of cancer-related hypercalcemia: clinicopharmacological and dose response analysis. Cancer Res. 46:4208–4212 [PubMed] [Google Scholar]
- 63. Weaver KD, et al. 2008. Ga3+ as a mechanistic probe in Fe3+ transport: characterization of Ga3+ interaction with FbpA. J. Biol. Inorg. Chem. 13:887–898 [DOI] [PubMed] [Google Scholar]
- 64. Weinberg ED. 2009. Iron availability and infection. Biochim. Biophys. Acta 1790:600–605 [DOI] [PubMed] [Google Scholar]
- 65. Yamamoto S, Okujo N, Kataoka H, Narimatsu S. 1999. Siderophore-mediated utilization of transferrin- and lactoferrin-bound iron by Acinetobacter baumannii. J. Health Sci. 45:297–302 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.