Abstract
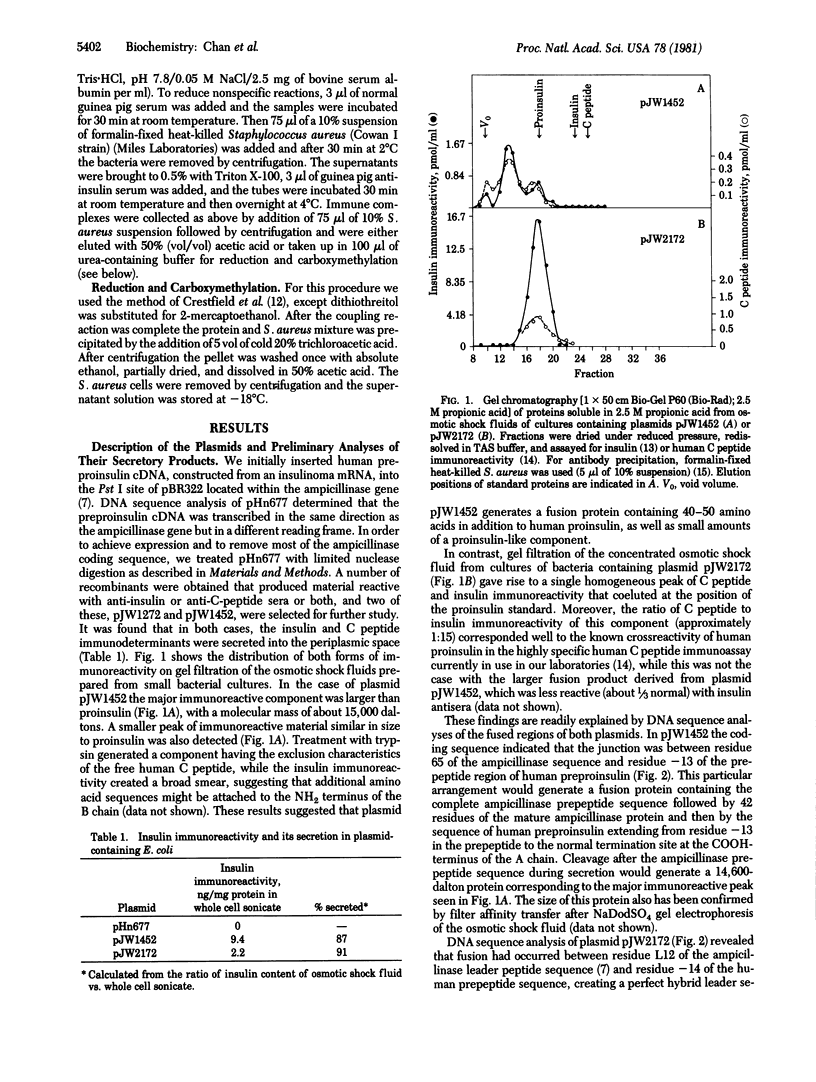
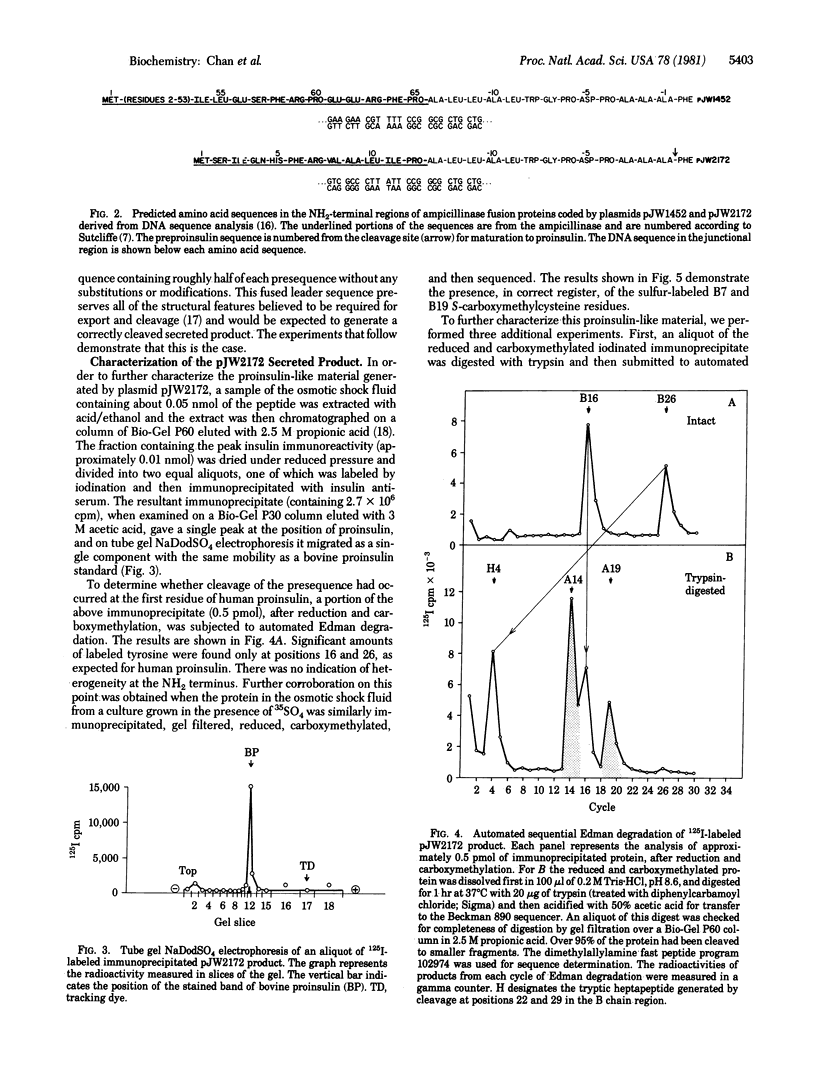
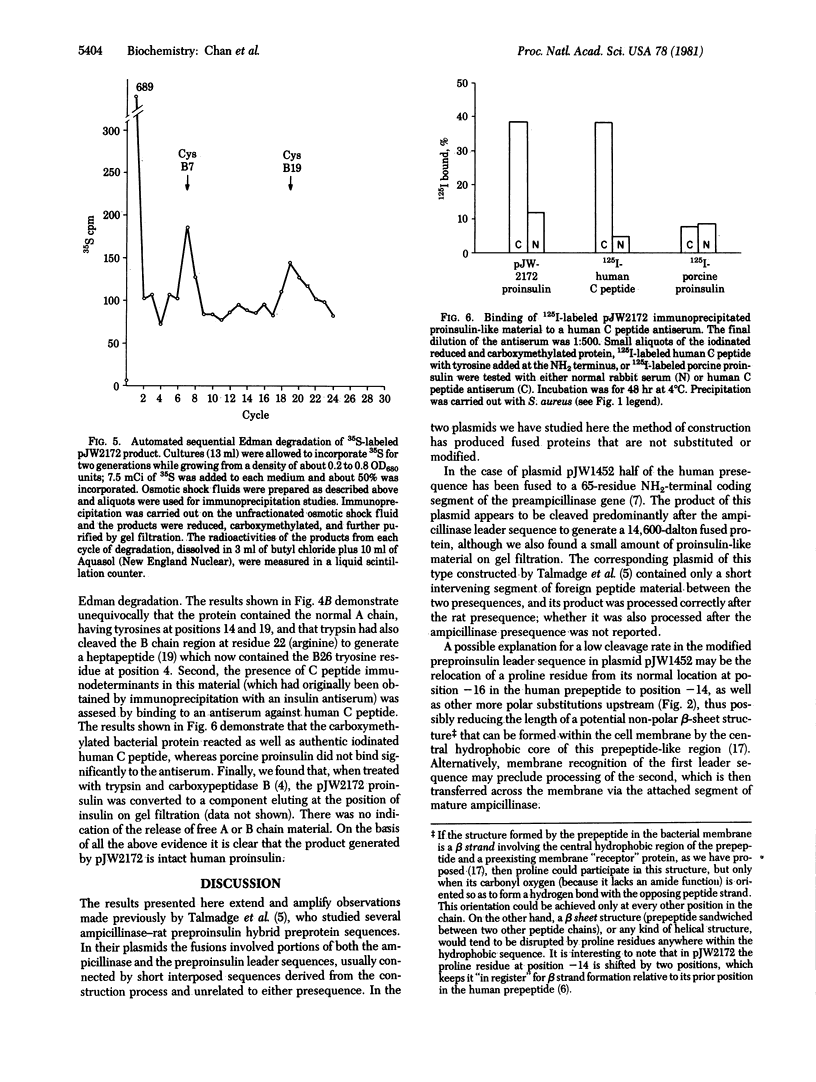
A plasmid containing human preproinsulin cDNA inserted into the endonuclease Pst I site of the ampicillinase gene of plasmid pBR322 was modified by excision of large portions of the ampicillinase-coding region to produce a variety of gene fusion combinations, many of which generated proteins detectable with antisera to insulin or human C peptide. In one case a perfect hybrid of the NH2-terminal half of the leader sequence of ampicillinase (residues -23 to -12) with the human preproinsulin prepeptide beginning at residue -13 was formed; the result was the synthesis and secretion of human proinsulin into the periplasmic space. We have characterized this protein immunologically and also by labeling it biosynthetically or by iodination followed by immunoprecipitation and automated amino acid sequence analysis. It contains the A and B chain regions of insulin as well as specific human C peptide immunodeterminants and is convertible to an insulin-like component by tryptic digestion. These results demonstrate that human proinsulin can be produced by bacteria and that this biosynthetic approach should prove feasible for the production of adequate amounts of human proinsulin for a variety of clinical studies and human insulin for therapeutic purposes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Swain W. F., Pictet R., Cordell B., Goodman H. M., Rutter W. J. Nucleotide sequence of a cDNA clone encoding human preproinsulin. Nature. 1979 Nov 29;282(5738):525–527. doi: 10.1038/282525a0. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER F. H., BAUM W. E. Rates of production of alanine and heptapeptide and of loss of biological activity during digestion of insulin with trypsin. J Biol Chem. 1962 Feb;237:409–412. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Chan S. J., Keim P., Steiner D. F. Cell-free synthesis of rat preproinsulins: characterization and partial amino acid sequence determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1964–1968. doi: 10.1073/pnas.73.6.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance R. E., Kroeff E. P., Hoffmann J. A., Frank B. H. Chemical, physical, and biologic properties of biosynthetic human insulin. Diabetes Care. 1981 Mar-Apr;4(2):147–154. doi: 10.2337/diacare.4.2.147. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C., Markussen J., Heding L. G., Naithani V. K., Kuzuya H., Blix P., Horwitz D. L., Rubenstein A. H. Characterization of seven C-peptide antisera. Diabetes. 1978;27 (Suppl 1):170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray O., Chang S. Molecular cloning and expression of Bacillus licheniformis beta-lactamase gene in Escherichia coli and Bacillus subtilis. J Bacteriol. 1981 Jan;145(1):422–428. doi: 10.1128/jb.145.1.422-428.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmler W., Peterson J. D., Steiner D. F. Studies on the conversion of proinsulin to insulin. I. Conversion in vitro with trypsin and carboxypeptidase B. J Biol Chem. 1971 Nov 25;246(22):6786–6791. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Steiner D. F., Horwitz D. L., Mako M. E., Block M. B., Starr J. I., Kuzuya H., Melani F. Clinical significance of circulating proinsulin and C-peptide. Recent Prog Horm Res. 1976;33:435–475. doi: 10.1016/b978-0-12-571133-3.50017-9. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Welbourne W. P., Mako M., Melani F., Steiner D. F. Comparative immunology of bovine, porcine and human proinsulins and C-peptides. Diabetes. 1970 Aug;19(8):546–553. doi: 10.2337/diab.19.8.546. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Clark J. L. The spontaneous reoxidation of reduced beef and rat proinsulins. Proc Natl Acad Sci U S A. 1968 Jun;60(2):622–629. doi: 10.1073/pnas.60.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Kaufman J., Gilbert W. Bacteria mature preproinsulin to proinsulin. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3988–3992. doi: 10.1073/pnas.77.7.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]


