Abstract
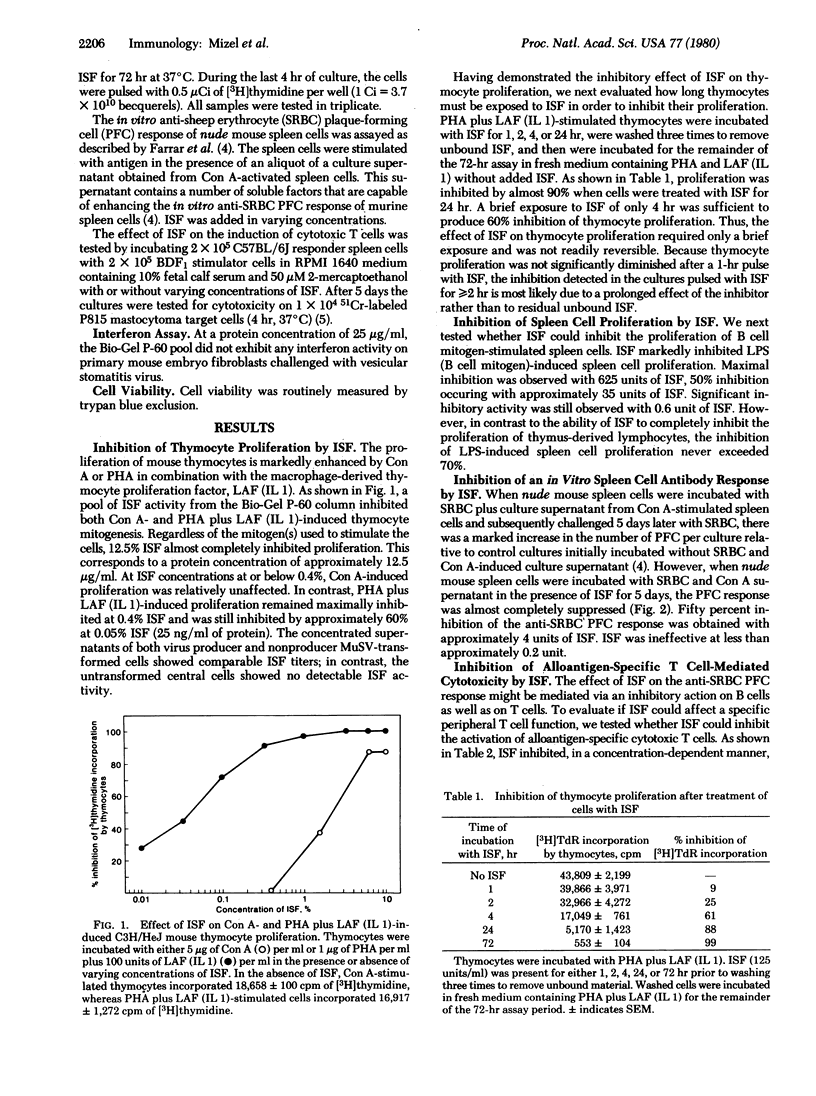
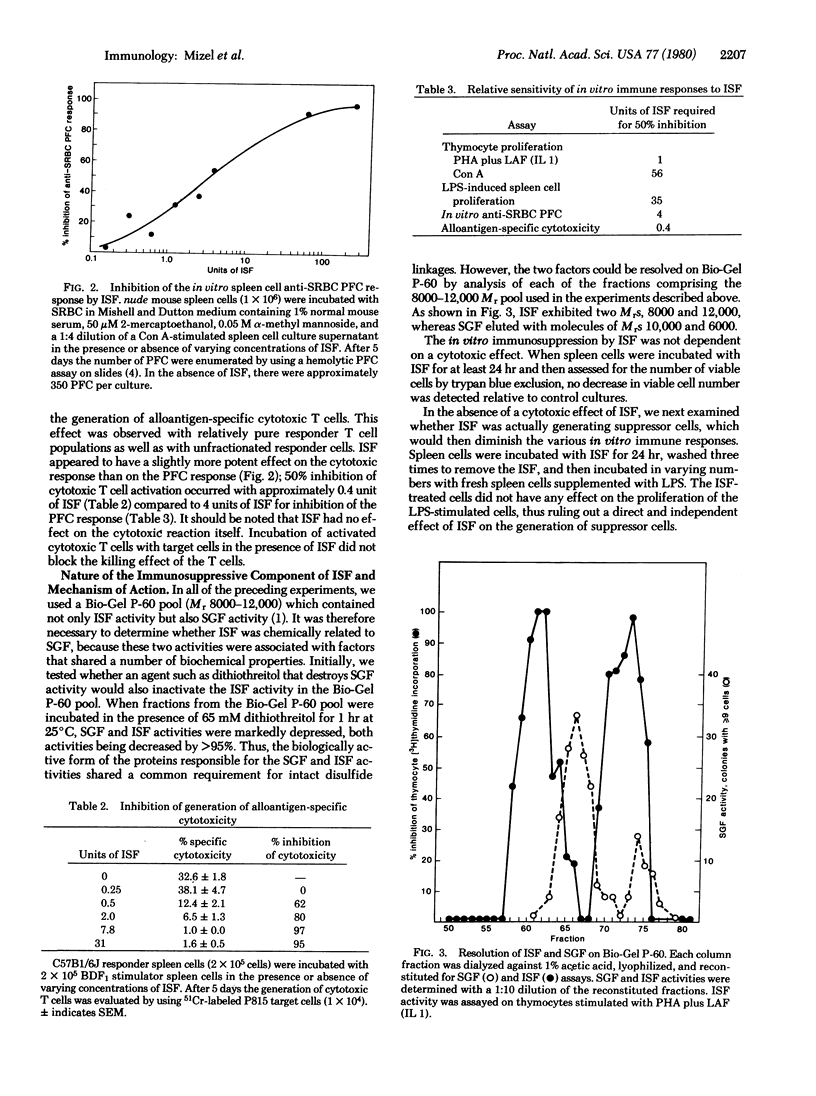
Murine sarcoma virus-transformed mouse fibroblasts produce potent immunosuppressive factors (ISF) in vitro. The partially purified ISF inhibited thymocyte proliferation induced by concanavalin A or phytohemagglutinin plus lymphocyte activating factor (Interleukin 1), lipopolysaccharide-induced spleen cell proliferation, the in vitro splenic anti-sheep erythrocyte plaque-forming cell response, and the generation of alloantigen-specific cytotoxic T cells. The effect of ISF on thymocyte proliferation was not readily reversible and required only a 4-hr exposure of the thymocytes to ISF to inhibit cell proliferation. Although ISF shares several biochemical properties with a murine sarcoma virus-transformed cell-derived sarcoma growth factor (e.g., acetic acid solubility and sensitivity to dithiothreitol), the two factors could be resolved by gel filtration on Bio-Gel P-60. Two peaks of ISF activity were found with apparent molecular weights of 12,000 and 8000. The results described here support the hypothesis that at least some of the ISF obtained from the serum of tumor-bearing hosts may be released by the tumor cells themselves. In view of the potent in vitro activity of the murine sarcoma virus-transformed fibroblast-derived ISF, it is quite possible that ISF-like molecules may play a role in subverting in vivo tumor rejection processes involving the immune system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrar J. J., Simon P. L., Koopman W. J., Fuller-Bonar J. Biochemical relationship of thymocyte mitogenic factor and factors enhancing humoral and cell-mediated immune responses. J Immunol. 1978 Oct;121(4):1353–1360. [PubMed] [Google Scholar]
- Farrar W. L., Mizel S. B., Farrar J. J. Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol. 1980 Mar;124(3):1371–1377. [PubMed] [Google Scholar]
- Mizel S. B. Physicochemical characterization of lymphocyte-activating factor (LAF). J Immunol. 1979 Jun;122(6):2167–2172. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Macrophage migratory dysfunction in cancer. A mechanism for subversion of surveillance. Am J Pathol. 1977 Sep;88(3):727–739. [PMC free article] [PubMed] [Google Scholar]
- Wood G. W. Suppression of Moloney sarcoma virus immunity following sensitization with attenuated virus. Cancer Res. 1976 Dec;36(12):4552–4557. [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]


