Abstract
Pancreatic islets are critical for glucose homeostasis via the regulated secretion of insulin and other hormones. We propose a novel mechanism that regulates insulin secretion from β-cells within mouse pancreatic islets: a dopaminergic negative feedback acting on insulin secretion. We show that islets are a site of dopamine synthesis and accumulation outside the central nervous system. We show that both dopamine and its precursor l-dopa inhibit glucose-stimulated insulin secretion, and this inhibition correlates with a reduction in frequency of the intracellular [Ca2+] oscillations. We further show that the effects of dopamine are abolished by a specific antagonist of the dopamine receptor D3. Because the dopamine transporter and dopamine receptors are expressed in the islets, we propose that cosecretion of dopamine with insulin activates receptors on the β-cell surface. D3 receptor activation results in changes in intracellular [Ca2+] dynamics, which, in turn, lead to lowered insulin secretion. Because blocking dopaminergic negative feedback increases insulin secretion, expanding the knowledge of this pathway in β-cells might offer a potential new target for the treatment of type 2 diabetes.
Insulin secretion from pancreatic β-cells is a fundamental process that is required to maintain glucose homeostasis in a healthy individual. Impairments in the mechanisms that control insulin secretion result in complications ranging from glucose intolerance to overt type 2 diabetes (1–3). The islets of Langerhans are interspersed in the exocrine pancreas and are made up of four principal cell types (α, β, δ, and PP) that synthesize hormones (glucagon, insulin, somatostatin, and pancreatic polypeptide, respectively) required to control blood glucose concentration (4). The islet is a micro-organ. The cells in an islet are chemically and electrically connected, and they affect each other by paracrine signaling (5–7). When blood glucose concentration increases, the islets respond by secreting insulin from the β-cells. On the other hand, when glucose concentration drops, insulin secretion decreases and the counteracting hormone, glucagon, is secreted from the α-cells. This basic response is modulated by many other stimuli (8, 9). The islets receive multiple signals from the bloodstream and from sympathetic and parasympathetic nerve terminals (10). Each of these signals can act through specific receptors expressed in the islet cells (8, 9). At any given time, the β-cells integrate all the stimuli to produce a finely tuned insulin secretion rate.
Dopamine is a neurotransmitter that plays a fundamental role in many specific regions of the brain (11). Loss of dopaminergic neurons results in Parkinson's disease (12), and imbalance in dopamine signaling is thought to be one cause of schizophrenia (13–15). Dopamine signals through a family of five G protein-coupled receptors named D1, D2, D3, D4, and D5 (11). Although the first report of biogenic amines in pancreatic islets dates to 1963 (16), we still have little understanding of the mechanism of action and physiological relevance of dopamine in the mouse pancreatic islet. Islets cells were classified as amine precursor uptake and decarboxylation cells based on cytochemical and ultrastructural similarities with other polypeptide hormone-producing cells (17). Our appreciation of dopamine action in the islet has been complicated because both stimulation and inhibition of insulin secretion have been reported: rat islets perfused with l-dopa showed potentiated glucose-stimulated insulin secretion (GSIS) (18), whereas mice injected with l-dopa showed lowered GSIS (19, 20). Studies in mouse showed that acute administration of l-dopa produced dopamine accumulation in islets, but the site of dopamine synthesis was not addressed (19, 21). Because of species variation, studies performed in golden hamster, guinea pig, and rabbit (22–27) cannot be automatically extended to the mouse (28). However, aromatic l-amino-acid decarboxylase and monoamine oxidase activities were characterized in mouse islet homogenates (20, 29, 30), but no direct measurement of dopamine accumulation was done. Vesicular monoamine transporter type 2 has also been reported in rodent islets (31, 32). Rubí et al. (33) first showed expression of dopamine receptor D2 (DRD2) in the rat β-cell tumor cell line INS1-E, and described the inhibition of cytoplasmic Ca2+ activity and insulin secretion by dopamine. Two following studies reported opposite roles for DRD2 in regulating insulin secretion showing both inhibition and stimulation (34, 35). Despite the presence of dopaminergic machinery in the β-cells, however, it is not known where dopamine could originate to stimulate islets in a living mouse. Dopamine does not cross the blood-brain barrier, and although there are peripheral sources of dopamine in the body (36–39), circulating dopamine levels in the plasma are too low to activate its receptors (38, 40, 41). Although there is a high degree of innervation in the islets, there are no reports of dopaminergic neurons innervating them (10).
We tested the hypothesis that islet β-cells synthesize dopamine from circulating l-dopa and that a dopaminergic feedback loop modulates the response of β-cells to glucose by regulated secretion of dopamine from the same islet cells. Although parts of this hypothesis have been previously proposed, it has not been rigorously tested in intact islets. In neurons, dopamine is more than a neurotransmitter; it is a neuromodulator that “alters the responses of target neurons to other neurotransmitters in a manner that depends on the functional status of these neurons” (42). We propose a similar function where dopamine modulates β-cell responses to glucose. During GSIS, dopamine is cosecreted, and it signals to the β-cells through a dopamine receptor, which exerts a continuous inhibition on insulin secretion. To test this model, we measured the dopamine content of islets, and our results confirm that the islet of Langerhans is a dopamine-producing tissue. Dopamine secretion from isolated islets shows that this secretion is regulated and that it is not a passive diffusion event. We confirmed by Western blot that dopamine receptors and the dopamine transporter (DAT) are present in the islet cells, and we show that dopamine receptors are localized on the plasma membrane of β-cells. Measurements of the intracellular calcium concentration ([Ca2+]i) oscillations in isolated islets show that the oscillation frequency strongly correlates with insulin secretion, which suggests that dopamine modulates GSIS by regulating the frequency of [Ca2+]i oscillations. Also we show that the effect of dopamine on [Ca2+]i oscillations can be prevented by blocking the D3 receptor but not by blocking the D2. The same results are observed when measuring the effects of dopamine on GSIS. Indeed blocking the D3 receptor on naïve untreated islets significantly increases their GSIS, supporting the hypothesis that this negative feedback is present in vivo. Similarly measurement of the effect of l-dopa on GSIS show that we can see an effect at concentrations that are comparable to the reported concentration in the plasma, thus supporting the hypothesis that this dopaminergic modulation of GSIS is present in vivo too.
Results
The effects of l-dopa on pancreatic islet dopamine content in vitro and in vivo
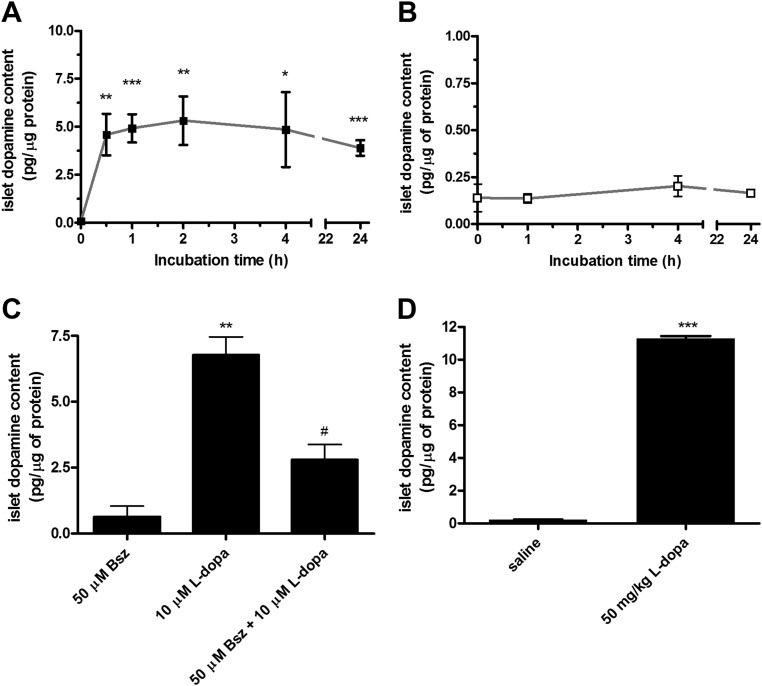
Incubation of isolated islets in medium supplemented with 10 μm l-dopa produced a rapid and saturable increase in their dopamine content (Fig. 1A) as compared with incubation in regular medium that left the dopamine content unchanged over the 24 h of culture (Fig. 1B). When measured immediately after isolation, the amount of dopamine in the islets was 0.161 ± 0.020 pg/μg of protein (n = 13), and it increased to 4.76 ± 0.48 pg/μg of protein (n = 28) after incubation with l-dopa for 30 min.
Fig. 1.
The effects of l-dopa on pancreatic islet dopamine content in vitro and in vivo. A and B, The dopamine content of pancreatic islets was measured immediately after isolation and after incubation in presence (■) (A) (n = 4–7) or absence (□) (B) (n = 3–4) of 10 μm l-dopa for different time intervals. *, P < 0.05; **, P < 0.01; ***, P < 0.001 vs. incubation time = 0 h. C, The dopamine content of pancreatic islets was measured after a 30-min incubation in presence of 50 μm benserazide, 10 μm l-dopa, and a combination of 50 μm benserazide + 10 μm l-dopa (n = 2–3). **, P < 0.01 vs. 50 μm benserazide; #, P < 0.05 vs. 10 μm l-dopa. D, The dopamine content of pancreatic islets was measured immediately after isolation from mice that have received an ip injection of saline solution or 50 mg/kg of l-dopa 30 min before surgery (n = 2). ***, P < 0.001 vs. saline.
Aromatic l-amino-acid decarboxylase is the enzyme that converts cytosolic l-dopa into dopamine, and its presence and activity in islet homogenates has been reported (29, 30); the drug benserazide inhibits aromatic l-amino-acid decarboxylase activity. We incubated isolated islets in medium with 10 μm l-dopa in the presence and absence of 50 μm benserazide to show that isolated islets were indeed producing dopamine from the available l-dopa in the medium; the results show that 50 μm benserazide produced a 60% reduction of the l-dopa induced dopamine accumulation (Fig. 1C).
Next, we measured the l-dopa-induced dopamine accumulation in the pancreatic islets in vivo. We treated mice with an ip injection of l-dopa at a dose of 50 mg/kg, or an injection of physiological saline, and we harvested pancreatic islets 30 min after the injection to measure their dopamine content. l-dopa-injected mice had significantly higher islet dopamine content compared with the saline-injected animals [11.29 ± 0.14 pg/μg of protein (n = 2) compared with 0.228 ± 0.025 pg/μg of protein (n =2)] (Fig. 1D), and this value is twice as high as the one measured in the in vitro experiments.
The effects of l-dopa-induced dopamine accumulation on GSIS
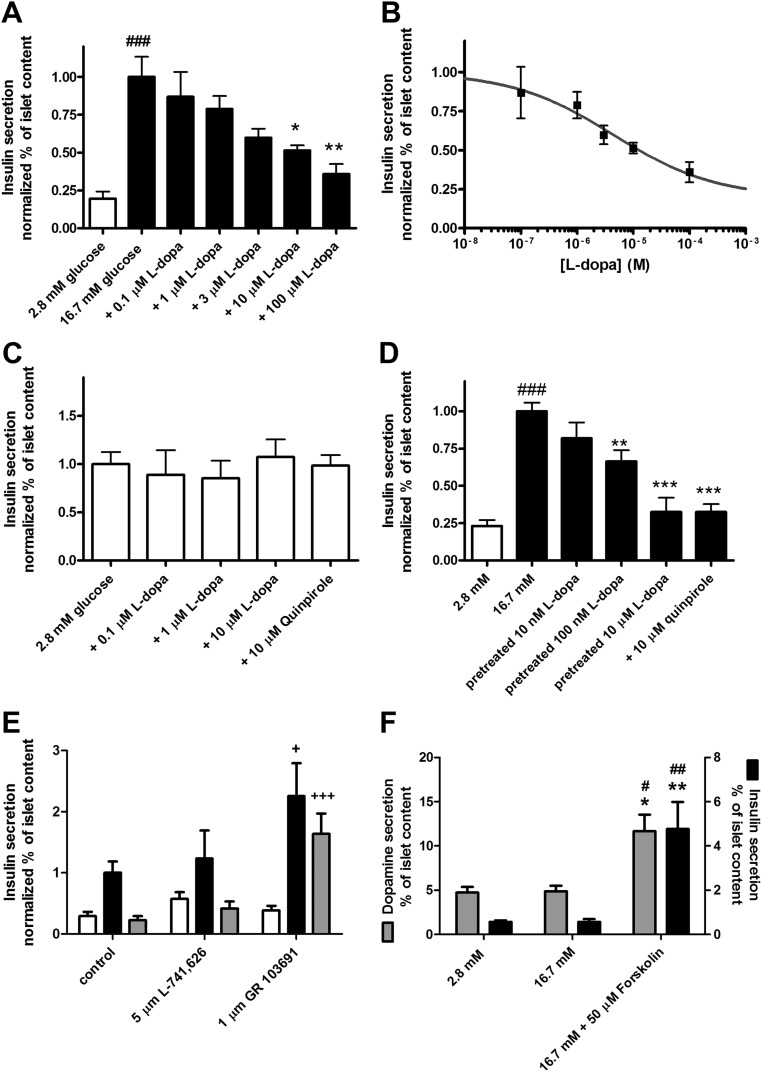
We next investigated the effects of l-dopa-induced dopamine accumulation on GSIS from the intact pancreatic islets. Static incubation experiments (Fig. 2A) show that increasing concentrations of l-dopa (0.1 μm, 1 μm, 10 μm, 100 μm) mixed with glucose stimulus (16.7 mm glucose) significantly inhibited GSIS. This effect was dose dependent with an estimated EC50 = 4.4 μm (Fig. 2B). In nonstimulatory conditions (2.8 mm glucose) l-dopa treatment did not change insulin secretion (Fig. 2C); addition of 10 μm quinpirole (a D2/D3/D4 agonist) to the 2.8 mm glucose did not change insulin secretion compared with the untreated islets (Fig. 2C).
Fig. 2.
The effects of l-dopa-induced dopamine accumulation and of dopamine receptor antagonists on glucose-stimulated insulin secretion. A, Insulin secretion measured at 2.8 mm glucose, 16.7 mm glucose, and 16.7 mm glucose plus increasing concentrations of l-dopa as indicated (n = 5–13). B, Sigmoidal dose-response curve fit of insulin secretion stimulated by 16.7 mm glucose in presence of 0.1 μm, 1 μm, 3 μm, 10 μm, and 100 μm l-dopa; R2 = 0.97; gray line indicates, best-fit EC50 = 4.4 μm (n = 5–6). C, Insulin secretion measured at 2.8 mm glucose, and 2.8 mm glucose + 0.1 μm, 1 μm, 10 μm l-dopa, and 10 μm quinpirole (n = 4–12). D, Insulin secretion measured at 2.8 mm glucose, 16.7 mm glucose, 16.7 mm glucose after pretreatment with 10 nm, 100 nm and 10 μm l-dopa, 16.7 mm glucose + 10 μm quinpirole (n = 4–11). E, Insulin secretion measured at 2.8 mm glucose (white bar), 16.7 mm glucose (black bar), and 16.7 mm glucose + 10 μm dopamine (gray bar). The selective antagonist for dopamine receptor D2 (L-741,626), or for dopamine receptor D3 (GR 103691) was added to the three stimuli as indicated (n = 6–8). F, Dopamine secretion and insulin secretion from pancreatic islets measured at 2.8 mm glucose, 16.7 mm glucose, 16.7 mm glucose + 50 μm forskolin (n = 4–5). ##, P < 0.01; ###, P < 0.0001 vs. 2.8 mm glucose; *, P < 0.05; **, P < 0.01; ***, P < 0.0001 vs. 16.7 mm glucose; +, P < 0.05; +++, P < 0.001 vs. the respective untreated control.
In our hypothesis, the inhibition of GSIS is produced via the increased dopamine level in the islets and the subsequent increase in dopamine signaling during insulin secretion. We performed insulin secretion experiments to measure GSIS in the presence of elevated islet dopamine content but in the absence of l-dopa. In contrast to what was done for Fig. 2A, the islets were treated with l-dopa before the insulin secretion experiment to increase their dopamine content. Islets were pretreated with three different concentrations of l-dopa for 60 min and then stimulated with glucose alone. Pretreatment with 100 nm and 10 μm l-dopa significantly inhibited GSIS, and addition of the D2/D3/D4 agonist quinpirole produced a comparable inhibition of GSIS (Fig. 2D). Based on previous results (33), we expected that the D2 receptor would play an important role in GSIS inhibition. We used selective antagonists for the D2 or the D3 receptor to determine their effects on GSIS on untreated islets and their efficacy in blocking the inhibitory action of dopamine (Fig. 2E). The D2 antagonist did not change the insulin secretion at 2.8 mm glucose and at 16.7 mm glucose when compared with the untreated control islets. Also the D2 antagonist did not block the effect of 10 μm dopamine added to the 16.7 mm glucose. On the contrary the D3 antagonist produced a statistically significant 2-fold increase in insulin secretion at 16.7 mm glucose, and it abolished the inhibitory effect of 10 μm dopamine.
We quantified the amount of secreted dopamine during GSIS in islets pretreated with 10 μm l-dopa, and stimulated with three different conditions (Fig. 2F). When stimulated with 2.8 mm glucose, the islets secreted 4.73 ± 0.65% of their dopamine content (n = 5). Stimulation with 16.7 mm glucose resulted in the secretion of 4.88 ± 0.61% of islet dopamine content (n = 5). When the islets were maximally stimulated with 16.7 mm glucose and 50 μm forskolin, they secreted 11.7 ± 1.9% of their dopamine content (n = 6). The insulin secretion from the same experimental groups of islets (Fig. 2F) showed an inhibition of GSIS, with no statistically significant difference between insulin secretion at 2.8 mm glucose vs. 16.7 mm glucose [0.57 ± 0.07% of islet insulin content (n =5) and 0.57 ± 0.12% of islet insulin content (n = 5)], consistent with Fig. 2D. Insulin secretion was maximally stimulated by 16.7 mm glucose + 50 μm forskolin (4.8 ± 1.2% of islet insulin content; n = 4).
The effects of dopamine and l-dopa on the redox state of pancreatic islets
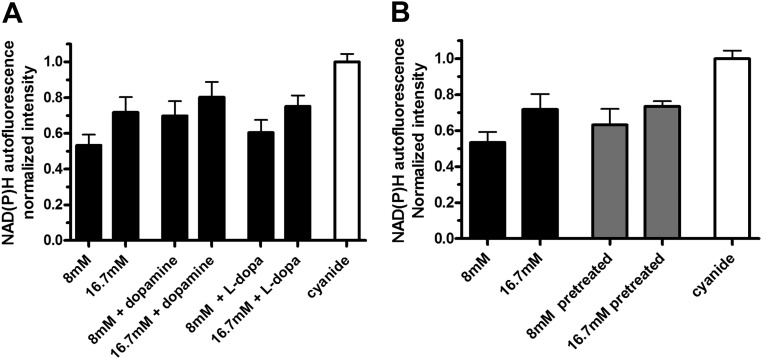
Having observed that l-dopa and dopamine inhibited GSIS, we wanted to determine whether that was consequence of an impairment of β-cell metabolic function. Because the downstream effects of dopamine receptors activation in islet cells have not been extensively studied, we examined the possibility that increased activity of the aromatic l-amino-acid decarboxylase enzyme to convert excess l-dopa to dopamine could change the islet redox state. Such a change would lead to reduced ATP/ADP ratio, and result in lowered GSIS. We used two-photon excitation microscopy to excite autofluorescence from reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate (collectively indicated as NAD(P)H) to monitor the islet redox state (43). The NAD(P)H signal for a given stimulus was normalized to the maximum level obtained by applying 3 mm sodium cyanide. Cyanide shifts the redox status of cells by blocking the mitochondrial electron transport chain, thus converting all NAD(P)+ to NAD(P)H (44).
We did not detect any statistically significant decrease in NAD(P)H signal between 8 mm glucose vs. 8 mm glucose + 10 μm dopamine or vs. 8 mm glucose + 10 μm l-dopa (Fig. 3A). Similarly there was no difference in signal at 16.7 mm glucose vs. 16 mm glucose +10 μm dopamine or vs. 16.7 mm glucose + 10 μm l-dopa (Fig. 3A). Also we tested whether increasing the dopamine content of the islet had an effect on the islet redox state. Islets were treated with 10 μm l-dopa for 30 min before the experiment to increase their dopamine content. Then they were stimulated with 8 mm or 16.7 mm glucose. Again we found no decrease in their NAD(P)H signal as compared with the response from untreated islets (Fig. 3B). This suggests that inhibitory actions of l-dopa and dopamine on GSIS are not caused by a decrease in NAD(P)H.
Fig. 3.
The effects of l-dopa and dopamine on the redox state of pancreatic islets. A, NAD(P)H autofluorescence from isolated islets was measured at 8 mm glucose and at 16.7 mm glucose and with 10 μm dopamine or 10 μm l-dopa as indicated; results are normalized to the maximum signal obtained with 3 mm sodium cyanide (n =2–4). B, Islets were incubated with or without 10 μm l-dopa for 30 min before the experiment after which NAD(P)H autofluorescence was measured at 8 mm and 16.7 mm glucose (n = 3–4).
The effects of dopamine and l-dopa on [Ca2+]i oscillation frequency in isolated islets
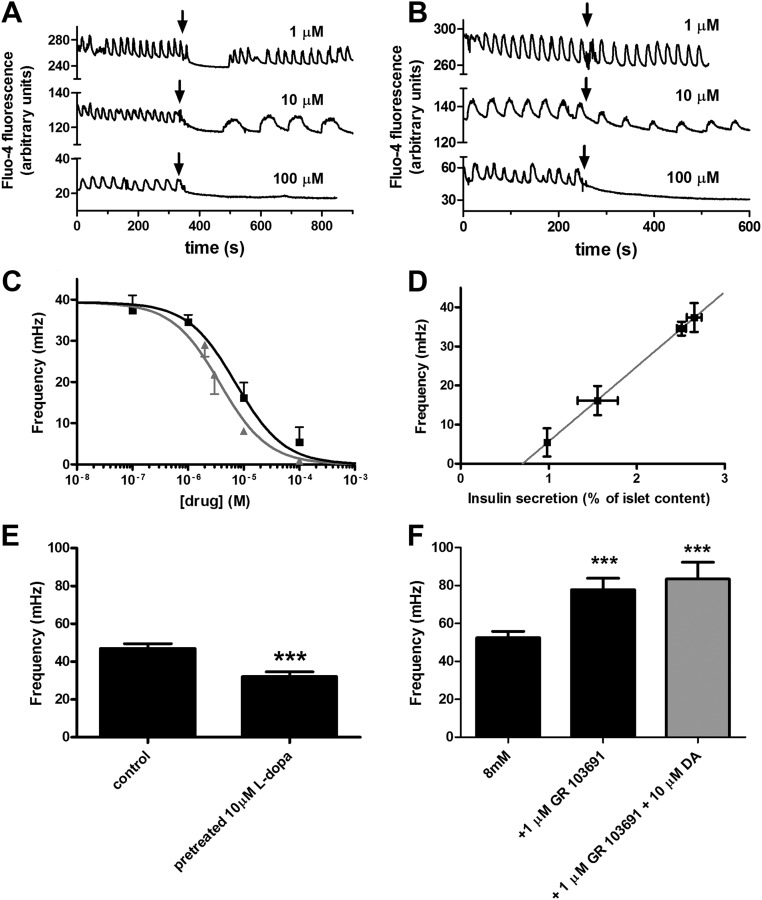
We monitored intracellular calcium levels of β-cell in intact islets using the fluorescent indicator Fluo-4 AM. In an intact pancreatic islet, β-cells respond to a step increase in glucose concentration with a coordinated and periodic increase in [Ca2+]i. These [Ca2+]i oscillations have a characteristic frequency that is a consequence of the electrical and metabolic properties of the islet (45). We stimulated isolated islets with a glucose concentration shift from 2 mm to 8 mm and found [Ca2+]i oscillations with a period of 27.19 ± 0.98 s (Fig. 4, A and B), that is a frequency of 39.3 ± 1.3 mHz (Fig. 4B). When we stimulated the islets with 8 mm glucose + dopamine (Fig. 4A) or l-dopa (Fig. 4B), we consistently observed a decrease in the [Ca2+]i oscillation frequency. We quantified the effect of dopamine and l-dopa on the [Ca2+]i oscillation frequency. Treating islets with l-dopa or dopamine during the glucose stimulation decreased [Ca2+]i oscillation frequency in a dose-dependent manner (Fig. 4C). A sigmoidal fit of the data gave an estimated EC50 for l-dopa and dopamine of 7.2 μm and 3.8 μm, respectively. We found a very significant correlation (P < 0.0001) between the effect of l-dopa on insulin secretion and the effect on [Ca2+]i oscillation frequency (Fig. 4D). We hypothesize that l-dopa produces this effect after it is converted to dopamine, so we measured the [Ca2+]i oscillation frequency in islets that were pretreated with l-dopa (Fig. 4E). Islets that were treated with 10 μm l-dopa for 30 min before stimulation with 8 mm glucose similarly had a slower [Ca2+]i oscillation frequency (32.11 ± 2.5 mHz; n = 8) compared with control (47.1 ± 2.4 mHz; n = 8; P < 0.001). We also tested whether a D3 antagonist exhibited an effect on the [Ca2+]i oscillation frequency. Indeed, the D3 antagonist produced a significant increase in the [Ca2+]i oscillation frequency and abolished the effect of dopamine (Fig. 4F).
Fig. 4.
The effects of dopamine and l-dopa on [Ca2+]i oscillation frequency in isolated islets. A, Representative patterns of [Ca2+]i oscillations from a single islet before and after dopamine stimulus. The typical oscillation pattern, stimulated by increasing glucose concentration from 2 mm to 8 mm, is shown in in the first 300 sec; the pattern changed after the addition of dopamine at the indicated concentration. Black arrows indicate the time when dopamine was added; the three plots are offset for an easier comparison. B, Representative patterns of [Ca2+]i oscillations from a single islet before and after l-dopa stimulus. The oscillations were triggered by increasing glucose concentration from 2 mm to 8 mm; black arrows indicate the time when l-dopa was added. C, Sigmoidal dose-response curve fit of [Ca2+]i oscillation frequency in response to treatment with dopamine (▴) or l-dopa (■) in conjunction with 8 mm glucose stimulus; gray line indicates, best-fit EC50 = 3.8 μm, R2 = 0.78 (n = 4–7); black line indicates, best-fit EC50 = 7.2 μm, R2 = 0.89 (n = 5–14); The difference between EC50 values is statistically significant with a P < 0.05. D, Plot of [Ca2+]i oscillation frequency vs. insulin secretion from two independents sets of experiments; the gray line represents the linear fit of the data (R2 = 0.999); Pearson's correlation coefficient r = 1 with P < 0.0001. E, The [Ca2+]i oscillation frequency of control islets vs. islets with elevated dopamine content resulting from a pretreatment with l-dopa as indicated (n = 8); ***, P < 0.001. F, The [Ca2+]i oscillation frequency of control islets vs. islets treated with D3 antagonist (GR 103691) or a mixture of D3 antagonist and dopamine as indicated (n = 9–14); ***, P < 0.001. DA, Dopamine; s, second.
Immunodetection of dopamine receptors and DAT in pancreatic islet lysate
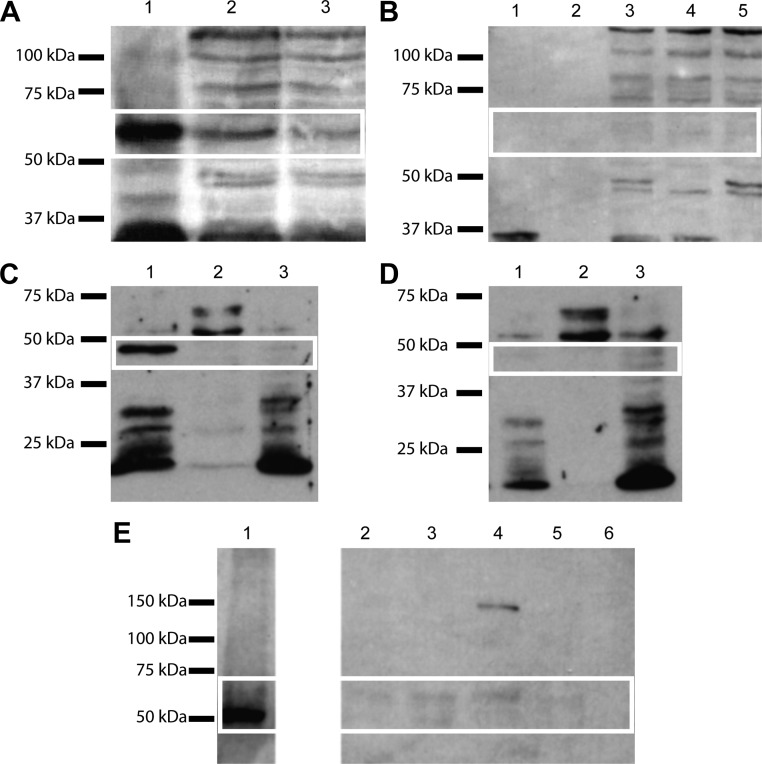
We directly tested for the presence of dopamine receptors and transporters in islets by performing Western blot experiments. A band of approximately 58 kDa was detected in the positive control (mouse brain extract) and in the islet lysate (Fig. 5A). This is in good agreement with the molecular weight reported in the literature for DRD2, but several other bands were present in the islet lysate lanes and so we performed another experiment to discriminate between specific and nonspecific interaction and confirm that the 58-kDa band in the islet lane was indeed DRD2. We neutralized the primary antibody with its own immunogen, the 28-amino acid peptide from the third intracellular loop of DRD2. Under this condition the band at 58 kDa was not detectable in any lane whereas the other nonspecific bands were still present (Fig. 5B). We compared the band intensity from the brain extract with the one from the islet lysate, normalizing the result for the loaded amount of total protein. We obtained an estimate for DRD2 protein content in the islets to be 17% ± 3% (n = 2) of the level found in brain. Because the DRD3-specific antagonist was shown to eliminate dopamine modulation of insulin secretion, we also investigated the expression of this receptor subtype. We detected a band of approximately 48 kDa corresponding to DRD3 in the membrane fraction of the islet lysate (Fig. 5C), and we confirmed the specificity of this signal by neutralization of the antibody with its specific immunogen peptide, resulting in the disappearance of the band (Fig. 5D). Similar Western blot procedures using a DAT antibody showed a single band of 50–62 kDa representing DAT expression in the islets (Fig. 5E).
Fig. 5.
Immunodetection of dopamine receptors and DAT in pancreatic islets. A, Immunoblot for DRD2: lane 1, mouse brain extract (25 μg total protein); lane 2, pancreatic islets lysate (54 μg total protein); lane 3, pancreatic islets lysate (45 μg total protein); the white box overlay highlights the 58-kDa band present in all lanes. B, Control immunoblot for DRD2 using the blocking peptide for the primary antibody: lane 1, mouse brain extract (20 μg total protein); lane 2, empty; lanes 3–5, pancreatic islets lysate (20 μg total protein); the white box overlay highlights the 58-kDa band missing in all lanes. C, Immunoblot for DRD3: lane 1, islet membrane fraction (18 μg); lane 2, islet cytosolic fraction (18 μg); lane 3, islet nuclear fraction (18 μg); white box overlay highlights the 48-kDa band present in the islet membrane fraction. D, Control immunoblot for DRD3: lane 1, islet membrane fraction (18 μg); lane 2, islet cytosolic fraction (18 μg); lane 3, islet nuclear fraction (18 μg): white box indicates the absence of the 48-kDa band in the islet membrane fraction. E, Immunoblot for DAT: lane 1, mouse brain extract (10 μg total protein); lanes 2–5, pancreatic islets lysate (76, 73, 46, and 24 islets, respectively); lane 6, empty.
Live subcellular localization of DRD2L-mVenus
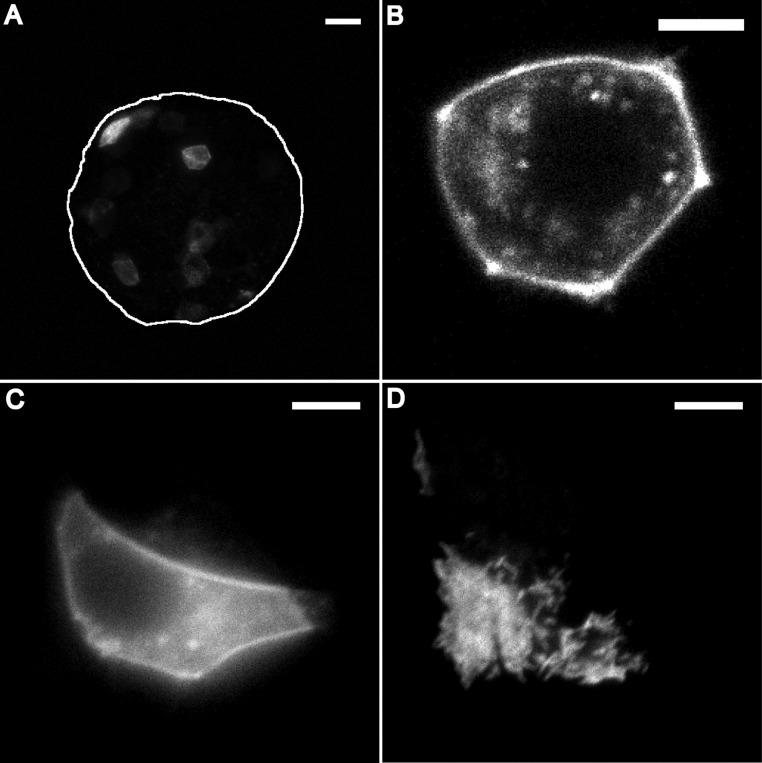
One previous report (33) suggested that dopamine receptors are only found in secretory granules, but, according to our model, receptors must be on the plasma membrane. To determine whether dopamine receptors are present on the plasma membrane, we used adeno-associated virus (AAV) vector to express DRD2L tagged with the fluorescent protein mVenus in islet cells. This transduction allowed us to image DRD2L-mVenus distribution in live islet cells. Multiple cells per islet showed fluorescence 48 h after the transduction (Fig. 6A). When we imaged these islet cells at higher spatial resolution, they all showed fluorescence associated with their plasma membrane (Fig. 6B), supporting the assumption that DRD2 is present on the plasma membrane of live islet cells. We confirmed this result performing total internal reflection fluorescence (TIRF) imaging on single MIN6 cells that were transduced with DRD2L-mVenus. The plasma membrane localization of DRD2L-mVenus appeared as a bright contour line when a cell is imaged with a wide-field microscope (Fig. 6C), similar to what was observed in the islet with a confocal microscope (Fig. 6B). By TIRF microscopy, membrane localization of the DRD2L-mVenus is confirmed by the presence of fluorescence in all the cellular regions that are in contact with the coverslip (Fig. 6D). AAV vectors containing enhanced green fluorescent protein alone were used as a control. The islet cells transduced with enhanced green fluorescent protein showed diffuse fluorescence with no membrane labeling.
Fig. 6.
Live subcellular localization of DRD2L-mVenus and quantification of dopamine secretion. A, Sum intensity projection of 47 confocal images of an islet expressing DRD2-mVenus (representative image of 33 cells from seven islets); white line indicates the islet outline; scale bar, 20 μm. B, Confocal image of a single cell from the islet shown in panel A; scale bar, 5 μm. C, Wide-field fluorescence image of a MIN6 expressing DRD2L-mVenus; scale bar, 5 μm. D, TIRF image of the same field of view shown in panel C; scale bar, 5 μm.
Discussion
Characterization of pancreatic islets as a dopamine-producing tissue
Previous studies have shown that dopamine can inhibit GSIS in isolated islets (33), but there is no consensus on either the availability or origin of any dopamine that can act on islets in vivo. Even when dopamine accumulation was qualitatively found in mouse β-cells after an l-dopa injection to the animal (19), the site of synthesis was not addressed. This has led to a conundrum because the islets are sensitive to dopamine, but the dopamine concentration in the plasma is too low (0.67 ± 0.21 nm in C57Bl6 mice) to trigger its receptor (40, 41), and dopaminergic innervation of the pancreas has not been reported. Thus, islet sensitivity to exogenous dopamine has not generally been considered physiologically relevant. We tested the hypothesis that the islet itself produces dopamine from circulating l-dopa (32, 46), which has not yet been rigorously examined in a single species. We addressed the question of dopamine synthesis and secretion in mouse islet cells and how dopamine regulates β-cell function. By focusing our study on isolated mouse islets, we could measure dopamine accumulation due solely to synthetic activity of the islets (Fig. 1), excluding the contribution of other tissues (19). Two previous determinations of mouse pancreatic islet dopamine content were performed by homogenizing islets immediately after their isolation and resulted in a higher dopamine concentration compared with our study (21, 47). Indeed, the comparison between our in vitro and in vivo results for the l-dopa induced dopamine accumulation shows that when l-dopa is injected in the mouse, the isolated islets contain twice as much dopamine as measured in the in vitro experiment (Fig. 1B). There are two likely explanations for this difference. One is the lack of efficient perfusion of the islets in culture compared with in vivo islets, which are provided with a rich vasculature. Alternatively, possible contamination of freshly isolated islets with dopamine-rich acinar tissue can also explain the observed difference. Because we did not observe any statistically significant change in the islet dopamine content that can be attributed to culture, and we can assume that any contribution of severed adrenergic fibers is diminished due to degradation over time, we conclude that we are measuring dopamine produced in the islet cells. For the same reason we can also exclude production by the nerve fibers of norepinephrine and epinephrine that could affect GSIS from our isolated islets.
Our data show that freshly isolated islets contain dopamine at a level of approximately 0.5 pg/islet. This value should reflect as closely as possible the in vivo condition of an islet in its native environment. The corresponding estimate for dopamine concentration in a single islet would be approximately 7 μm, using an average islet diameter of 100 μm. This level of dopamine would be consistent with a biologically relevant negative feedback loop as we propose, because at this concentration dopamine affects [Ca2+]i (Fig. 4C). When l-dopa was increased in vitro, we measured a rapid 30-fold increase in the islet dopamine content (Fig. 1A). Similarly, when circulating l-dopa levels were raised by exogenous administration in vivo, we saw a 50-fold increase in dopamine concentration (Fig. 1D), which corresponds to a robust inhibition of GSIS. We observed a dose-dependent inhibition of GSIS by combining l-dopa and glucose (Fig 2, A and B), but based on the results in Fig. 2D we conclude that this effect was due to increased dopamine content, and not a direct pharmacological effect of l-dopa on GSIS.
We observed that treatment with 10 nm l-dopa, which is comparable to 5 nm plasma concentration of l-dopa in the mouse (41, 48), produced a trend toward GSIS inhibition but did not reach statistical significance for the number of observations used (n = 7). This result is due, at least in part, to the limitations of in vitro settings vs. physiological situation. In the animal, the islet is constantly exposed to l-dopa, whereas the in vitro experiments depend on an acute treatment. It is also known that l-dopa is quickly oxidized in aqueous solution; therefore, ascorbic acid is often added as a preservative. In our experiments, though, we could not add ascorbic acid because it affects GSIS directly (49). Thus, the effective concentration of l-dopa in the final solution may be diminished by oxidation, and this effect would particularly affect the results at lower l-dopa concentrations. Neither l-dopa nor the dopamine receptor agonist quinpirole altered basal insulin secretion at low glucose concentration, supporting the hypothesis that dopamine must be secreted by the islet to produce its effect. Further, activation of dopamine receptors does not produce any effect if the [Ca2+]i is not elevated by other processes.
Rubi et al. (33) showed that quinpirole recapitulated the inhibitory effect of dopamine on GSIS, and they concluded that the D2-like receptors mediated the effects of dopamine (33). By using receptor-selective antagonists, however, we show that only the receptor D3 is involved in dopamine signaling in the islets (Fig. 2E). Blockade of D3 receptor produced significant increase of GSIS in untreated islets. This evidence indicates that our proposed dopamine negative feedback circuit regulates insulin secretion in the islets, and that the D3 antagonist disrupts such feedback. Accordingly, only the D3 antagonist rescued GSIS when exogenous dopamine was added to the islets (Fig. 2E).
Based on findings by Ericson et al. (19) using radiolabeled l-dopa, we hypothesized that dopamine is cosecreted with insulin, and we tested this concept by simultaneously measuring dopamine and insulin secretion under different conditions. These results (Fig. 2F) show that dopamine secretion follows insulin secretion, which supports the colocalization of the two substances in the same granules. In our experimental setting, we increased the islet dopamine content to enable the measurement of its secretion. However, this loading results in inhibition of GSIS (Fig. 2D), which explains the lack of insulin or dopamine secretion at high glucose (Fig. 2F). To overcome this dopamine-dependent inhibition of GSIS, we used forskolin to elevate cAMP and stimulate insulin secretion in the absence of extracellular calcium influx (50). In the presence of such stimulus, we measured a significant increase in insulin secretion and a corresponding increase in dopamine secretion (Fig. 2F).
The existence of GSIS regulation that relies on dopamine allows speculation regarding the high prevalence of diabetes in Parkinson patients (51). Because islets share the dopaminergic system with neurons, it is possible that the unknown cause of dopaminergic neuron loss underlying in Parkinson's disease could also cause the loss of β-cell function that results in type 2 diabetes. Therapy for Parkinson's disease consists of administration of l-dopa and benserazide to increase dopamine concentration in dopaminergic neurons and prevent peripheral conversion of l-dopa and its consequent side effects (52). However, in our experiments, benserazide did not completely halt the production of dopamine in the islets. We can speculate that if this holds true in patients, then the Parkinson's treatment regimen could partially inhibit GSIS and put a chronic stress on islet function that would exacerbate the association between type 2 diabetes and Parkinson's disease (51–54).
l-dopa and dopamine change calcium dynamics in the islets
We investigated the mechanism through which l-dopa and dopamine inhibit GSIS from intact pancreatic islets. We observed a dose dependent effect of dopamine and l-dopa on the frequency of [Ca2+]i oscillations in islets that correlates with the inhibition of GSIS. Although the [Ca2+]i oscillation frequency is not the only parameter that is changing, it is a robust measure because it does not depend on calcium dye loading, photobleaching, or focal drift during the imaging experiment. Because the elevation of [Ca2+]i is the trigger for insulin secretion in β-cells, we conclude that dopamine and l-dopa modulate/inhibit GSIS by changing the firing rate of the β-cells in their excited state. Blocking the D3 receptor increases the frequency of [Ca2+]i oscillations (Fig. 4F) and concomitantly increases insulin secretion (Fig. 2E). Importantly, blockade of the D3 receptor totally abolished the effect of dopamine on the [Ca2+]i oscillations. The exact molecular mechanism to produce this modulation on [Ca2+]i is yet to be defined. A direct effect of dopamine receptor activation on CaV1.2 and CaV1.3 channel conductance has been demonstrated in neurons (55, 56) and is the simplest explanation for the change in frequency that we observe. This direct relationship between dopamine receptor activation and reduced calcium channel conductance is supported by our forskolin treatment data, which showed that cAMP elevation can overcome dopamine-dependent inhibition of GSIS (Fig. 2F). However, other electrophysiological schemes, such as effects on K+ channel conductance could also explain these effects. We exclude short-term cytotoxic effect of l-dopa and dopamine because the redox-state of the islets is unchanged upon exposure to either compound (Fig. 3).
Expression of dopamine receptors and transporters in pancreatic islets and their relevance as target of antipsychotics
We performed Western blot experiments on isolated mouse islets, and detected both dopamine receptors D2 and D3, and the DAT. The presence of these proteins completes the picture of a functional dopaminergic system in the islets. Live cell fluorescence imaging of DRD2L-mVenus in islets and MIN6 cells (Fig. 6, A–D) show clear plasma membrane localization for the receptor in contrast to the previous literature that proposed intracellular localization of DRD2 (33). As we are overexpressing this receptor, we expect to observe a percentage of abnormal trafficking, but the fact that the majority of the signal is associated with the cell membrane lead us to conclude that the receptor is normally trafficked to the plasma membrane. The perinuclear distribution that can still be observed can be interpreted as immature peptide that has yet to be translocated.
In addition to being a dopamine-producing tissue, we propose that the islets of Langerhans should also be added to the list of dopamine-target tissues. This could be particularly important considering the number of studies showing associations between metabolic syndrome and antipsychotic therapy (57–61). For example, some side effects of dopamine agonists and antagonists could be independent from their action on the central nervous system but instead may be related to direct action on insulin secretion. Finally, the expression of DAT in the pancreatic islet provides a suggestive hypothesis relating type 2 diabetes and Parkinson's disease: we can speculate that the brain and endocrine pancreas are responding to the same insult, and that both tissues suffer same type of damage (i.e. loss of dopaminergic cells). If substance(s) that enter neurons via DAT is one cause of Parkinson's disease, then the same substance(s) could have equally deleterious effects on islet cells (62–64).
Proper insulin secretion requires integration of multiple signals by the β-cells. Knowing all the signals involved is important because it provides new targets to design drugs to treat type 2 diabetes. Although further studies will be necessary to enumerate all of the molecular mechanisms and all the components of it, this new dopaminergic negative feedback loop that contributes regulating insulin secretion offers another mechanism that could be further investigated and targeted in the quest for treatment of type 2 diabetes.
Materials and Methods
Islet isolation and culture
C57Bl/6 (Harlan Sprague Dawley, Inc., Indianapolis, IN) mice were used for these experiments. All animals were fed standard laboratory chow and cared for according to the guidelines of the Vanderbilt Institutional Animal Care and Use Committee. Mice (2–6 months of age) were anesthetized by ip injection of 0.05 ml of a ketamine (Bioniche Teoranta, Inverin, Co. Galway, Ireland) and xylazine (Lloyd Laboratories, Shenandoah, IA) mixture at a dose of 80 mg/ml and 20 mg/ml, respectively. The pancreas was quickly removed and the animal was euthanized. The islets of Langerhans were isolated in Hanks' balanced salt solution following a modified version of the protocol from Lacy and Kostianovsky (65). Subsequently, islets were handpicked under a dissecting microscope. Islets were cultured overnight in RPMI 1640 medium with glutamine (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 11 mm glucose, at 37 C in 5% CO2 humidified atmosphere. Islets for the dopamine assay were cultured overnight in islet medium with 2 mm glucose.
MIN6 cell culture
Mouse MIN6 cells (66) were maintained in sodium bicarbonate-buffered DMEM with the addition of 10% heat-inactivated fetal bovine serum, 50 μm β-mercaptoethanol, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured at 37 C in 5% CO2 humidified atmosphere.
Dopamine content assay
After being treated and incubated according to the experiment being performed, islets from a single mouse were transferred to a tube containing ice-cold PBS (Mediatech Inc., Manassas, VA) and rinsed once. Lysis was performed in 28 μl of minimal lysis buffer (5% glycerol, 1% Triton X-100, 100 mm NaCl, 1 mm EDTA, 4 mm Na2S2O5, 10 mm HCl) for 30 min on ice. The sample was snap frozen and thawed three times, and then sonicated for 5 min. Finally the sample was centrifuged at 13,000 relative centrifugal force for 10 min in a tabletop centrifuge, and the supernatant was collected for dopamine and protein determinations. Five microliters were used for protein determination in duplicate using Pierce 660nm protein assay reagent in a 96-well plate. The remaining sample was diluted to a final volume of 500 μl in minimal lysis buffer. The dopamine concentration was determined using the Dopamine Research ELISA (Rocky Mountains Diagnostics, Inc., Colorado Springs, CO). Each sample was split in half and processed in duplicate, and the concentration range of dopamine in the standard curve was 36–3600 pg. We used a Spectramax M5 plate reader (Molecular Devices, Sunnyvale, CA) to read the 96-well plates. The results were normalized to the sample protein content and expressed as (picograms of dopamine per μg of protein; n represents the number of mice used to test each condition.
Dopamine secretion assay
Islets from multiple mice were pooled and allowed to recover from the isolation procedure overnight in islet medium, at 37 C in 5% humidified CO2. On the next day they were divided in groups of 215 islets in Krebs Ringer Bicarbonate HEPES Buffer (KRBH) at 37 C in 5% humidified CO2. KRBH components were: 128.8 mm NaCl, 4.8 mm KCl, 1.2 mm KH2PO4, 1.2 mm MgSO4, 2.5 mm CaCl2, 5 mm NaHCO3, 10 mm HEPES, 0.1% BSA, pH 7.4. Each group was treated with 2.8 mm glucose +10 μm l-dopa for 40 min to increase the islet dopamine content. Each group was then transferred to 1.5 ml Eppendorf tubes contatining KRBH + 2.8 mm glucose for 20 min, to let the dopamine content equilibrate. Then each group was transferred to the 1.5-ml Eppendorf tube containing KRBH + the condition to be tested for the secretion experiments. Each group was incubated for 45 min. After incubation, the supernatant was collected, dopamine preservatives were added (1 mm EDTA, 4 mm Na2S2O5, 10 mm HCl), and this supernatant was used to measure secreted dopamine. The standard curve for these measurements had a 12–1200 pg range. A small fraction of the same supernatant was used to measure secreted insulin. The islets were processed to measure total dopamine and insulin content. Data from each group were normalized to the respective islet dopamine content and reported as the percentage of the islet dopamine content; n represents the number of experimental groups.
Insulin secretion assay
Islets were equilibrated for 1 h in KRBH. During the equilibration period glucose concentration was 2.8 mm. Islets were then transferred in tubes (four islets per tube) containing KRBH + the condition to be tested. The tubes were incubated at 37 C in a water bath for 45 min. A fraction of the supernatant was collected to determine secreted insulin, whereas TritonX-100 was added to the remaining volume at a final concentration of 1% to extract total islet insulin. The tubes were frozen overnight at −20 C. Insulin concentration was measured by RIA in the Vanderbilt Hormone Assay Core. Each condition was tested in triplicate and expressed as the percentage of secreted insulin relative to the total islet insulin content; n represents the number of mice used to test each condition.
NAD(P)H imaging
The combined reduced nicotinamide adenine dinucleotide autofluorescence and reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) was measured in single islets using two-photon excitation microscopy. We used a LSM710NLO laser-scanning microscope (Carl Zeiss, Inc., Thornwood, NY) and a Chameleon tunable laser (Coherent Inc., Santa Clara, CA). The excitation wavelength was set to 710 nm and power was set to 60 mW entering the LSM710 (∼5 mW at the sample). We used a Plan-Apochromat 20×/0.8 objective (Carl Zeiss, Inc.). Pixel size was 0.830 μm. NAD(P)H autofluorescence was collected through the LSM710 spectral detector with the bandpass set from 381–581 nm. The microscope stage was equipped with a temperature-controlled stage (Carl Zeiss, Inc.) to keep the islets at 37 C and 5% CO2 during the imaging experiments. The islets were transferred (10 islets per dish) to a 35-mm glass-bottomed dish (MatTek Corp., Ashland, MA) containing freshly prepared imaging media (125 mm NaCl, 5.7 mm KCl, 2.5 mm CaCl2, 1.2 mm MgCl2, 10 mm HEPES, 0.1% BSA, pH 7.4) with 2 mm glucose and equilibrated 30 min before the experiment. NAD(P)H images were acquired at 2 mm, 8 mm, and 16.7 mm glucose and at 16.7 mm glucose + 3 mm sodium cyanide, which gives a maximal value for normalization of the results. After this initial group, different groups of islets from the same mouse were then used to image NAD(P)H autofluorescence in the experimental conditions to be tested; n represents the number of mice on which all the conditions were tested.
Calcium imaging
Islets were labeled by incubation with 4 μm Fluo-4 AM (Invitrogen) in imaging medium with 2 mm glucose at room temperature for 45 min. The islets were then loaded in a simple microfluidic device on the microscope stage (67) and maintained at 37 C under humidified 5% CO2. The islets were constantly perfused with fresh imaging buffer containing the drug to be tested. We used a Plan-Apochromat 20×/0.8 objective (Zeiss Inc.) and 488-nm excitation laser at 0.3% of total power. The emission was collected from 492–622 nm. Pixel size was 0.830 μm. Images were acquired at a rate of one frame per second; n represents the number of islets.
DRD2-mVenus live imaging
Islets and MIN6 cell were transduced using AAV particles, for the expression of the fusion protein in which the long isoform of the human DRD2 (DRD2L) is fused to the yellow fluorescent protein mVenus. AAV for the expression of mVenus alone were used as a control. The AAV particles were a generous gift from Dr. Jonathan Javitch (Columbia University, New York, NY). Briefly the islets and the cells were exposed to the AAV for 18 h, and then they were cultured for 48 h in regular medium to obtain maximal expression before imaging. The islets were imaged on the LSM710 confocal microscope (Carl Zeiss, Inc.) using a Fluar 40× oil objective, with NA = 1.30. We used a 514 laser line to excite the mVenus fluorescence, and we collected the emission setting the bandpass from 518–613 nm. The pixel dwell time was 25.2 μsec. To image whole islets we acquired z-stack of images with a pixel size of 0.415 μm and 2 μm between each image in the stack. To image single cells in the islet, we changed the pixel size to 0.086 μm. For imaging experiments with MIN6 cells we used an Eclipse Ti microscope (Nikon,) equipped with a TIRF objective (ApoTirf 60× Oil DIC N2 NA = 1.49). The wide-field fluorescence images were acquired using a Xenon lamp excitation and a green fluorescent protein ex/em filter set. For the TIRF images the excitation was provided by a 488-nm diode laser whereas the same emission filter was used (505 bp). The acquisition time was 1 sec and the pixel size was 0.086 μm. All the live fluorescence experiments were performed at 37 C under humidified 5% CO2.
Image analysis
Image analysis was performed using ImageJ (68). For each image, background was subtracted, and regions of interest were drawn corresponding to the islets. The average intensity in these regions of interest was calculated for each frame. The intensity plots from calcium imaging experiments were subsequently processed for frequency analysis using freely available software (69).
Western blot
Islets were transferred to a tube and rinsed once in ice-cold PBS. The tube was kept on ice, and the lysis buffer was added. The lysis buffer had the following components: 150 mm NaCl; 1% TritonX-100; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 50 mm Tris, pH 8.0; 5 mm EDTA; 1 mm EGTA; 5 mm NaF; 1 mm Na3VO4; 1 mm phenylmethysulfonyl fluoride; and a cocktail of mammalian protease inhibitors (P8340 from Sigma, St Louis, MO). Lysis proceeded on ice for 45 min, followed by sonication for 5 min. The sample was centrifuged at 14000 relative centrifugal force for 30 min at 4 C. The supernatant was collected, assayed for protein concentration, and mixed with Laemli loading buffer. Mouse brain extract (B6928, Sigma) was used as positive control. The islet lysates and positive control were subjected to SDS-PAGE (10%) and then transferred onto nitrocellulose membrane. The membranes were blocked for 1 h at room temperature with Tris-buffered saline containing 0.1% Tween-20, 5% BSA, and 5 mm sodium azide. For DRD2 immunoblotting, the membranes were incubated with the rabbit anti-D2 polyclonal antibody (AB5084P; Millipore Corp., Bedford, MA) (1:1000) at 4 C overnight. Secondary incubation was done with goat antirabbit IgG horseradish peroxidase conjugate antibody (W4011; Promega Corp., Madison, WI) diluted 1:5000. The specific control peptide (AG221, Millipore) was used to neutralize the rabbit anti-D2 antibody in the control experiment. The same protocol was used for immunoblotting of DAT, but with a 1:1000 dilution of mouse anti-DAT monoclonal antibody (mAb16; generous gift from Dr. Roxanne A. Vaughan at University of North Dakota, Grand Forks, ND). The secondary antibody was a goat antimouse IgG horseradish peroxidase conjugated, diluted 1:5000. In all the experiments, signal detection was detected by chemiluminescence [ECL Plus system from GE Healthcare, Piscataway, NJ; and Kodak BioMax light film; Eastman Kodak, Rochester, NY).
For the DRD3 immunoblotting, the islets were homogenized in Dounce homogenizer in lysis buffer without detergents. The homogenate was centrifuged at 7000 relative centrifugal force for 5 min. The resulting pellet was dissolved in lysis buffer and used as the nuclear fraction. The supernatant was centrifuged at 500,000 relative centrifugal force for 10 min. The resulting pellet was dissolved in lysis buffer and used as the membrane fraction. The supernatant was used as the cytosolic fraction. The primary antibody used for this experiment was a rabbit polyclonal antibody (ab42114, from Abcam, Inc., Cambridge, MA) at a 1:750 dilution. The secondary antibody was the same used for the DRD2 immunoblotting. The control peptide used in the neutralization experiment was a 19-amino acid D3 peptide (ab128688, from Abcam, Inc.)
Statistical analyses
Data analyses were performed using GraphPad Prism version 4.03 for Windows, from GraphPad Software, San Diego CA, www.graphpad.com. Data are presented as mean ± sem. Significance was evaluated by Student's t test and defined as P < 0.05.
Acknowledgments
Mouse anti-DAT monoclonal antibody (mAb16) was generously provided by Dr. Roxanne Vaughan (Department of Biochemistry and Molecular Biology, University of North Dakota, Grand Forks, ND). AAV vector for expression of DRD2L-mVenus was a generous gift from Dr. Jonathan Javitch (Columbia University, New York, NY). We thank Drs. Jonathan Javitch, Zachary Freyberg (Columbia University), and Aurelio Galli (Vanderbilt University, Nashville, TN) for helpful feedback on the experimental design and data interpretation.
This work was supported by National Institutes of Health Grant DK08564. Equipment and technical assistance was provided by the Vanderbilt Diabetes Center Hormone Assay & Analytical Services Core (supported by National Institutes of Health Grant DK020593). This research was conducted in part at the Marine Biological Laboratory (Woods Hole, MA) and was partially supported by the Nikon Research Fellow Award (to D.W.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AAV
- Adeno-associated virus
- [Ca2+]i
- intracellular calcium concentration
- DAT
- dopamine transporter
- DRD2
- dopamine receptor D2
- GSIS
- glucose-stimulated insulin secretion
- KRBH
- Krebs Ringer bicarbonate HEPES buffer
- NAD(P)H
- reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate
- TIRF
- total internal reflection fluorescence.
References
- 1. Leahy JL. 2005. Pathogenesis of type 2 diabetes mellitus. Arch Med Res 36:197–209 [DOI] [PubMed] [Google Scholar]
- 2. Leahy JL, Hirsch IB, Peterson KA, Schneider D. 2010. Targeting β-cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab 95:4206–4216 [DOI] [PubMed] [Google Scholar]
- 3. Weyer C, Bogardus C, Mott DM, Pratley RE. 1999. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, Powers AC. 2005. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53:1087–1097 [DOI] [PubMed] [Google Scholar]
- 5. Benninger RK, Zhang M, Head WS, Satin LS, Piston DW. 2008. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J 95:5048–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hopcroft DW, Mason DR, Scott RS. 1985. Structure-function relationships in pancreatic islets: support for intraislet modulation of insulin secretion. Endocrinology 117:2073–2080 [DOI] [PubMed] [Google Scholar]
- 7. Kanno T, Gopel SO, Rorsman P, Wakui M. 2002. Cellular function in multicellular system for hormone-secretion: electrophysiological aspect of studies on α-, β- and δ-cells of the pancreatic islet. Neurosci Res 42:79–90 [DOI] [PubMed] [Google Scholar]
- 8. Ahrén B. 2009. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov 8:369–385 [DOI] [PubMed] [Google Scholar]
- 9. Winzell MS, Ahrén B. 2007. G-protein-coupled receptors and islet function-implications for treatment of type 2 diabetes. Pharmacol Ther 116:437–448 [DOI] [PubMed] [Google Scholar]
- 10. Miller RE. 1981. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev 2:471–494 [DOI] [PubMed] [Google Scholar]
- 11. Beaulieu JM, Gainetdinov RR. 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217 [DOI] [PubMed] [Google Scholar]
- 12. Wood-Kaczmar A, Gandhi S, Wood NW. 2006. Understanding the molecular causes of Parkinson's disease. Trends Mol Med 12:521–528 [DOI] [PubMed] [Google Scholar]
- 13. Abi-Dargham A. 2004. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol 7(Suppl 1):S1–S5 [DOI] [PubMed] [Google Scholar]
- 14. Grace AA. 2010. Dopamine system dysregulation by the ventral subiculum as the common pathophysiological basis for schizophrenia psychosis, psychostimulant abuse, and stress. Neurotox Res 18:367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodge DJ, Grace AA. 2010. Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci 29:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Falck B, Hellman B. 1963. Evidence for the presence of biogenic amines in pancreatic islets. Cell Mol Life Sci 19:139–140 [Google Scholar]
- 17. Pearse AG. 1968. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci 170:71–80 [DOI] [PubMed] [Google Scholar]
- 18. Ahrén B, Lundquist I. 1985. Effects of L-dopa-induced dopamine accumulation on 45Ca2+ efflux and insulin secretion in isolated rat islets. Pharmacology 30:71–82 [DOI] [PubMed] [Google Scholar]
- 19. Ericson LE, Håkanson R, Lundquist I. 1977. Accumulation of dopamine in mouse pancreatic B-cells following injection of L-DOPA. Localization to secretory granules and inhibition of insulin secretion. Diabetologia 13:117–124 [DOI] [PubMed] [Google Scholar]
- 20. Lundquist I, Panagiotidis G, Stenstrom̈ A. 1991. Effect of L-dopa administration on islet monoamine oxidase activity and glucose-induced insulin release in the mouse. Pancreas 6:522–527 [DOI] [PubMed] [Google Scholar]
- 21. Lundquist I, Ahrén B, Hansson C, Håkanson R. 1989. Monoamines in pancreatic islets of guinea pig, hamster, rat, and mouse determined by high performance liquid chromatography. Pancreas 4:662–667 [DOI] [PubMed] [Google Scholar]
- 22. Falck B, Hellman B. 1964. A fluorescent reaction for monoamines in the insulin producing cells of the guinea-pig. Acta Endocrinol (Copenh) 45:133–138 [DOI] [PubMed] [Google Scholar]
- 23. Håkanson R, Lundquist I, Rerup C. 1967. On the hyperglycaemic effect of DOPA and dopamine. Eur J Pharmacol 1:114–119 [DOI] [PubMed] [Google Scholar]
- 24. Cegrell L. 1968. The occurrence of biogenic monoamines in the mammalian endocrine pancreas. Acta Physiol Scand Suppl 314:1–60 [PubMed] [Google Scholar]
- 25. Feldman JM, Lebovitz HE. 1971. The nature of the interaction of amines with the pancreatic β cells to influence insulin secretion. J Pharmacol Exp Ther 179:56–65 [PubMed] [Google Scholar]
- 26. Quickel KE, Jr, Feldman JM, Lebovitz HE. 1971. Inhibition of insulin secretion by serotonin and dopamine: species variation. Endocrinology 89:1295–1302 [DOI] [PubMed] [Google Scholar]
- 27. Wilson JP, Downs RW, Feldman JM, Lebovitz HE. 1974. β Cell monoamines: further evidence for their role in modulating insulin secretion. Am J Physiol 227:305–312 [DOI] [PubMed] [Google Scholar]
- 28. Feldman JM. 1979. Species variation in the islets of Langerhans. Diabetologia 16:1–4 [DOI] [PubMed] [Google Scholar]
- 29. Lindström P. 1986. Aromatic-L-amino-acid decarboxylase activity in mouse pancreatic islets. Biochim Biophys Acta 884:276–281 [DOI] [PubMed] [Google Scholar]
- 30. Teitelman G, Joh TH, Reis DJ. 1981. Transformation of catecholaminergic precursors into glucagon (A) cells in mouse embryonic pancreas. Proc Natl Acad Sci USA 78:5225–5229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A. 2008. VMAT2 gene expression and function as it applies to imaging β-cell mass. J Mol Med 86:5–16 [DOI] [PubMed] [Google Scholar]
- 32. Raffo A, Hancock K, Polito T, Xie Y, Andan G, Witkowski P, Hardy M, Barba P, Ferrara C, Maffei A, Freeby M, Goland R, Leibel RL, Sweet IR, Harris PE. 2008. Role of vesicular monoamine transporter type 2 in rodent insulin secretion and glucose metabolism revealed by its specific antagonist tetrabenazine. J Endocrinol 198:41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. 2005. Dopamine D2-like receptors are expressed in pancreatic β cells and mediate inhibition of insulin secretion. J Biol Chem 280:36824–36832 [DOI] [PubMed] [Google Scholar]
- 34. Garcia-Tornadú I, Ornstein AM, Chamson-Reig A, Wheeler MB, Hill DJ, Arany E, Rubinstein M, Becu-Villalobos D. 2010. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology 151:1441–1450 [DOI] [PubMed] [Google Scholar]
- 35. Wu W, Shang J, Feng Y, Thompson CM, Horwitz S, Thompson JR, MacIntyre ED, Thornberry NA, Chapman K, Zhou YP, Howard AD, Li J. 2008. Identification of glucose-dependant insulin secretion targets in pancreatic β cells by combining defined-mechanism compound library screening and siRNA gene silencing. J Biomol Screen 13:128–134 [DOI] [PubMed] [Google Scholar]
- 36. Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B, Mezey E. 1997. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82:3864–3871 [DOI] [PubMed] [Google Scholar]
- 37. Eisenhofer G, Kopin IJ, Goldstein DS. 2004. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 56:331–349 [DOI] [PubMed] [Google Scholar]
- 38. Goldstein DS, Eisenhofer G, Kopin IJ. 2003. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther 305:800–811 [DOI] [PubMed] [Google Scholar]
- 39. Mezey E, Eisenhofer G, Harta G, Hansson S, Gould L, Hunyady B, Hoffman BJ. 1996. A novel nonneuronal catecholaminergic system: exocrine pancreas synthesizes and releases dopamine. Proc Natl Acad Sci USA 93:10377–10382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eldrup E. 2004. Significance and origin of DOPA, DOPAC, and dopamine-sulphate in plasma, tissues and cerebrospinal fluid. Dan Med Bull 51:34–62 [PubMed] [Google Scholar]
- 41. Keller NR, Diedrich A, Appalsamy M, Tuntrakool S, Lonce S, Finney C, Caron MG, Robertson D. 2004. Norepinephrine transporter-deficient mice exhibit excessive tachycardia and elevated blood pressure with wakefulness and activity. Circulation 110:1191–1196 [DOI] [PubMed] [Google Scholar]
- 42. Girault JA, Greengard P. 2004. The neurobiology of dopamine signaling. Arch Neurol 61:641–644 [DOI] [PubMed] [Google Scholar]
- 43. Piston DW, Knobel SM. 1999. Quantitative imaging of metabolism by two-photon excitation microscopy. Methods Enzymol 307:351–368 [DOI] [PubMed] [Google Scholar]
- 44. Eng J, Lynch RM, Balaban RS. 1989. Nicotinamide adenine dinucleotide fluorescence spectroscopy and imaging of isolated cardiac myocytes. Biophys J 55:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. MacDonald PE, Rorsman P. 2006. Oscillations, intercellular coupling, and insulin secretion in pancreatic β cells. Plos Biol 4:167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rubí B, Maechler P. 2010. Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance. Endocrinology 151:5570–5581 [DOI] [PubMed] [Google Scholar]
- 47. Hansen SE, Hedeskov CJ. 1977. Simultaneous determination of the content of serotonin, dopamine, noradrenaline and adrenaline in pancreatic islets isolated from fed and starved mice. Acta Endocrinol (Copenh) 86:820–832 [DOI] [PubMed] [Google Scholar]
- 48. Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. 2002. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology 143:4520–4526 [DOI] [PubMed] [Google Scholar]
- 49. Bergsten P, Moura AS, Atwater I, Levine M. 1994. Ascorbic acid and insulin secretion in pancreatic islets. J Biol Chem 269:1041–1045 [PubMed] [Google Scholar]
- 50. Komatsu M, Schermerhorn T, Aizawa T, Sharp GW. 1995. Glucose stimulation of insulin release in the absence of extracellular Ca2+ and in the absence of any increase in intracellular Ca2+ in rat pancreatic islets. Proc Natl Acad Sci USA 92:10728–10732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sandyk R. 1993. The relationship between diabetes mellitus and Parkinson's disease. Int J Neurosci 69:125–130 [DOI] [PubMed] [Google Scholar]
- 52. Sirtori CR, Bolme P, Azarnoff DL. 1972. Metabolic responses to acute and chronic L-dopa administration in patients with parkinsonism. N Engl J Med 287:729–733 [DOI] [PubMed] [Google Scholar]
- 53. Craft S, Watson GS. 2004. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3:169–178 [DOI] [PubMed] [Google Scholar]
- 54. Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. 2008. Prospective cohort study of type 2 diabetes and the risk of Parkinson's disease. Diabetes Care 31:2003–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. 2005. G-Protein-coupled receptor modulation of striatal CaV1.3 L-Type Ca2+ channels is dependent on a shank-binding domain. J Neurosci 25:1050–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hernández-López S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. 2000. D2 Dopamine receptors in striatal medium spiny neurons reduce L-Type Ca2+ currents and excitability via a novel PLCβ1-IP3-calcineurin-signaling cascade. J Neurosci 20:8987–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barrett E, Blonde L, Clement S, Davis J, Devlin J, Kone J, Klein S, Torrey W. 2004. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 27:596–601 [DOI] [PubMed] [Google Scholar]
- 58. Girgis RR, Javitch JA, Lieberman JA. 2008. Antipsychotic drug mechanisms: links between therapeutic effects, metabolic side effects and the insulin signaling pathway. Mol Psychiatry 13:918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Melkersson K, Dahl ML. 2004. Adverse metabolic effects associated with atypical antipsychotics: literature review and clinical implications. Drugs 64:701–723 [DOI] [PubMed] [Google Scholar]
- 60. Miller LJ. 2009. Management of atypical antipsychotic drug-induced weight gain: focus on metformin. Pharmacotherapy 29:725–735 [DOI] [PubMed] [Google Scholar]
- 61. Pramyothin P, Khaodhiar L. 2010. Metabolic syndrome with the atypical antipsychotics. Curr Opin Endocrinol Diabetes Obes 17:460–466 [DOI] [PubMed] [Google Scholar]
- 62. Horowitz MP, Greenamyre JT. 2010. Gene-environment interactions in Parkinson's disease: the importance of animal modeling. Clin Pharmacol Ther 88:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanyal J, Chakraborty DP, Sarkar B, Banerjee TK, Mukherjee SC, Ray BC, Rao VR. 2010. Environmental and familial risk factors of Parkinsons disease: case-control study. Can J Neurol Sci 37:637–642 [DOI] [PubMed] [Google Scholar]
- 64. Vance JM, Ali S, Bradley WG, Singer C, Di Monte DA. 2010. Gene-environment interactions in Parkinson's disease and other forms of parkinsonism. Neurotoxicology 31:598–602 [DOI] [PubMed] [Google Scholar]
- 65. Lacy PE, Kostianovsky M. 1967. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 16:35–39 [DOI] [PubMed] [Google Scholar]
- 66. Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. 1990. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology 127:126–132 [DOI] [PubMed] [Google Scholar]
- 67. Easley CJ, Benninger RK, Shaver JH, Steven Head W, Piston DW. 2009. Rapid and inexpensive fabrication of polymeric microfluidic devices via toner transfer masking. Lab Chip 9:1119–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rasband WS. 1997–2009. ImageJ, U S National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/ [Google Scholar]
- 69. Uhlén P. 2004. Spectral analysis of calcium oscillations. Sci STKE 2004:pl15. [DOI] [PubMed] [Google Scholar]