Abstract
Steroid-refractory graft-versus-host disease causes significant morbidity and mortality after allogeneic stem cell transplantation. The pathomechanism of steroid resistance is currently not understood, but it has been suggested that endothelial cell dysfunction plays a role. Endothelial thrombomodulin was quantified along with histological markers of epithelial damage and cytotoxic T cells in colon biopsies from 51 allografted patients, and retrospectively correlated with response to steroids and survival. Loss of endothelial thrombomodulin was the strongest predictor of response to steroids (P=0.02) and nonrelapse mortality (P=0.01) in multivariate analyses adjusting for T-cell infiltrates, histological grading, vessel density, disease status, donor type, and conditioning therapy. Our data provide evidence that at disease onset, loss of endothelial thrombomodulin expression rather than excessive T-cell infiltration associates with steroid-refractory graft-versus-host disease and mortality. Prospective histological investigations are now warranted to improve diagnosis and prognostication of this core complication of stem cell transplantation.
Key words: allogeneic stem cell transplantation, acute GvHD, thrombomodulin, endothelial cells, steroid-resistance, cytotoxic T cells, TIA-1
Introduction
Graft-versus-host disease (GvHD) is a severe complication of allogeneic stem cell transplantation (SCT). In particular, its steroid-refractory form represents a major reason for the significant morbidity and mortality associated with this procedure.1,2 Understanding the pathomechanism of steroid-refractoriness is one of the most important tasks of current transplantationrelated clinical research.
GvHD is triggered by alloreactive donor T lymphocytes that attack host epithelial cells in skin, liver and gastrointestinal tract.3 In addition, there is growing evidence that vascular endothelial cells can also be targeted and severely damaged during acute GvHD.4-6 Endothelial cell damage can manifest clinically as capillary leak syndrome, thrombotic microangiopathies, and/or veno-occlusive disease, i.e. typical complications observed in the context of SCT.5 Endothelial cell dysfunction can be detected by elevated serum thrombomodulin (sTM), and high sTM levels have been reported to be associated with complications after SCT.7,8
Thrombomodulin (TM) is expressed on the surface of endothelial cells and mediates an anti-apoptotic signal via activation of protein C and cleavage of PAR-1.9 Vessels of solid organ transplants undergoing acute rejection show depletion of endothelial TM,10 and so did endothelial cells in radiation induced colitis11 and inflammatory bowel disease.12,13 However, so far, no histological studies on endothelial TM expression during GvHD have been reported.
The aim of the present study was to assess endothelial TM expression in histological gut tissue sections obtained from patients at the onset of GvHD-typical symptoms in order to corroborate our serological findings of the endothelium being involved in the pathogenesis of steroid resistant GvHD.7 Therefore, we evaluated whether TM along with other immunohistochemical markers could be used to distinguish a steroid-resistant from a steroid-sensitive course of GvHD by means of histology.
Design and Methods
Patient eligibility
A total of 738 patients were allografted in our institution between 1998 and 2010. In a retrospective observational study, the rate of intestinal GvHD was 23.8% with 9.9% steroid-refractory courses. All patients eligible for the present study fulfilled the following criteria: 1) colon biopsies having been performed on suspicion of gastrointestinal tract GvHD; and 2) availability of surplus tissue samples for additional histopathological examinations as required for this study. Informed consent was obtained from all patients with approval of the local ethics committee.
Steroid-resistance and graft-versus-host disease grading
GvHD was clinically graded using standard criteria.14 Steroid-refractory GvHD was defined as lack of clinical response to standard steroid therapy (2 × 1 mg/kg), and the need for salvage immunosuppressive therapy or no salvage therapy due to early death
Histological procedures and immunohistochemistry
Histological GvHD grading was performed according to standard criteria.15-17 In addition, the following features were quantified on 3 representative high power fields (HPF): number of crypts, number of apoptotic bodies within the colonic epithelium, number of T-cell intracellular antigen 1 (TIA1)-positive cytotoxic T lymphocytes (CTL), and number of CD34-stained vessels. All histological tests were performed and evaluated independently by 2 pathologists (MA and TLo) who were blinded for the clinical data.
For immunohistochemistry, mouse-anti-thrombomodulin-mAb (clone 1009, 1:40, DAKO), mouse-anti-TIA1-mAb (clone 2G9, 1:200, Beckman-Coulter, Brea, USA), and mouse-anti-CD34-antibody (clone QBend10, 1:25, DAKO) were used along with the LSAB2 Kit (DAKO) and the chromogen AEC (3-amino-9-ethylcarbazole).
Endothelial thrombomodulin (eTM) expression
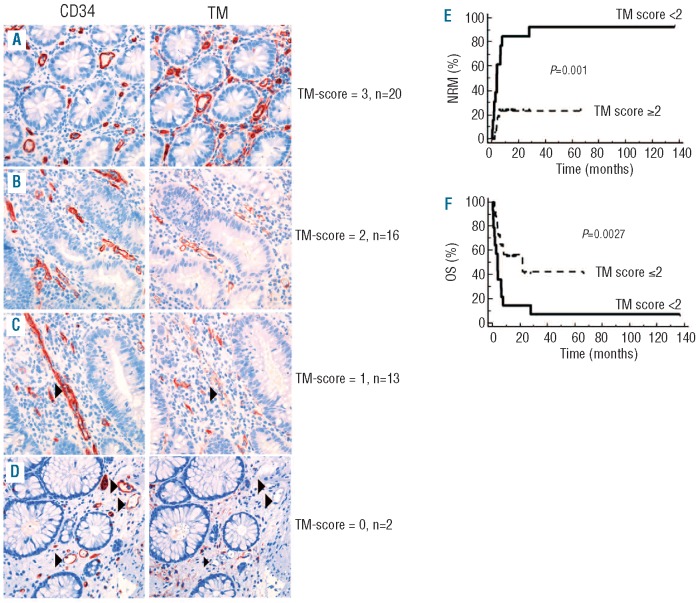
eTM expression was assessed using a semi-quantitative scoring system. eTM expression was evaluated in the same three areas of colon mucosa that were used for quantification of microvessels (numbers of microvessels per HPF). The intensity of eTM expression was assessed qualitatively and semi-quantitatively as negative (0), weak (1), moderate (2) and strong (3) for each HPF (Figure 1). The difference between weak and moderate was defined by continuous versus discontinuous eTM staining on a single vessel, i.e. cases with loss of TM in few endothelial cells resulting in a discontinuous staining of the vessel line were scored as ‘weak’ (1); cases with at least 3 completely TM negative vessels were scored as ‘negative’ (0). Means of 3 evaluated HPFs were calculated and used for further analysis.
Figure 1.
Loss of endothelial thrombomodulin expression in colon mucosa of GvHD-patients predicts NRM and OS. Representative examples of eTM in colon biopsies taken for suspected GVHD after SCT (400x original magnification). Matched serial sections of the same biopsy immunostained for eTM (left column) and CD34 (right column). Case (A), all CD34+ vessels strongly express eTM (eTM intensity=3). Case (B), moderate eTM (eTM intensity=2). Case (C), loss of eTM expression on single endothelial cells resulting in patchy staining was judged as weak expression (eTM intensity=1). Case (D), absent expression (eTM intensity= 0) required at least 3 vessels completely negative for eTM staining (corresponding vessels in CD34 and eTM-stained sections marked by arrowheads). eTM-positive mucosal myofibroblasts served as internal control. (E and F) Univariate analyses of influence on (E) non-relapse mortality (NRM) and (F) overall survival (OS) following biopsy comparing patients categorized according to the eTM score (eTM score<2: black line, eTM score≥2: dashed line) evaluated before start of immunosuppressive treatment for GvHD. x axis: time (months) following biopsy.
The normal range of eTM expression was evaluated on normal colon biopsies (n=20) taken from healthy individuals during a preventive endoscopy. The vessels of normal colon mucosa displayed moderate (18 of 20) or strong (2 of 20) TM expression. Accordingly, eTM expression was judged as negative if the TM-staining was weak (1) or absent (0).
Statistical analysis
Histological parameters were compared using either the Mann- Whitney or the Kruskal-Wallis test. Categorical data of patients' characteristics were compared using the two-tailed Fisher's exact test or the χ2 test. Multivariate analysis of histological parameters with regard to steroid response was made by logistical regression analysis.
Survival data were analyzed as of November 2010. Survival curve estimation for overall survival (OS) was made by the Kaplan and Meier method using the log rank test to compare survival times (time from biopsy to death from any cause). Non-relapse mortality (NRM) was analyzed according to the time from biopsy to death in the absence of relapse.18,19 Multivariate analysis of OS was made by Cox's regression model. Calculations were made using R (version 2.7.2), and SPSS (version 16.0, Chicago, USA) and MedCalc Software.
Results and Discussion
Patients
Fifty-one patients biopsied for suspected gut GvHD fulfilled the eligibility criteria for this study. Of these, 40 patients had histologically proven GvHD and were subsequently treated with high-dose steroids (1-2 mg/kg body weight/d). Response to high-dose steroid therapy was observed in 16 of 40 patients (steroid-sensitive group), whereas 24 of 40 patients did not respond and were subjected to salvage immunosuppressive therapy (steroidrefractory group). The remaining 11 patients had no histological signs of GvHD and served as control.
Patients' characteristics are shown in the Online Supplementary Table S1. There was no significant difference in disease- and transplantation-related variables between steroid-sensitive and steroid-refractory patients (Online Supplementary Appendix).
Graft-versus-host disease and clinical outcome
Comparison of steroid-refractory and steroid-sensitive intestinal GvHD patients at disease onset revealed a trend toward higher clinical grade GvHD (P=0.09) and a higher proportion of patients with multi-organ involvement in steroid-refractory disease: skin (P=0.08), liver (bilirubin levels, P=0.04) (Online Supplementary Table S2).
Patients with steroid-refractory GvHD had a significantly higher NRM (P<0.001) compared with patients who had steroid-sensitive GvHD or no GvHD, resulting in an overall survival disadvantage of the steroid-refractory group (P=0.02). There was no difference in NRM or OS between control patients and patients with steroid-sensitive GvHD (Online Supplementary Figure S1).
Histological grading and infiltrating cytotoxic T/NK-cells at disease onset
There was a significant difference in numbers of apoptotic bodies per crypt between control patients without histological evidence of GvHD (median 0.0/crypt) and patients with either sensitive (median 0.2/crypt) or refractory (median 0.6/crypt) GvHD (P<0.001) (Online Supplementary Figure S2A). There was no difference in the degree of crypt loss between control patients without histological evidence of GvHD (median number of crypts 19.4/HPF) (Online Supplementary Figure S2B) and patients with either sensitive (median 16/HPF) or refractory (median 16/HPF) GvHD. There was no difference in histological GVHD grading at disease onset between sensitive and refractory patients (P=0.74) (Online Supplementary Figure S2C).
The numbers of infiltrating T-cell intracellular antigen (TIA)-1 positive cytotoxic T/NK-cells were stained and quantified immunohistochemically. TIA-1 positive cytotoxic cells were found to infiltrate the epithelial cell layer and associate with apoptotic bodies inside the crypts (Online Supplementary Figure S3). However, there was no significant difference in counts of TIA-1 positive cytotoxic cells between biopsy samples of steroid-sensitive and steroidrefractory patients.
Loss of endothelial thrombomodulin correlates with steroid-refractory graft-versus-host disease and predicts survival
We quantified mucosal microvessels by CD34 expression analyses and assessed TM expression on endothelial cells (Online Supplementary Figure S4A-D). Loss of eTM expression was observed in 14 of 24 steroid-refractory patients (58%), while eTM expression was maintained in all steroid-sensitive patients (Online Supplementary Figure S4B); (Fisher's exact test, P<0.001, Online Supplementary Table S3). In cases classified as eTM-negative, loss of thrombomodulin was detectable in every single biopsy, independent of the localization.
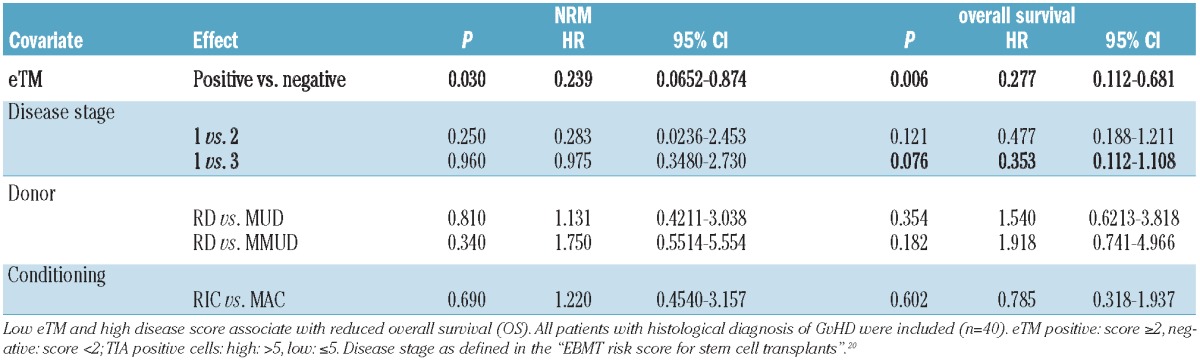
Multivariate analyses demonstrate that eTM expression is an independent predictor of response to steroid therapy within the group of patients with histologically proven GvHD (Table 1). Using clinical parameters as confounders, we found that loss of eTM expression was significantly associated with NRM (P=0.030) and OS (P=0.006) in patients diagnosed for intestinal GvHD (Table 2, Figure 1E and F).
Table 1.
Multivariable analysis with histological variables for end point steroid response.
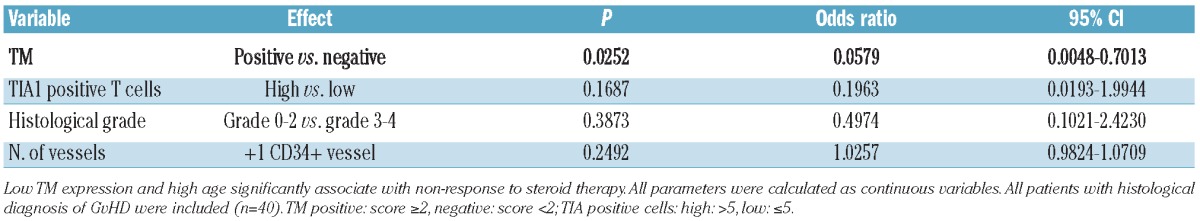
Table 2.
Multivariable analysis with clinical variables for end points NRM and overall survival.
Discussion
Although it is well documented that endothelial damage can play a role in GvHD pathogenesis,4,21,22 only recently has evidence been found that endothelial involvement may be a hallmark of steroid-refractory GvHD, whereas its contribution in sensitive GvHD is much less prominent.7 These findings, however, were largely based on serological analyses focusing on sTM and angiopoietin-2.7
The present histological study identified loss of endothelial TM as a negative predictor of non-response to steroid therapy and, consecutively, of NRM. The overlap between the patient populations analyzed in this and the preceding serological study was small due to limited sample availability for immunohistochemistry and serology. Nevertheless, both analyses were in agreement in suggesting that endothelial damage as indicated by loss of TM expression is almost completely restricted to refractory GvHD, whereas there is no difference in T-cell activity between patients with refractory and those with sensitive GvHD.
It is known that loss of eTM expression represents endothelial dysfunction and this has previously been reported in different contexts, such as radiogenic colitis,11 inflammatory bowel disease12,13 or rejection of transplanted solid organs.10,23 Loss of eTM was shown to correlate with enhanced sTM levels.12,13 Therefore, loss of eTM expression may be caused by different mechanisms and is neither specific nor predictive for GvHD. However, the association between steroid resistance and eTM loss suggests that endothelial dysfunction is an important player in the pathogenesis of steroid resistance.
Although the differences observed between non-GvHD controls and GvHD patients (i.e. apoptotic bodies, number of TIA-1 cells, number of vessels) support the validity of our findings, we are well aware that this study included only a small number of patients, and confirmation of our findings in larger and independent patient cohorts is mandatory before any changes in clinical practice can be developed from these results.
In summary, these data support the hypothesis that endothelial cell dysfunction is associated with steroid resistance in GvHD. These findings require validation in prospective trials and may open up exciting new avenues to develop novel interventional approaches to prevent this core complication of allogeneic SCT and to promote its timely diagnosis.
Acknowledgments
this work was supported by grants from the Helmholtz-Alliance for Immunotherapy of Cancer and the Wilhelm-Sander-Stiftung. The authors thank Charlotte Wahrendorf and the team of NCT-Gewebebank Heidelberg for their technical assistance.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Bolanos-Meade J, Vogelsang GB. Novel strategies for steroid-refractory acute graftversus- host disease. Curr Opin Hematol. 2005;12(1):40-4 [DOI] [PubMed] [Google Scholar]
- 2.Levine JE, Logan B, Wu J, Alousi AM, Ho V, Bolanos-Meade J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara JL, Levine JE, Reddy P, Holler E. Graftversus-host disease. Lancet. 2009;373(9674):1550-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holler E, Kolb HJ, Hiller E, Mraz W, Lehmacher W, Gleixner B, et al. Microangiopathy in patients on cyclosporine prophylaxis who developed acute graft-versus- host disease after HLA-identical bone marrow transplantation. Blood. 1989;73(7):2018-24 [PubMed] [Google Scholar]
- 5.Nurnberger W, Michelmann I, Burdach S, Gobel U. Endothelial dysfunction after bone marrow transplantation: increase of soluble thrombomodulin and PAI-1 in patients with multiple transplant-related complications. Ann Hematol. 1998;76(2):61-5 [DOI] [PubMed] [Google Scholar]
- 6.Penack O, Socie G, van den Brink MR. The importance of neovascularization and its inhibition for allogeneic hematopoietic stem cell transplantation. Blood. 2011;117(16):4181-9 [DOI] [PubMed] [Google Scholar]
- 7.Luft T, Dietrich S, Falk CS, Benner A, Conzelmann M, Hess M, et al. Steroid-refractory GVHD: T-cell attack within a vulnerable endothelial system. Blood. 2011;118(6):1685-92 [DOI] [PubMed] [Google Scholar]
- 8.Testa S, Manna A, Porcellini A, Maffi F, Morstabilini G, Denti N, et al. Increased plasma level of vascular endothelial glycoprotein thrombomodulin as an early indicator of endothelial damage in bone marrow transplantation. Bone Marrow Transplant. 1996;18(2):383-8 [PubMed] [Google Scholar]
- 9.Isermann B, Sood R, Pawlinski R, Zogg M, Kalloway S, Degen JL, et al. The thrombomodulin-protein C system is essential for the maintenance of pregnancy. Nat Med. 2003;9(3):331-7 [DOI] [PubMed] [Google Scholar]
- 10.Labarrere CA, Pitts D, Halbrook H, Faulk WP. Natural anticoagulant pathways in normal and transplanted human hearts. J Heart Lung Transplant. 1992;11(2 Pt 1):342-7 [PubMed] [Google Scholar]
- 11.Richter KK, Fink LM, Hughes BM, Sung CC, Hauer-Jensen M. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy? Radiother Oncol 1997;44(1):65-71 [DOI] [PubMed] [Google Scholar]
- 12.Boehme MW, Autschbach F, Zuna I, Scherbaum WA, Stange E, Raeth U, et al. Elevated serum levels and reduced immunohistochemical expression of thrombomodulin in active ulcerative colitis. Gastroenterology. 1997;113(1):107-17 [DOI] [PubMed] [Google Scholar]
- 13.Faioni EM, Ferrero S, Fontana G, Gianelli U, Ciulla MM, Vecchi M, et al. Expression of endothelial protein C receptor and thrombomodulin in the intestinal tissue of patients with inflammatory bowel disease. Crit Care Med. 2004;32(5 Suppl):S266-70 [DOI] [PubMed] [Google Scholar]
- 14.Sullivan K. Graft-versus-host-disease. In: Thomas ED, ed Hematopoietic Cell Transplantation. Boston MA: Blackwell Science, 1999515-36 [Google Scholar]
- 15.Epstein RJ, McDonald GB, Sale GE, Shulman HM, Thomas ED. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980;78(4):764-71 [PubMed] [Google Scholar]
- 16.Lerner KG, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6(4):367-71 [PubMed] [Google Scholar]
- 17.Washington K, Jagasia M. Pathology of graftversus-host disease in the gastrointestinal tract. Hum Pathol. 2009;40(7):909-17 [DOI] [PubMed] [Google Scholar]
- 18.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381-7 [DOI] [PubMed] [Google Scholar]
- 19.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45(9):1388-95 [DOI] [PubMed] [Google Scholar]
- 20.Gratwohl A, Stern M, Brand R, Apperley J, Baldomero H, de Witte T, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer. 2009;115(20):4715-26 [DOI] [PubMed] [Google Scholar]
- 21.Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(2):129-38 [DOI] [PubMed] [Google Scholar]
- 22.Ertault-Daneshpouy M, Leboeuf C, Lemann M, Bouhidel F, Ades L, Gluckman E, et al. Pericapillary hemorrhage as criterion of severe human digestive graft-versus-host disease. Blood. 2004;103(12):4681-4 [DOI] [PubMed] [Google Scholar]
- 23.Labarrere CA, Faulk WP. Microvascular perturbations in human allografts: analogies in preeclamptic placentae. Am J Reprod Immunol. 1992;27(3-4):109-16 [DOI] [PubMed] [Google Scholar]