Abstract
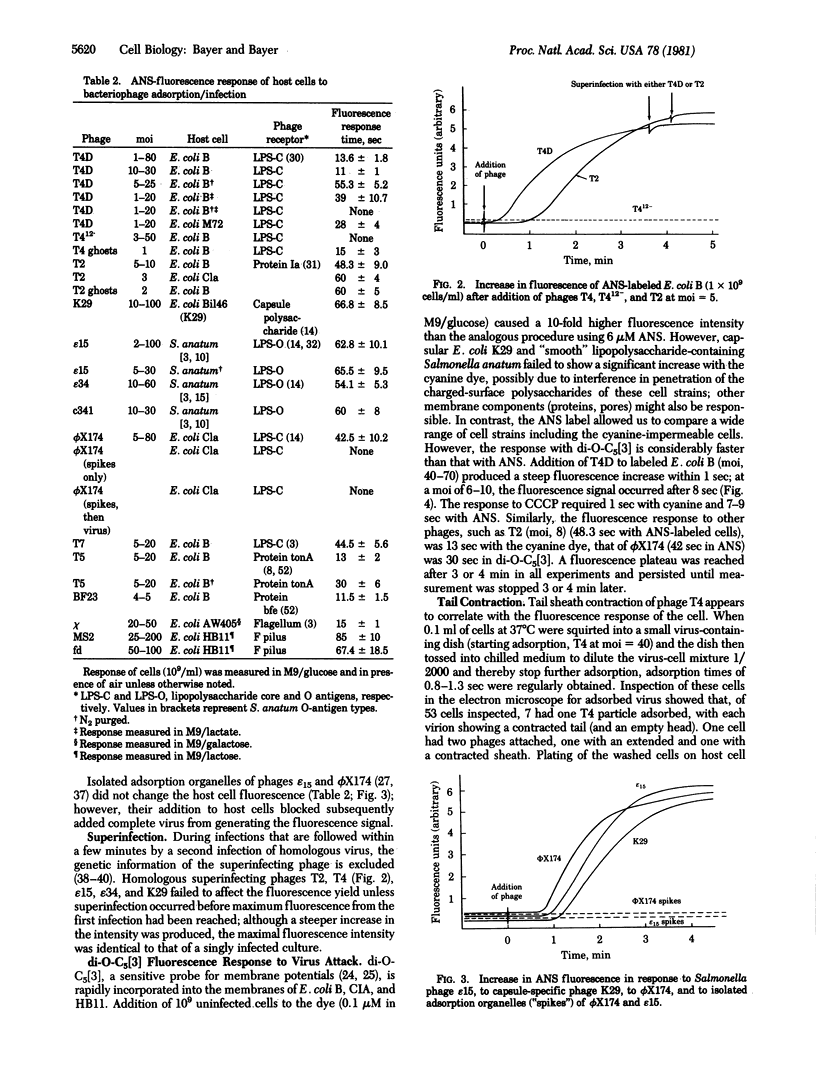
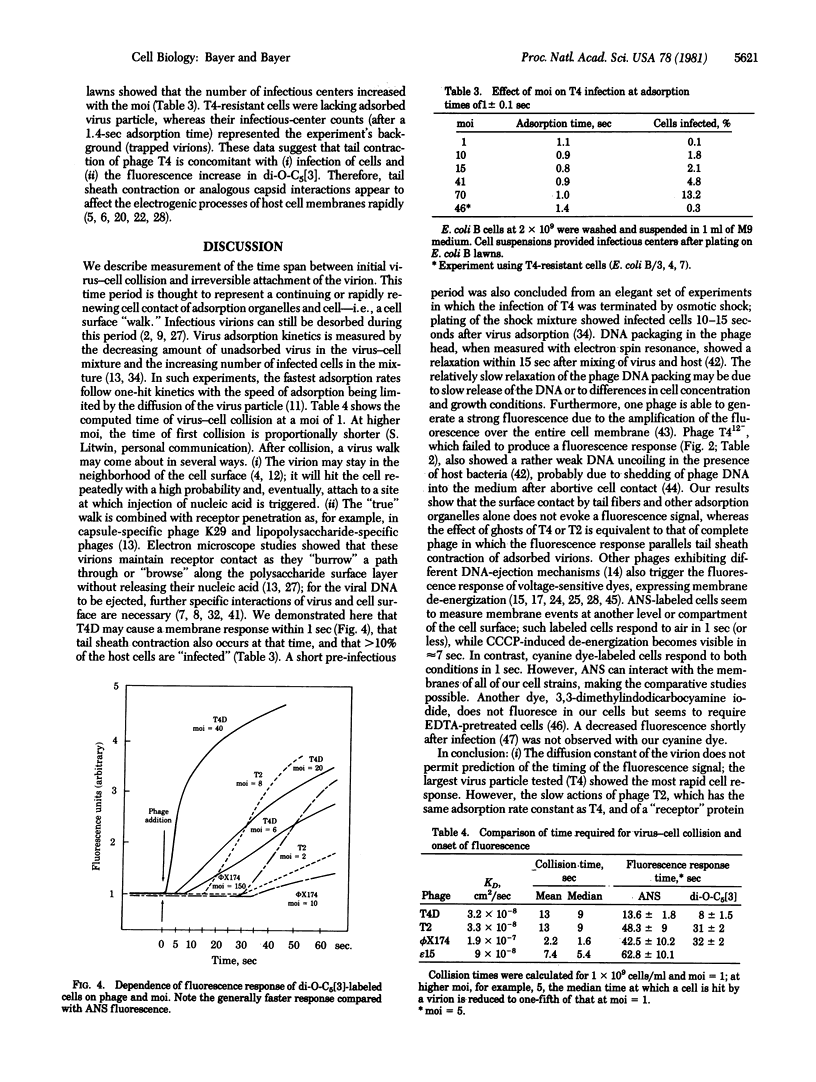
After collision with their host cells, virus particles may remain mobile on cell surfaces until they become attached at firm binding sites. We propose that a virion will arrive within a typical median time at such a site, generating a membrane signal such as an increased membrane fluorescence in cells labeled with the voltage-sensitive dyes 8-anilino-1-naphthalene-sulfonate (Mg-salt) (ANS), N-phenylnaphthylamine (NPA), or 3,3'-dipentyl-2,2'-oxacarbocyanine (di-O-C5[3]). We found that the time span between virus adsorption and fluorescence response varies widely among phages and also depends on bacterial strain, metabolic state, and type of dye. di-O-C5[3]-labeled cells react within 1 sec to uncouplers such as carbonyl cyanide m-chlorophenylhydrazone (CCCP). Cells labeled with ANS and NPA react to CCCP in 4-6 sec. Bacteriophages T4, T5, chi, and BF23, added to ANS-labeled cells, change the fluorescence in 9-15 sec. T-even ghosts cause a response at identical times. Baseplate-defective phage mutant T412- and isolated adsorption organelles of smaller viruses fail to cause an effect. di-O-C5[3]-labeled cells respond to T4 at a multiplicity of infection greater than or equal to 40 within 1 sec. A longer time (8 sec) is required at lower multiplicities. The receptor-degrading phages epsilon 15, epsilon 34, c 341, and K29 need the longest time (1 min for ANS) to cause a fluorescence increase. We suggest that the delayed fluorescence response is concomitant with the surface "walk" of the virion, which is terminated at an injection site. T4 tail sheath contraction coincides with the onset of the membrane fluorescence response.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON T. F. The morphology and osmotic properties of bacteriophage systems. Cold Spring Harb Symp Quant Biol. 1953;18:197–203. doi: 10.1101/sqb.1953.018.01.030. [DOI] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., DeBlois R. W. Diffusion constant and dimension of bacteriophage phi X174 as determined by self-beat laser light spectroscopy and electron microscopy. J Virol. 1974 Oct;14(4):975–980. doi: 10.1128/jvi.14.4.975-980.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Starkey T. W. The adsorption of bacteriophage phi X174 and its interaction with Escherichia coli; a kinetic and morphological study. Virology. 1972 Jul;49(1):236–256. doi: 10.1016/s0042-6822(72)80026-6. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Takeda K., Uetake H. Effects of receptor destruction by Salmonella bacteriophages epsilon 15 and c341. Virology. 1980 Sep;105(2):328–337. doi: 10.1016/0042-6822(80)90034-3. [DOI] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H., Bayer M. H. Penetration of the polysaccharide capsule of Escherichia coli (Bi161/42) by bacteriophage K29. Virology. 1979 Apr 15;94(1):95–118. doi: 10.1016/0042-6822(79)90441-0. [DOI] [PubMed] [Google Scholar]
- Benz W. C., Goldberg E. B. Interactions between modified phage T4 particles and spheroplasts. Virology. 1973 May;53(1):225–235. doi: 10.1016/0042-6822(73)90481-9. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berget P. B., Poteete A. R. Structure and functions of the bacteriophage P22 tail protein. J Virol. 1980 Apr;34(1):234–243. doi: 10.1128/jvi.34.1.234-243.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Oldmixon E. Relationship between steps in 8-anilino-1-naphthalene sulfonate (ANS) fluorescence and changes in the energized membrane state and in intracellular and extracellular adenosine 5'-triphosphate (ATP) levels following bacteriophage T5 infection of Escherichia coli. J Supramol Struct. 1979;10(3):329–347. doi: 10.1002/jss.400100305. [DOI] [PubMed] [Google Scholar]
- Brewer G. J. The state of energization of the membrane of Escherichia coli as affected by physiological conditions and colicin K. Biochemistry. 1976 Apr 6;15(7):1387–1392. doi: 10.1021/bi00652a006. [DOI] [PubMed] [Google Scholar]
- Crawford J. T., Goldberg E. B. The function of tail fibers in triggering baseplate expansion of bacteriophage T4. J Mol Biol. 1980 Jun 5;139(4):679–690. doi: 10.1016/0022-2836(80)90054-6. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Lenk E. V., Kikuchi Y., King J. Molecular reorganization in the hexagon to star transition of the baseplate of bacteriophage T4. J Mol Biol. 1977 Nov 5;116(3):489–523. doi: 10.1016/0022-2836(77)90081-x. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- FRASER W. J. Intestinal obstruction by gall-stone. Br J Surg. 1954 Sep;42(172):210–212. doi: 10.1002/bjs.18004217214. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Yamada H., Mizushima S. Interaction of bacteriophage T4 with reconstituted cell envelopes of Escherichia coli K-12. J Bacteriol. 1979 Dec;140(3):1071–1080. doi: 10.1128/jb.140.3.1071-1080.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J., Duckworth D. H. Fluorescence changes of a membrane-bound dye during bacteriophage T5 infection of Escherichia coli. J Virol. 1980 Jan;33(1):553–556. doi: 10.1128/jvi.33.1.553-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griniuviene B., Dzheia P., Grinius L. Anilinonaphthalenesulfonate as a fluorescent probe of the energized membrane state in Escherichia coli cells and sonicated membrane particles. Biochem Biophys Res Commun. 1975 May 19;64(2):790–796. doi: 10.1016/0006-291x(75)90390-3. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Shechter E., Iwatsubo M., Ranck J. L., Luzzati V. Correlations between structure and spectroscopic properties in membrane model systems. Tryptophan and I-anilino-8-naphthalene sulfonate fluorescence in protein-lipid-water phases. Biochim Biophys Acta. 1970;219(1):1–10. doi: 10.1016/0005-2736(70)90055-6. [DOI] [PubMed] [Google Scholar]
- Hancock R. W., Braun V. Nature of the energy requirement for the irreversible adsorption of bacteriophages T1 and phi80 to Escherichia coli. J Bacteriol. 1976 Feb;125(2):409–415. doi: 10.1128/jb.125.2.409-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Fluorescence studies on first steps of phage-host interactions. Virology. 1974 Mar;58(1):310–312. doi: 10.1016/0042-6822(74)90167-6. [DOI] [PubMed] [Google Scholar]
- Hantke K. Major outer membrane proteins of E. coli K12 serve as receptors for the phages T2 (protein Ia) and 434 (protein Ib). Mol Gen Genet. 1978 Aug 17;164(2):131–135. doi: 10.1007/BF00267377. [DOI] [PubMed] [Google Scholar]
- Heller K., Braun V. Accelerated adsorption of bacteriophage T5 to Escherichia coli F, resulting from reversible tail fiber-lipopolysaccharide binding. J Bacteriol. 1979 Jul;139(1):32–38. doi: 10.1128/jb.139.1.32-38.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Kauffmann M., Pfenning-Yeh M., Ponta H., Herrlich P., Schweiger M. A virus-specified mechanism for the prevention of multiple infection--T7- and T3-mutual and superinfection exclusion. Mol Gen Genet. 1976 Dec 22;149(3):243–249. doi: 10.1007/BF00268524. [DOI] [PubMed] [Google Scholar]
- King J. Assembly of the tail of bacteriophage T4. J Mol Biol. 1968 Mar 14;32(2):231–262. doi: 10.1016/0022-2836(68)90007-7. [DOI] [PubMed] [Google Scholar]
- Labedan B., Goldberg E. B. Requirement for membrane potential in injection of phage T4 DNA. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4669–4673. doi: 10.1073/pnas.76.9.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedan B., Letellier L. Membrane potential changes during the first steps of coliphage infection. Proc Natl Acad Sci U S A. 1981 Jan;78(1):215–219. doi: 10.1073/pnas.78.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Litwin S. Monte Carlo simulation of particle adsorption rates at high cell concentration. Biophys J. 1980 Aug;31(2):271–277. doi: 10.1016/S0006-3495(80)85056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D. J., Bode V. C. Events in lambda injection between phage adsorption and DNA entry. Virology. 1976 Jul 1;72(1):154–166. doi: 10.1016/0042-6822(76)90320-2. [DOI] [PubMed] [Google Scholar]
- Maeda T., Ohnishi S. I., Yanagida M. Infection-triggered release of tempocholine from bacteriophage G4 studied by electron spin resonance. FEBS Lett. 1978 May 1;89(1):29–32. doi: 10.1016/0014-5793(78)80515-8. [DOI] [PubMed] [Google Scholar]
- McConnell M., Reznick A., Wright A. Studies on the initial interactions of bacteriophage epsilon15 with its host cell, Salmonella anatum. Virology. 1979 Apr 15;94(1):10–23. doi: 10.1016/0042-6822(79)90434-3. [DOI] [PubMed] [Google Scholar]
- Nieva-Gomez D., Gennis R. B. Affinity of intact Escherichia coli for hydrophobic membrane probes is a function of the physiological state of the cells. Proc Natl Acad Sci U S A. 1977 May;74(5):1811–1815. doi: 10.1073/pnas.74.5.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Prehm P., Jann B., Jann K., Schmidt G., Stirm S. On a bacteriophage T3 and T4 receptor region within the cell wall lipopolysaccharide of Escherichia coli B. J Mol Biol. 1976 Feb 25;101(2):277–281. doi: 10.1016/0022-2836(76)90377-6. [DOI] [PubMed] [Google Scholar]
- Reeves J. P., Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. VII. Fluorescence of 1-anilino-8-naphthalenesulfonate during D-lactate oxidation by membrane vesicles from Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6204–6211. [PubMed] [Google Scholar]
- Schwartz M. The adsorption of coliphage lambda to its host: effect of variations in the surface density of receptor and in phage-receptor affinity. J Mol Biol. 1976 May 25;103(3):521–536. doi: 10.1016/0022-2836(76)90215-1. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin E. K., Martin D. L., Shain W., Gallin J. I. Interaction of chemotactic factors with human polymorphonuclear leukocytes: studies using a membrane potential-sensitive cyanine dye. J Membr Biol. 1980;52(3):257–272. doi: 10.1007/BF01869194. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Swan J. G., Flatgaard J. E. Functional defects in T4 bacteriophages lacking the gene 11 and gene 12 products. Virology. 1970 May;41(1):77–90. doi: 10.1016/0042-6822(70)90056-5. [DOI] [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Waggoner A. Optical probes of membrane potential. J Membr Biol. 1976 Jun 30;27(4):317–334. doi: 10.1007/BF01869143. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Ponta H., Schweiger M. Development of Escherichia coli virus T1. The role of the proton-motive force. J Biol Chem. 1980 Jan 25;255(2):534–539. [PubMed] [Google Scholar]
- Weiss M. J., Luria S. E. Reduction of membrane potential, an immediate effect of colicin K. Proc Natl Acad Sci U S A. 1978 May;75(5):2483–2487. doi: 10.1073/pnas.75.5.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]


