Abstract
Apurinic/apyrimidinic endonuclease 1 (Ape1/Ref-1) is a multifunctional protein critical for cellular survival. Its involvement in adaptive survival responses includes key roles in redox sensing, transcriptional regulation and repair of DNA damage via the base excision repair (BER) pathway. Ape1 is abundant in most cell types and central in integrating the first BER step catalyzed by different DNA glycosylases. BER is the main process for removal of oxidative DNA lesions in post mitotic brain cells, and after ischemic brain injury preservation of Ape1 coincides with neuronal survival, while its loss has been associated with neuronal death. Here, we report that in cultured primary neurons, diminution of cellular ATP by either oligomycin or H2O2, is accompanied by depletion of nuclear Ape1, while other BER proteins are unaffected and retain their nuclear localization under these conditions. Importantly, while H2O2 induces γH2AX phosphorylation, indicative of chromatin rearrangements in response to DNA damage, oligomycin does not. Furthermore, despite comparable diminution of ATP content, H2O2 and oligomycin differentially affect critical parameters of mitochondrial respiration that ultimately determine cellular ATP content. Taken together, our findings demonstrate that in neurons, nuclear compartmentalization of Ape1 depends on ATP and loss of nuclear Ape1 reflects disruption of neuronal energy homeostasis. Energy crisis is a hallmark of stroke and other ischemic/hypoxic brain injuries. In vivo studies have shown that Ape1 deficit precedes neuronal loss in injured brain regions. Thus, our findings bring to light the possibility that energy failure-induced Ape1 depletion triggers neuronal death in ischemic brain injuries.
Keywords: Ape1, ATP, neuron, base excision repair, DNA damage, mitochondrial respiration, ischemia
INTRODUCTION
Apurinic/apyrimidinic endonuclease 1 (Ape1) is a pleiotropic protein involved in multiple processes which govern cellular growth and death [1–3]. Ape1 protein is essential for survival, and early embryonic lethal when genetically eliminated [4, 5]. Its primary role in the base excision repair (BER) process includes endonuclease and exonuclease enzymatic activities [6, 7]. As an endonuclease Ape1 recognizes abasic sites generated by different DNA glycosylases, and cleaves the sugar-phosphate backbone 5′ to the lesion, generating a 3′-hydroxyl group which serves substrate for subsequent DNA repair synthesis. Less extensively studied activities of Ape1, include redox sensitive transcriptional regulation and signaling in adaptive survival and growth processes, as well as in cell death [8–10]. Of particular interest in this context is Ape1 n-terminal domain, which is absent in bacterial ortholog and implicated in regulatory functions in eukaryotes [10–13]. Ape1 is subject of diverse posttranslational modification [12, 14] and more recently was found a target for degradation via the ubiquitination pathway [15].
In the mammalian brain, Ape1 is abundantly expressed and via the base excision repair (BER) process plays a central role in maintenance of genomic integrity in post mitotic neurons [16–20]. In rodent models of cerebral ischemia, preservation of nuclear Ape1 was linked with neuronal survival, whereas loss of Ape1 coincided with neuronal death [21–24]. More recently, neuroprotection by elevated Ape1 was reported in a setting of cerebral ischemia [25]. In vitro, protection by elevated Ape1 from glutamate-induced DNA damage, was observed [26], while reduction of Ape1 endonuclease activity by Cdk5-mediated phosphorylation resulted in neuronal death [27].
In view of acute neuronal sensitivity to loss or impairment of Ape1, and since nuclear localization of Ape1 is critical for execution of its essential functions, we asked what mechanisms might trigger deleterious losses of nuclear Ape1 under compromised conditions. Because an earlier report suggested that supplemental ATP facilitates nuclear translocation of Ape1 [28], we investigated whether reduced cellular ATP levels might fail to sustain the appropriate nuclear localization of Ape1. We administered two distinct challenges, which decreased ATP content to a similar extent in cultured primary neurons: oligomycin, an inhibitor of mitochondrial ATP synthase [29], and H2O2, an oxidant which compromises mitochondrial function. We found that both treatments caused comparable decreases in cellular ATP and induced significant depletion of nuclear Ape1 protein and abasic site incision activity. In sharp contrast, nuclear localization of other BER enzymes remained unperturbed under these conditions, indicating that Ape1 is differentially affected by energy status of the cell. Differential sensitivity of Ape1 to energy homeostasis supports a specialized role for Ape1, which is distinct from its role in BER process.
MATERIALS AND METHODS
Preparation and treatment of neuronal cultures
All procedures involving mice handling were approved by the IACUC at the University of Texas Medical Branch. Primary neurons were prepared from cortices of C57BL6 mice at embryonic day 17 and cultured as described with some modifications [30–32]. Briefly, cortical tissues were separated, meninges removed, cortices mechanically disrupted in calcium-magnesium free Dulbecco’s Phosphate Buffered Saline (DPBS), incubated with 1 mg/ml papain (Worthington, Lakewood, NJ) in Hibernate A Medium (Brain Bits LLC, Springfield, IL, USA) at 30°C for 9 minutes followed by an addition of 3 μg/ml DNase I (Roche Diagnostics, Indianapolis, IN, USA) for further 3-minute incubation at 25°C. Suspension was triturated, passed through a 70 μm strainer and centrifuged at 250 g for 8 minutes (ILC Centra CL2). Supernatants were discarded and pellets were resuspended with DPBS containing 30 μg/ml DNAseI. Subsequent pellets were washed, resuspended in neurobasal medium supplemented with 2% (v/v) B-27 supplement, 0.5 mM L-glutamine (Glutamax®) and seeded in poly-L-Lysine-coated (P899, Sigma, St. Louis, MO, USA) culture plates at 1.5 × 105/cm2. Medium was replaced after 30 minutes and cultures maintained at 37°C in humidified 95% air/5% CO2 incubator; cultures yield >98% neurons. On the seventh day in culture (DIV7), neurons were incubated with 25 μM and 50 μM hydrogen peroxide (EMD Gibbstown, NJ, USA) or 1 nM and 2.5 nM Oligomycin (Sigma, St. Louis, MO, USA) and processed for the various assays. Reagents for cell culture were from Invitrogen/GIBCO (Grand Island, NY, USA).
Immunocytochemistry
Neurons were seeded on coverslips in 12- or 24-well plates, treated on DIV7, fixed with 4% paraformaldehyde (Sigma) for 30 minutes at 37°C, washed twice with PBS and permeabilized with 0.1% Triton (X-100)/0.1% sodium citrate/PBS for 9 min. Nonspecific binding was blocked with 3% BSA/1% donkey serum/PBS for 40 min at 37°C and coverslips incubated with primary antibodies diluted in 1.5% BSA/0.5% donkey serum in PBS for 1.5 h at 37°C as follows: rabbit anti-Map2 (1:1000, Chemicon, AB 5622) in combination with mouse anti-ser139 phosphorylated γH2AX (1:3000, Upstate (05636), mouse anti-APE (1:100,000, Novus biological, NB 100–116), mouse anti-Ligase3 (1:3000, BD Transduction Laboratories, #611876), rabbit anti-XRCC1 (1:400, Santa Cruz, sc -11429), followed by 3 washes with 1% BSA in PBS and incubated 45 minutes in the dark at 37°C with Alexa dye conjugated secondary antibodies (Molecular Probes/Invitrogen): Alexa Fluor® 488 donkey anti-rabbit IgG, Alexa Fluor®594 donkey anti-mouse, Alexa Fluor® 488- donkey anti-mouse IgG, Alexa Fluor® 594-donkey anti-rabbit IgG. Coverslips were washed with PBS (3x/5 min), mounted with Prolong®gold antifade (Invitrogen) and examined using IX71 Olympus fluorescence microscope equipped with QIC-F-M-12-C cooled digital camera (QImaging, Surrey, BC, Canada) with QCapture Pro 5 software.
Measurement of intracellular ATP
ATP content was determined with ATP Bioluminescence Assay Kit HS II (Roche Molecular Biochemicals, Indianapolis, IN, USA) according to manufacturer with some modifications. Briefly, on DIV7, neurons were washed with cold PBS, collected in 150 μl of cell lysis reagent and mixed with equal amount of dilution buffer. Lysates were centrifuged at 16,000 g/8 minutes at 4°C, supernatants (50 ul) transferred to black microtiter plates (Greiner Bio-one, 655090), and mixed with equal amount of luciferase reagent [32]. Measurements of luminescence were at 5 s integration time (TECAN Genios plate Reader). ATP amounts were calculated from log-log graph generated for ATP standard using Magellan™ software. ATP amounts were normalized to protein and presented as percent relative to control. ATP average for controls was ~35 nmoles/mg protein, consistent with reported range [33]. Standard curve linear range was 10−6 to 10−10 M.
Nuclear extracts
Nuclear extracts were prepared as we described [20]. Briefly, on DIV7 neurons were gently collected in PBS, pelleted and suspended in cold hypotonic buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 2 μg/ml Pepstatin, and Complete Protease Inhibitor Cocktail Tablet; Roche Diagnostics) followed by 30 min on ice. Swollen cells were disrupted by homogenization and spun at 800 g for 3 min; nuclear pellets were gently washed, resuspended in high-salt buffer (420 mM NaCl) and extracted at 4°C for 30 min with gentle agitation. Extracts were cleared by centrifugation (18 000 g, 10 min), made 15% glycerol and stored at −80°C.
In vitro DNA Repair assays
Abasic site incision and nick ligation reactions were assembled with duplex oligonucleotide substrates carrying the respective adducts. Nick ligation substrate was prepared by annealing two top 21- and 24-nt oligonucleotides (5′-ATTACGAATGCCCAC ACC GCC -3′ and 5′-GGCGCCCACCACCACTAGCTGGCC-3′) with complementary 45-mer oligonucleotide (3′-TAATGCTTACGGGTGTGGCGGCCGCGGGTGGTGGTGATCGACCGG-5′). To model abasic site, an analog-tetrahydrofuran (THF) was used (Cat #3854; Trevigen,) and substrate prepared by annealing 20-mer carrying centrally located THF (5′-CCTGCCCTG(THF)GCAGCTGTGC-3′) with complementary 20-mer (3′-CGGACGGGACACGTCGACACG-5′). Top oligomers were 5′-end-labeled with T4 polynucleotide kinase in the presence of [γ-32P] ATP, annealed and catalytic activities determined from conversion rates of substrate to product. Nick ligation reactions were assembled with DIV7 neuronal nuclear extracts (1.5 μg), 20 fmol end-labeled duplex substrate, 5 mM ATP in buffer (50 mM Tris–HCl pH 8.0, 10 mM KCl, 60 mM NaCl, 10 mM MgCl2, 1 mM DTT, 1% glycerol) in 20 μl at 37°C. Reactions were terminated with loading buffer (96% formamide, 20 mM EDTA, pH 8, 0.025% bromophenol blue, and 0.025% xylene cyanol) and heated at 98°C for 10 min prior to loading, as we described [20, 31]. For abasic site incision assays, reactions were assembled with 20 ng nuclear extract (buffer: 66 mM Tris–HCl, pH 7.6, 1 mM DTT, 1 mM MgCl2, 0.2 mM EDTA, 0.1 mg/ml BSA) and terminated with loading buffer without heating. Recombinant hApe1 (Trevigen, Gaithesburg, MD, USA) served as positive control. Reaction mixtures were resolved in 14% and 16% polyacrylamide, respectively, 7 M urea gels in Tris-Borate buffer, pH 8.3 at 14 mA; products were visualized by autoradiography and quantified on Phosphorimager. Products generated in at least three independent reactions were used to calculate mean ± SEM.
Measurements of oxygen consumption rates in primary neuronal cultures
Mitochondrial respiration in cultured neurons was examined using the Seahorse XF24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA) that measures in real time oxygen consumption [34]. All procedures were done according to manufacturer’s instructions: neurons were seeded at 3.3 × 105/cm2 in neurobasal medium on XF24 V7 assay plates coated with Poly-D-Lysine (Sigma); on DIV7 following treatment, growth medium was replaced with unbuffered Dulbecco’s modified Eagle’s medium (DMEM, Seahorse Bioscience) supplemented with 5 mM pyruvate, 25 mM glucose and 2 mM Glutamax (all media and reagents were adjusted to pH 7.4 at that time) and incubated in CO2 free incubator at 37°C for 1 hour prior to XF24 measurements. Assay protocol was implemented by the XF24 Reader software Version 1.7 to sequentially measure oxygen consumption rates (OCR). Three measurements at 5-minute intervals were taken for each segment of the assay. To obtain respiration profiles for control and treated cultures, baseline OCR measurements were followed by sequential automated addition of reagents through the ports of XF24 cartridges to final concentrations of 1.2 μM Oligomycin (O4876, Sigma), 0.4 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), (C2920, Sigma) and 2.7 μM antimycin A (A8674, Sigma); these concentrations were determined by titration under our experimental conditions. Modulations of mitochondrial activities by these reagents translate into changes in OCR, enabling computation of relative changes versus control, induced by a given treatment [35–37].
Statistical analysis
Data are presented as mean ± SEM from 3–6 independent experiments, as indicated. Two-tailed Student’s t-test was used to analyze the difference between the two groups. P≤0.05 was accepted as statistically significant.
RESULTS
1. Comparable decreases in ATP content in cultured primary neurons are produced by H2O2 and oligomycin
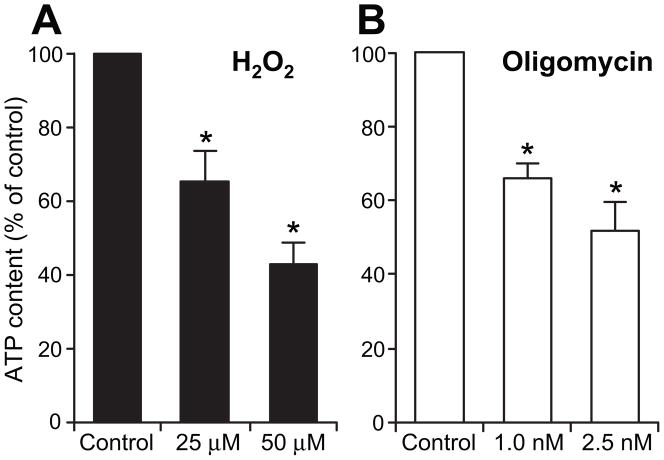
Oligomycin, an inhibitor of mitochondrial ATP-synthase, blocks oxidative phosphorylation and reduces cellular ATP content in a dose- and time-dependent manner. We have determined that a 3-hour incubation of primary neuronal cultures with low concentrations of 1- and 2.5 nM oligomycin leads to dose-dependent reduction of 30%- and nearly 50% in ATP content, respectively (Fig 1). Similarly, 3-hour incubation with 25 μM and 50 μM H2O2 resulted in dose dependent ~30% and ~55% reduction in ATP (Fig 1).
Figure 1. Reduction of neuronal ATP content by H2O2 and oligomycin exposures.
Cellular ATP content was measured in DIV7 neurons incubated with indicated concentrations of H2O2 and oligomycin. Cultures were lysed and ATP content determined using ATP Bioluminescence Assay and normalized to protein amount. ATP content was significantly reduced: 3-hour exposure to 25 μM H2O2 resulted in ~30% reduction, whereas exposure to 50 μM, reduced ATP content by ~55%. Exposures to 1 nM and 2.5 nM oligomycin resulted in 30% and nearly 50% reduction, respectively. Values are presented as means ± SEM relative to non-treated controls for 6 independent experiments; *P<0.05 versus control.
2. H2O2 but not oligomycin exposures induce DNA damage in neurons
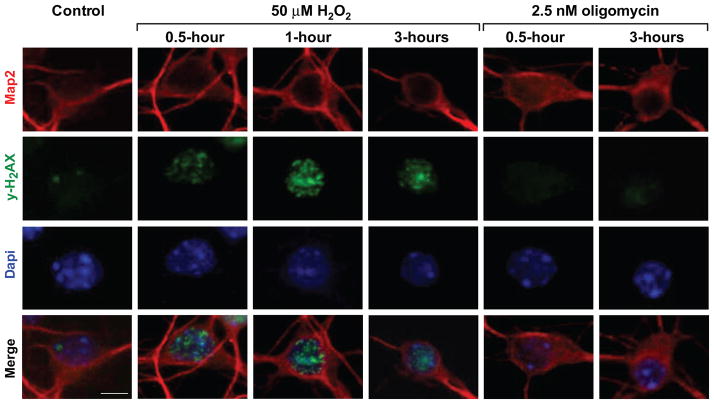
Since in neurons, exposures in this range of H2O2, lead to formation of oxidative DNA damage [31], we asked whether oligomycin at concentrations, which exert similar changes in ATP content, might also induce DNA damage. This was assessed by monitoring emergence of immunoreactivity for H2AX phosphorylated at serine 139, which reflects chromatin changes in response to different types of DNA damage [38]. In the course of H2O2 and oligomycin exposures, changes in neuronal morphology including, rounding of nuclei (DAPI, blue) were observed (Fig 2). As expected, H2O2 exposure induced foci positive for phosphorylated γH2AX (Fig 2, green). Foci were detected already after 5 minutes (not shown), with intensification of γH2AX immunoreactivity within 30 and 60 minutes, indicative of progression of chromatin modifications associated with DNA damage/repair processes; the lower dose of 25 μM H2O2 induced lesser changes and in fewer neurons (not shown). In contrast, no induction of γH2AX positive foci was observed during treatment with 2.5 nM oligomycin (right panel), demonstrating that in neurons, ATP reduction by oligomycin does not generate DNA damage.
Figure 2. Induction of γH2AX phosphorylation by exposure to H2O2 but not to oligomycin.
Representative Immunofluorescent images of DIV7 neurons reacted with anti Map2 (neuronal marker, red) and anti-γH2AX (phosphorylated at serine 139, green; nuclei stain blue with DAPI) are shown. γH2AX positive foci emerge and intensify in the course of exposure to 50 μM H2O2 (green), indicative of chromatin modifications associated with formation and processing of DNA damage. In contrast, γH2AX immunoreactivity was undetectable in the course of exposure to 2.5 nM oligomycin, indicating that significant DNA damage is not induced under these conditions (right), bar=6 μm.
3. H2O2 and oligomycin exposures cause depletion of nuclear Ape1 in primary neurons
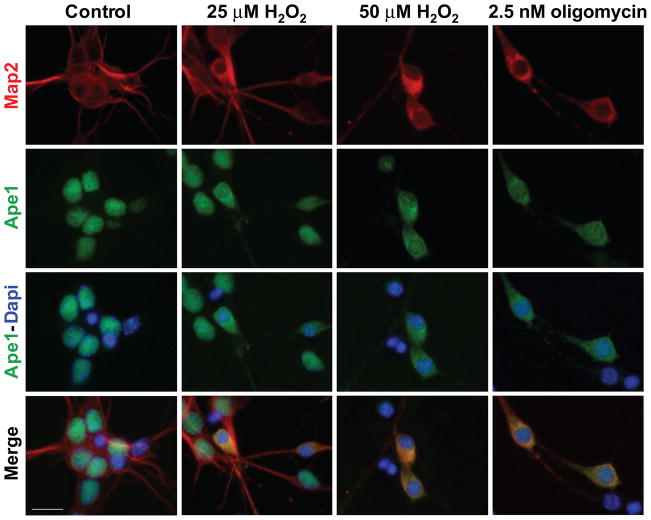
Because in neurons oxidative DNA damage is primarily processed by the BER pathway, we examined the level and distribution of the central BER enzyme, Ape1, before and during H2O2 and oligomycin exposures. In non-exposed control neurons, immunofluorescent staining (Fig 3, left panel) revealed substantial Ape1 immunoreactivity in nuclear compartment (neurons are identified by Map2 in red, whereas nuclear Ape1 is observed in green). Following a 3-hour exposure to 50 μM H2O2, neuronal nuclei became rounded with diminishing nuclear Ape1, while cytoplasmic Ape1 immunoreactivity concomitantly increased. Exposure to the lower concentration of 25 μM H2O2 resulted in lesser nuclear depletion of Ape1 and in fewer neurons, when compared to 50 μM (31.8%±3.4% and 72.4%±3.8%, respectively). Likewise, depletion of nuclear Ape1 was observed in neurons incubated for 3 hours with 2.5 nM oligomycin (61.3%±3.3), whereas the lower, 1 nM concentration of oligomycin, did not have significant effect (not shown).
Figure 3. Depletion of nuclear Ape1 by exposure of neurons to H2O2 and oligomycin.
Immunofluorescent images of primary neuronal cultures are shown. Neuronal soma and extensions are visualized by Map2 immunoreactivity (red), whereas Ape1 is observed in green (nuclei stain blue with DAPI; bar=12 μm). On DIV7, cultures were exposed to H2O2 or oligomycin at indicated concentrations. Under control conditions (left panel), neuronal extensions are robust (Map2, red) and nuclei show uniform Ape1 immunoreactivity (green), which overlaps with DAPI (blue). In addition, few condensed apoptotic nuclei, which are inherent to primary neuronal cultures and stain with DAPI alone, are also observed. Following incubations with the higher concentration of either H2O2 (50 μM) or oligomycin (2.5 nM), neuronal extensions are shortened, nuclei become rounded and Ape1 immunoreactivity (green) increases in cytoplasm and decreases in nuclear compartment. Following exposure to 25 μM H2O2 ~30% of neurons undergo changes, while in the case of 50 μM H2O2 or 2.5 nM oligomycin, the proportion of affected cells increases to ~70% and 60%, respectively. Concomitantly, nuclear Ape1 immunoreactivity (green) decreases and shifts to cytoplasm. In merged images, yellow color (bottom) reflects an overlap of cytoplasmic Ape1 (green) and Map2 (red).
4. Nuclear localization of base excision repair proteins, DNA ligase 3 and Xrcc1, is not altered by exposures to either H2O2 or oligomycin
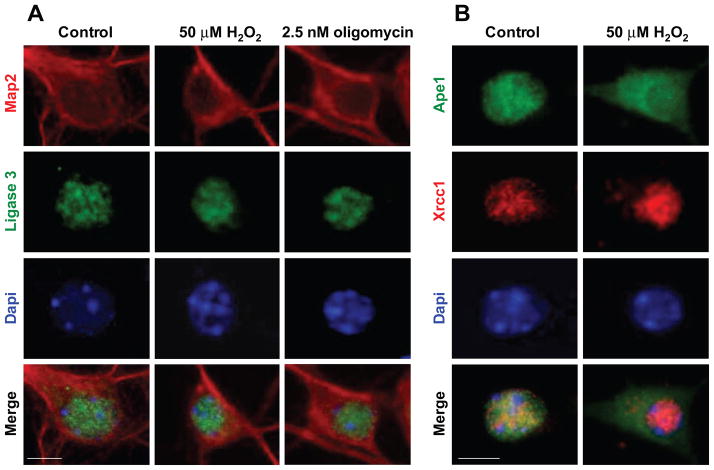
In view of observed changes in subcellular localization of neuronal Ape1, we examined status of other BER proteins before and during H2O2 and oligomycin exposures (Fig 4). As expected, in control cultures, immunoreactivity for key BER proteins DNA ligase 3, x-ray cross complementation group 1 (Xrcc1) and DNA polymerase beta (pol-β) was localized in nuclear compartment (Fig 4A, neurons are identified by Map2 in red; ligase 3 immunoreactivity is green). Nuclear localization of ligase3 was not altered after 3-hour exposure to either H2O2 or oligomycin (Fig 4A). Likewise, immunoreactivity for the BER scaffold protein Xrcc1 (Fig 4B, red) and DNA polymerase beta (not shown) was fully retained in neuronal nuclei under these conditions. This was visualized by double staining for Xrcc1 and Ape1 (Fig 4B), with Xrcc1 immunoreactivity fully retained in nucleus (red), while nuclear Ape1 (green) was depleted and shifted to cytoplasmic compartment (merged image, bottom right).
Figure 4. Nuclear localization of BER proteins, ligase 3 and Xrcc1 is maintained, while nuclear Ape1 is depleted following H2O2 and oligomycin exposures.
(A) Immunofluorescent images of DIV7 neurons reacted with anti Map2 antibody (red) and anti-ligase3 (green), show nuclear immunoreactivity of ligase 3, which coincides with DAPI (blue; scale bar=6 μm). Nuclear localization of ligase 3 (green, left panel) was not altered by either 50 μM H2O2 or 2.5 nM oligomycin exposures, and fully retained in the nuclear compartment (middle and right panels). (B) Xrcc1 is retained while Ape1 is depleted from nuclei and accumulates in cytoplasm following H2O2 exposure. Following exposure to H2O2, Xrcc1 immunoreactivity is retained in nucleus (red), while Ape1 immunoreactivity (green) shifts to cytoplasm (top, right); consequently, no overlap of Xrcc1 and Ape1 immunoreactivity is detected in merged images after treatment (bottom, right).
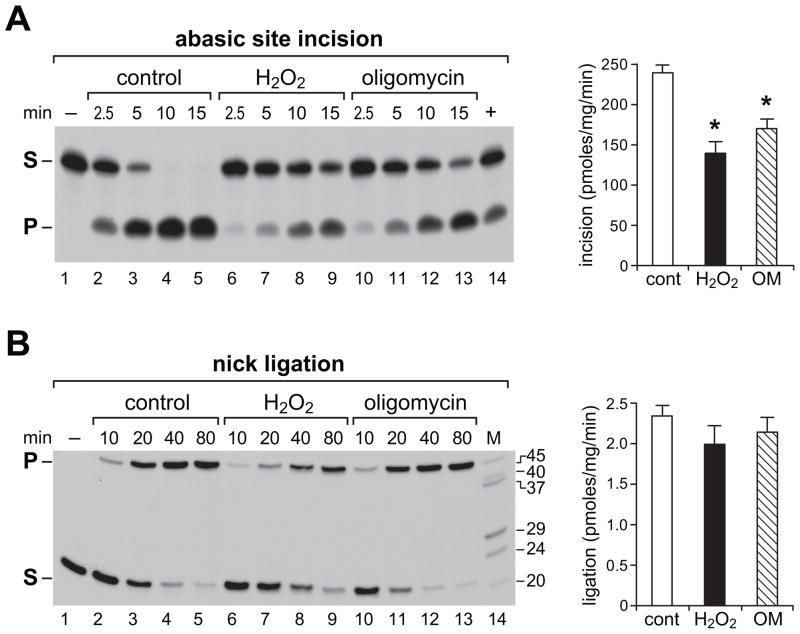
5. Abasic site incision activity but not nick ligation is diminished following exposure of primary neurons to H2O2 and oligomycin
Next, we asked to what extent nuclear depletion of Ape1 following H2O2 or oligomycin exposure is reflected in reduction of abasic site incision activity. In neuronal BER, abasic sites are targeted primarily by Ape1 endonuclease, whereas nick ligation is catalyzed mainly by DNA ligase 3. To compare the effects of H2O2 and oligomycin on abasic site incision and nick ligation in nuclear extracts from DIV7 neurons, we measured conversion rates of radioactively labeled, adduct-carrying oligonucleotide substrates into predicted products (Fig 5). Compared to control neurons (Fig 5A, lanes 2–5), abasic site incision was significantly reduced by either H2O2 (Fig 5A, lanes 6–9) or oligomycin (Fig 5A, lanes 10–13), consistent with depletion of nuclear Ape1, observed as reduced nuclear immunoreactivity (Fig 3). In contrast, no significant reduction in nuclear nick ligation activity was observed following same treatments (Fig 5B). This further agrees with immunofluorescent localization analyses (Fig 4), showing no evidence of nuclear depletion of DNA ligase 3 or of the closely associated scaffold protein, Xrcc1, following either H2O2 or oligomycin exposures. Bar graphs show incision and ligation activities calculated from average product yields generated in linear range of each reaction presented as mean ± SEM.
Figure 5. Abasic site incision and nick ligation activities in nuclear extracts from DIV7 neurons are differentially affected by exposures to H2O2 and oligomycin.
Reactions were assembled with radioactively labeled substrates. Products resolved in denaturing polyacrylamide gels were visualized by autoradiography; representative autoradiograms are shown. Time points are indicated and a substrate-only lane is included (lane 1). (A) Incision assay of abasic site (THF) carrying substrate. Reaction with recombinant human Ape1 serves as positive control (lane 14). 5′-end labeled 20-mer substrate (S) and the 9-mer incision product (P) are indicated. (B) Nick ligation assay. 5′-end labeled 21-mer substrate (S) and ligated 45-mer product (P) are indicated. Labeled oligonucleotides resolved in parallel provide size markers (M). Bar graphs represent product yields calculated from phosphorimager values and expressed as pmoles/min/mg protein. Values for three or four independent assays for each substrate in the linear range of reaction were used to obtain mean ± SEM. *P<0.05 versus control.
6. Mitochondrial respiration in primary neurons is differentially affected by exposures to H2O2 and oligomycin
Because our study revealed comparable effects of oligomycin and H2O2 on neuronal ATP content, as well as on subcellular localization of Ape1, while only H2O2 treatment induced DNA damage, we proceeded to investigate modulations of critical bioenergetic parameters, which might be contributing to these differences. Since in neurons majority of ATP is generated by oxidative phosphorylation, we asked how a 3-hour exposure to either H2O2 or oligomycin affects different parameters of mitochondrial respiration. This was approached using the XF24 extracellular flux analyzer (Seahorse Bioscience), which supports real time measurements of oxygen consumption rates (OCR) in living cells [34]. We compared respective contributions of the different oxygen consuming processes (bioenergetic profile), i.e., baseline cellular respiration, ATP synthesis, proton leak, FCCP response and non-mitochondrial oxygen consumption, in normal and challenged cultures (Fig 6). Neuronal baseline rates of oxygen consumption and changes in responses to various mitochondrial effectors were first determined under normal culture conditions. The average baseline OCR for DIV7 neurons seeded at 105/well measured ~200 pmoles O2/min (Fig 6A, circles). The portion of oxygen consumption devoted to ATP synthesis (which is eliminated by oligomycin supplied at a concentration titrated for immediate-upon addition effect) was ~65%, while the remaining ~35% fed into proton leak (~15%) and non-mitochondrial respiration (~20%), which is measurable after administration of the potent inhibitor of complex III, antimycin A. Maximal respiratory capacity was measured after addition of FCCP, an ionophore which carries protons across the membrane independently of ATP synthesis and thus reveals the cell type-specific maximal OCR. The difference between maximal OCR and baseline OCR is defined as spare respiratory capacity (SRC). In the case of DIV7 neurons, OCR feeding the spare respiratory capacity was at ~60% above baseline. Next, bioenergetic profiles were determined for neurons challenged by exposures to 25 μM (solid triangles) and 50 μM (solid squares) H2O2 (Fig 6A). Baseline OCR was reduced ~20% by 50 μM but not by 25 μM H2O2 (Fig 6B). Oxygen consumption associated with ATP synthesis was significantly reduced by 50 μM H2O2, while oxygen consumption feeding the proton leak increased: a nearly 65% decrease in ATP synthesis-linked OCR was measured, OCR portion associated with proton leak increased by 150% compared to proton leak measured under control conditions, and respiratory reserve capacity measured after addition of FCCP, was completely eliminated and dropped below baseline level (Fig 6B). Similar trend of changes was noted following exposure to 25 μM H2O2, however, the magnitude was smaller, with ~30% decrease in ATP synthesis-linked OCR, ~100% increase in proton leak and ~60% reduction in respiratory reserve capacity (Fig 6B).
Figure 6. Differential effects of H2O2 and oligomycin on mitochondrial respiratory profiles in primary neurons.
On DIV7 neurons were exposed on to H2O2 or oligomycin and along with non-treated control neurons, processed for measurements of oxygen consumption rates (OCR) with XF24 analyzer. Respiratory profile established for non-treated control neurons (A & C, circles) showed baseline OCR of approximately 200 pmoles O2/min/105 neurons. Sequential, in port additions of modifiers of mitochondrial activities (downward arrows), revealed that under normal conditions ~65% of consumed oxygen feeds into ATP synthesis, proton leak is limited to ~15% of oxygen consumption, spare respiratory capacity is at ~60%, while non mitochondrial respiration accounts for ~20% of total oxygen consumption. Respiratory profile was changed following a 3-hour exposure to 25 μM H2O2 (A, solid triangles) and to a greater extent following exposure to 50 μM H2O2 (A, solid squares). Changes in respiratory profile emerged also after exposure to 1 nM oligomycin (C, open triangles) and to a much greater extent after a 2.5 nM treatment (C, open squares). The extent of changes induced by each treatment versus respective control, was calculated for the different segments of respiratory profiles (baseline, ATP synthesis, proton leak and spare respiratory capacity) and presented as means ± SEM of 4–5 independent experiments for each treatment: H2O2 (B, solid bars) and oligomycin (D, open bars). * P<0.05 versus respective control.
Measurements of respiratory parameters after exposure to 2.5 nM oligomycin (Fig 6C) revealed fundamental differences when compared to controls and cultures treated with H2O2: baseline oxygen consumption was reduced by nearly 70% (open squares), such that the portion corresponding to ATP synthesis-linked oxygen consumption, was practically eliminated (Fig 6D); as expected, a further-in port addition of oligomycin did not exert significant changes in OCR. Interestingly, respiratory reserve capacity, i.e., capability to increase OCR in response to the ionophore, FCCP, was retained (Fig 6C, D). This suggests that mitochondria were not significantly impaired by incubation with the low 2.5 nM dose of oligomycin and that the inhibition of ATP synthesis accounts for the measured decrease in OCR. Exposure to 1 nM oligomycin (triangles) had a significantly lesser effect on OCR profile (Fig 6C, D).
DISCUSSION
We have shown that in neurons, diminution of ATP content leads to nuclear depletion of the DNA repair/redox sensing protein, Ape1/Ref-1. An equivalent diminution of neuronal ATP was produced by two distinct reagents, oligomycin and H2O2, which in the latter case was accompanied by formation of DNA damage. Bioenergetic profiles revealed that despite comparable decreases in ATP content, the underlying mitochondrial events were quite different. Oxygen consumption measurements showed in the case of oligomycin, an inhibitor of mitochondrial ATP synthase, reduced baseline oxygen consumption rate (OCR), reflecting elimination of oxygen consumption coupled to ATP synthesis. This was accompanied by a nearly 50% ATP deficit, relative to normal steady state ATP levels. No significant increases in proton leak or decreases in spare respiratory capacity, which typically are associated with increased production of ROS [39] were observed. Accordingly, formation of DNA damage was not detected with this treatment. Although the extent of ATP reduction by H2O2 was quite similar, the resultant bioenergetic profile was fundamentally different. While, baseline OCR was only marginally reduced, OCR linked with ATP synthesis significantly decreased, with concomitant sharp increase in oxygen feeding into proton leak. Increased proton leak has been associated with elevated ROS and formation of DNA damage. Moreover, spare respiratory capacity, which reflects mitochondrial proficiency in adaptation to increased energy demands [36], was eliminated with maximal OCR falling below baseline respiration. Notwithstanding, a similar reduction in ATP, a shift in Ape1 subcellular localization and a reduction in nuclear activity of Ape1, have emerged as common end points of these fundamentally different challenges, suggesting that in neurons, Ape1 localization and thereby its function depend on ATP availability. Ameliorative effects of exogenous augmentation of ATP levels observed in in vitro models of neuronal injury [40] are consistent with this scenario.
Ape1 is essential for cellular survival and embryonic lethal in knockout mouse models [41]. It is ubiquitously expressed and critical in the mammalian brain [25]; in rodent models of stroke, loss of Ape1 coincides with neuronal death in ischemic core and penumbra, while Ape1 preservation is consistent with neuronal survival [21–24]. Here, we show that reagents, which diminish neuronal ATP, albeit by distinct mechanisms, lead to comparable depletion of nuclear Ape1. Since the magnitude of ATP deficit is similar with these treatments, it is plausible that energy status governs proper Ape1 localization and thereby sustains Ape1 function in neurons. Remarkably, nuclear levels, catalytic activity and localization of other key proteins, which participate in the base excision repair process in neurons [18], are not altered by these treatments. This difference is consistent with documented involvement of Ape1 in diverse cellular processes in addition to DNA repair, and might reveal a novel role for Ape1 in sensing energy status in neurons.
Trafficking of Ape1 between cellular compartments has been observed by others: reports describe translocations of Ape1 into nucleus, mitochondria or cytoplasm induced by diverse stimuli, reflecting involvement in broad range of responses to different types of stressors [28, 42–47]. Interestingly, reliance of Ape1 translocation into the nucleus on adequate ATP levels has been noted by Pines et al. [28]. These investigators found that exogenous ATP stimulates cytoplasm to nucleus Ape1 movement and thereby, exerts protection from H2O2-induced cell death. Ameliorative augmentation of nuclear Ape1 levels and subsequent efficient removal of DNA damage, were observed also following mild glutamate stimulation of cortical neurons [26]. Mild stimulation of glutamate receptors activates mitochondrial respiration and increases ATP. It is therefore plausible, that after transient glutamate challenge, elevated ATP facilitates nuclear translocation of Ape1 to augment oxidative DNA damage repair capacity in neurons.
Our findings showing depletion of nuclear Ape1 are also in agreement with nuclear emptying, that was observed in human glioblastoma cell line after treatment with anti cancer compound, which blocks the redox function of Ape1 [43]. Ape1 redox functions, involved in cell survival, adaptation and proliferation processes, require particular cysteine residues in the protein [48]. Specified cysteines were reported to mediate nuclear to cytoplasm translocation of Ape1 following exposure to nitric oxide and S-nitrosation, which could be reversed by reductants [47]. Interestingly, altered Ape1 was observed in fibroblasts derived from donors with Gaucher disease, which is a storage disorder with altered intracellular redox. Gaucher patients’ fibroblasts, showed reduced levels of nuclear Ape1, with concomitant increase in Ape1 cytoplasmic content, increased cellular sensitivity and inability to respond to H2O2 challenge [45]. Similarly, Wu et al., [44] noted predominantly cytoplasmic Ape1 localization in lung tumors. Although not a subject of the current study, it is plausible that specialized tumor-cell energy environment triggers depletion of nuclear Ape1 to further undermine genomic integrity in cancer cells.
Loss of neurons in brains injured by stroke is a major contributor to irreversibility of stroke injury and poor patient prognosis. In ischemic/hypoxic models of brain injury, ATP production is compromised early on. Moreover, ischemic activation of PARP-1 exacerbates brain injury via consumption of ATP, while disruption of the PARP1 gene is protective [49]. In rodent models of stroke, Ape1 depletion was identified as an early predictor of neuronal loss, yet the mechanisms which trigger Ape1 disappearance have not been elucidated. Here, we link for the first time ATP deficit in neurons with the failure of Ape1 to maintain its nuclear localization and subsequent inability to fulfill critical functions, which take place in the nuclear compartment and are indispensible for neuronal survival. Thus, depletion of nuclear Ape1 and loss of its appropriate localization might represent an early manifestation of deleterious energy deficits associated with hypoxic/ischemic brain injuries.
Highlights.
Ape1/Ref-1 is a redox sensing/DNA repair protein indispensable for cell survival.
In vivo models of ischemic brain reveal that loss of Ape1 precedes neuronal death.
We show that in primary neurons, ATP deficit leads to depletion of nuclear Ape1.
Neuronal Ape1 depends on energy homeostasis for nuclear localization and function.
Energy failure-induced Ape1 loss might trigger neuronal death in ischemic brain.
Acknowledgments
This work was supported by National Institutes of Health grants NS039449 and ES014613 and Shriners Hospitals for Children grant SHG8670 to EWE. We thank Eileen Figueroa and Steve Schuenke for help with manuscript preparation. The funding organizations played no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Abbreviations
- Ape1
Apurinic/apyrimidinic endonuclease 1
- BER
base excision repair
- DIV
day in vitro
- OCR
oxygen consumption rate
- SRC
spare respiratory capacity
- Ref-1
redox factor-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhakat KK, Mantha AK, Mitra S. Transcriptional regulatory functions of mammalian AP-endonuclease (APE1/Ref-1), an essential multifunctional protein. Antioxid Redox Signal. 2009;11:621–638. doi: 10.1089/ars.2008.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28:375–395. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig DL, MacInnes MA, Takiguchi Y, Purtymun PE, Henrie M, Flannery M, Meneses J, Pedersen RA, Chen DJ. A murine AP-endonuclease gene-targeted deficiency with post-implantation embryonic progression and ionizing radiation sensitivity. Mutat Res. 1998;409:17–29. doi: 10.1016/s0921-8777(98)00039-1. [DOI] [PubMed] [Google Scholar]
- 5.Xanthoudakis S, Smeyne RJ, Wallace JD, Curran T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc Natl Acad Sci U S A. 1996;93:8919–8923. doi: 10.1073/pnas.93.17.8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demple B, Sung JS. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA Repair (Amst) 2005;4:1442–1449. doi: 10.1016/j.dnarep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Sukhanova MV, Khodyreva SN, Lebedeva NA, Prasad R, Wilson SH, Lavrik OI. Human base excision repair enzymes apurinic/apyrimidinic endonuclease1 (APE1), DNA polymerase beta and poly(ADP-ribose) polymerase 1: interplay between strand-displacement DNA synthesis and proofreading exonuclease activity. Nucleic Acids Res. 2005;33:1222–1229. doi: 10.1093/nar/gki266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jayaraman L, Murthy KG, Zhu C, Curran T, Xanthoudakis S, Prives C. Identification of redox/repair protein Ref-1 as a potent activator of p53. Genes Dev. 1997;11:558–570. doi: 10.1101/gad.11.5.558. [DOI] [PubMed] [Google Scholar]
- 9.Xanthoudakis S, Miao G, Wang F, Pan YC, Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xanthoudakis S, Miao GG, Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A. 1994;91:23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demple B, Herman T, Chen DS. Cloning and expression of APE, the cDNA encoding the major human apurinic endonuclease: definition of a family of DNA repair enzymes. Proc Natl Acad Sci U S A. 1991;88:11450–11454. doi: 10.1073/pnas.88.24.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YJ, Kim D, Illuzzi JL, Delaplane S, Su D, Bernier M, Gross ML, Georgiadis MM, Wilson DM., 3rd S-glutathionylation of cysteine 99 in the APE1 protein impairs abasic endonuclease activity. J Mol Biol. 2011;414:313–326. doi: 10.1016/j.jmb.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robson CN, Milne AM, Pappin DJ, Hickson ID. Isolation of cDNA clones encoding an enzyme from bovine cells that repairs oxidative DNA damage in vitro: homology with bacterial repair enzymes. Nucleic Acids Res. 1991;19:1087–1092. doi: 10.1093/nar/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busso CS, Lake MW, Izumi T. Posttranslational modification of mammalian AP endonuclease (APE1) Cell Mol Life Sci. 2010;67:3609–3620. doi: 10.1007/s00018-010-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busso CS, Iwakuma T, Izumi T. Ubiquitination of mammalian AP endonuclease (APE1) regulated by the p53-MDM2 signaling pathway. Oncogene. 2009;28:1616–1625. doi: 10.1038/onc.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin LH, Cao S, Yu L, Cui J, Hamilton WJ, Liu PK. Up-regulation of base excision repair activity for 8-hydroxy-2′-deoxyguanosine in the mouse brain after forebrain ischemia-reperfusion. J Neurochem. 2000;74:1098–1105. doi: 10.1046/j.1471-4159.2000.741098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PK, Hsu CY, Dizdaroglu M, Floyd RA, Kow YW, Karakaya A, Rabow LE, Cui JK. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J Neurosci. 1996;16:6795–6806. doi: 10.1523/JNEUROSCI.16-21-06795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Englander EW. Brain capacity for repair of oxidatively damaged DNA and preservation of neuronal function. Mech Ageing Dev. 2008;129:475–482. doi: 10.1016/j.mad.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore N, Okocha F, Cui JK, Liu PK. Homogeneous repair of nuclear genes after experimental stroke. J Neurochem. 2002;80:111–118. doi: 10.1046/j.0022-3042.2001.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei W, Englander EW. DNA polymerase beta-catalyzed-PCNA independent long patch base excision repair synthesis: a mechanism for repair of oxidatively damaged DNA ends in post-mitotic brain. J Neurochem. 2008;107:734–744. doi: 10.1111/j.1471-4159.2008.05644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Early decrease of apurinic/apyrimidinic endonuclease expression after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:495–501. doi: 10.1097/00004647-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Kawase M, Fujimura M, Morita-Fujimura Y, Chan PH. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats: implication of the failure of DNA repair in neuronal apoptosis. Stroke. 1999;30:441–448. doi: 10.1161/01.str.30.2.441. discussion 449. [DOI] [PubMed] [Google Scholar]
- 23.Walton M, Lawlor P, Sirimanne E, Williams C, Gluckman P, Dragunow M. Loss of Ref-1 protein expression precedes DNA fragmentation in apoptotic neurons. Brain Res Mol Brain Res. 1997;44:167–170. doi: 10.1016/s0169-328x(96)00291-4. [DOI] [PubMed] [Google Scholar]
- 24.Lewen A, Sugawara T, Gasche Y, Fujimura M, Chan PH. Oxidative cellular damage and the reduction of APE/Ref-1 expression after experimental traumatic brain injury. Neurobiol Dis. 2001;8:380–390. doi: 10.1006/nbdi.2001.0396. [DOI] [PubMed] [Google Scholar]
- 25.Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, Cao G, Bennett MV, Chen J. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Natl Acad Sci U S A. 2010;107:3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JL, Tadokoro T, Keijzers G, Mattson MP, Bohr VA. Neurons efficiently repair glutamate-induced oxidative DNA damage by a process involving CREB-mediated up-regulation of apurinic endonuclease 1. J Biol Chem. 2010;285:28191–28199. doi: 10.1074/jbc.M109.082883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang E, Qu D, Zhang Y, Venderova K, Haque ME, Rousseaux MW, Slack RS, Woulfe JM, Park DS. The role of Cdk5-mediated apurinic/apyrimidinic endonuclease 1 phosphorylation in neuronal death. Nat Cell Biol. 2010;12:563–571. doi: 10.1038/ncb2058. [DOI] [PubMed] [Google Scholar]
- 28.Pines A, Perrone L, Bivi N, Romanello M, Damante G, Gulisano M, Kelley MR, Quadrifoglio F, Tell G. Activation of APE1/Ref-1 is dependent on reactive oxygen species generated after purinergic receptor stimulation by ATP. Nucleic Acids Res. 2005;33:4379–4394. doi: 10.1093/nar/gki751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehninger AL. Oxidative phosphorylation, mitoehondrial structure, and the compartmentation of respiratory metabolism. In: Lehninger AL, editor. Biochemistry. New York: Worth Publishers Inc; 1970. pp. 509–542. [Google Scholar]
- 30.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Swiercz R, Englander EW. Elevated metals compromise repair of oxidative DNA damage via the base excision repair pathway: implications of pathologic iron overload in the brain on integrity of neuronal DNA. J Neurochem. 2009;110:1774–1783. doi: 10.1111/j.1471-4159.2009.06271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Misiak M, Beyer C, Arnold S. Cytochrome c oxidase isoform IV-2 is involved in 3-nitropropionic acid-induced toxicity in striatal astrocytes. Glia. 2009;57:1480–1491. doi: 10.1002/glia.20864. [DOI] [PubMed] [Google Scholar]
- 33.Wang YH, Borkan SC. Prior heat stress enhances survival of renal epithelial cells after ATP depletion. Am J Physiol. 1996;270:F1057–1065. doi: 10.1152/ajprenal.1996.270.6.F1057. [DOI] [PubMed] [Google Scholar]
- 34.Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe Y, Sakairi T, Kajiyama H, Shrivastav S, Beeson C, Kopp JB. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol. 2010;299:C464–476. doi: 10.1152/ajpcell.00563.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13:1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 39.Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Schock SC, Munyao N, Yakubchyk Y, Sabourin LA, Hakim AM, Ventureyra EC, Thompson CS. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res. 2007;1168:129–138. doi: 10.1016/j.brainres.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 41.Friedberg EC, Meira LB. Database of mouse strains carrying targeted mutations in genes affecting biological responses to DNA damage Version 7. DNA Repair (Amst) 2006;5:189–209. doi: 10.1016/j.dnarep.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Frossi B, Tell G, Spessotto P, Colombatti A, Vitale G, Pucillo C. H(2)O(2) induces translocation of APE/Ref-1 to mitochondria in the Raji B-cell line. J Cell Physiol. 2002;193:180–186. doi: 10.1002/jcp.10159. [DOI] [PubMed] [Google Scholar]
- 43.Vascotto C, Bisetto E, Li M, Zeef LA, D’Ambrosio C, Domenis R, Comelli M, Delneri D, Scaloni A, Altieri F, Mavelli I, Quadrifoglio F, Kelley MR, Tell G. Knock-in reconstitution studies reveal an unexpected role of Cys-65 in regulating APE1/Ref-1 subcellular trafficking and function. Mol Biol Cell. 2011;22:3887–3901. doi: 10.1091/mbc.E11-05-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu HH, Cheng YW, Chang JT, Wu TC, Liu WS, Chen CY, Lee H. Subcellular localization of apurinic endonuclease 1 promotes lung tumor aggressiveness via NF-kappaB activation. Oncogene. 2010;29:4330–4340. doi: 10.1038/onc.2010.178. [DOI] [PubMed] [Google Scholar]
- 45.Deganuto M, Pittis MG, Pines A, Dominissini S, Kelley MR, Garcia R, Quadrifoglio F, Bembi B, Tell G. Altered intracellular redox status in Gaucher disease fibroblasts and impairment of adaptive response against oxidative stress. J Cell Physiol. 2007;212:223–235. doi: 10.1002/jcp.21023. [DOI] [PubMed] [Google Scholar]
- 46.Fan Z, Beresford PJ, Zhang D, Xu Z, Novina CD, Yoshida A, Pommier Y, Lieberman J. Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme A. Nat Immunol. 2003;4:145–153. doi: 10.1038/ni885. [DOI] [PubMed] [Google Scholar]
- 47.Qu J, Liu GH, Huang B, Chen C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of cysteines 93 and 310. Nucleic Acids Res. 2007;35:2522–2532. doi: 10.1093/nar/gkl1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo M, Zhang J, He H, Su D, Chen Q, Gross ML, Kelley MR, Georgiadis MM. Characterization of the redox activity and disulfide bond formation in apurinic/apyrimidinic endonuclease. Biochemistry. 2012;51:695–705. doi: 10.1021/bi201034z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3:1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]