Abstract
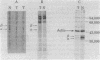
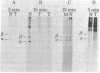
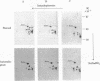
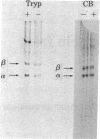
The steady-state level and synthesis of a pair of polypeptides of Mr 33,000 and 35,000 in chicken embryo fibroblasts (CEF) transformed by Rous sarcoma virus (RSV) are significantly decreased relative to normal CEF; however, the decrease is more pronounced in the case of the Mr 35,000 polypeptide. These polypeptides have been identified as the alpha and beta subunits of CEF tropomyosin by selective staining with tropomyosin antibody, two-dimensional gel electrophoresis, partial peptide analysis, and solubility properties. The decrease in tropomyosin is shown to be a transformation-specific phenomenon in that it does not occur after infection with a virus deleted in src sequences. Decreased synthesis of tropomyosin is also observed in quail cells transformed by MC29 (a retrovirus with a different onc gene than that in RSV) and also in chemically transformed quail cells. The decreased in tropomyosin is probably not a direct result of the disruption of the microfilament system in transformed cells because disruption of the microfilament system with trypsin or cytochalasin B in normal CEF does not lead to a decrease in tropomyosin synthesis. A decrease in tropomyosin in CEF after transformation may be a result of a pleiotropic effect that results in the transcriptional inactivation not only of the tropomyosin gene but also of the fibronectin and procollagen genes described by others.
Full text
PDF
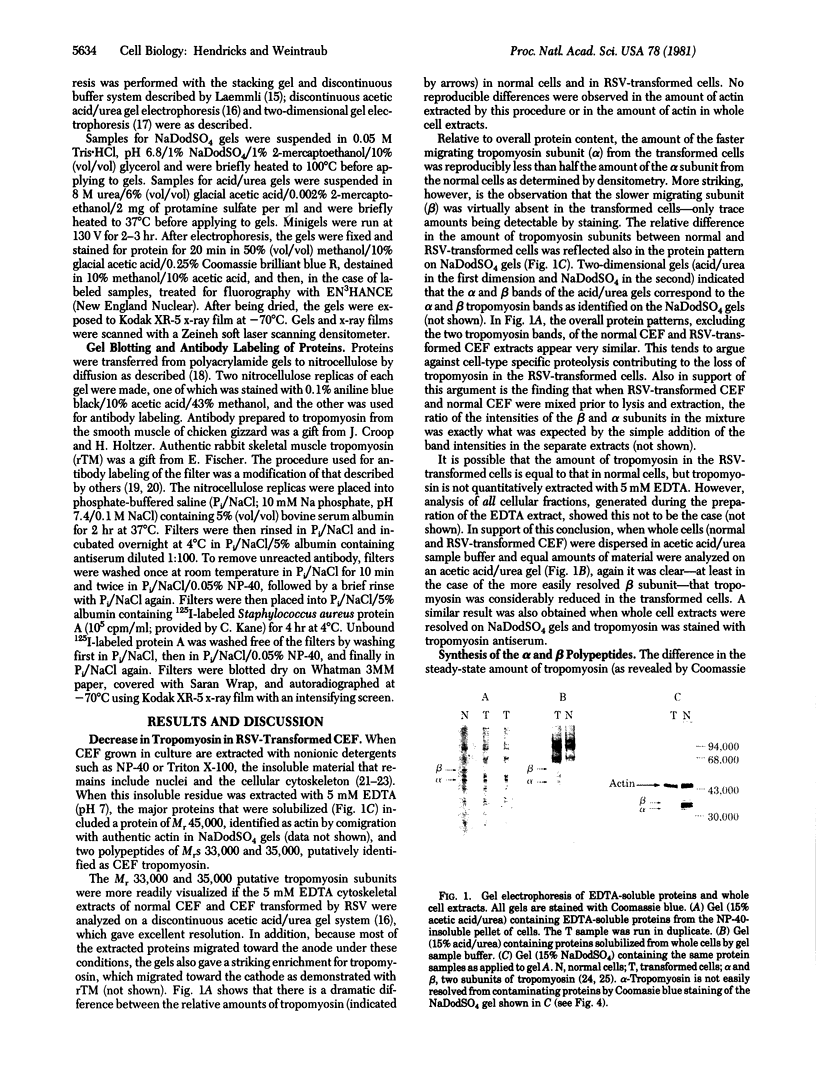

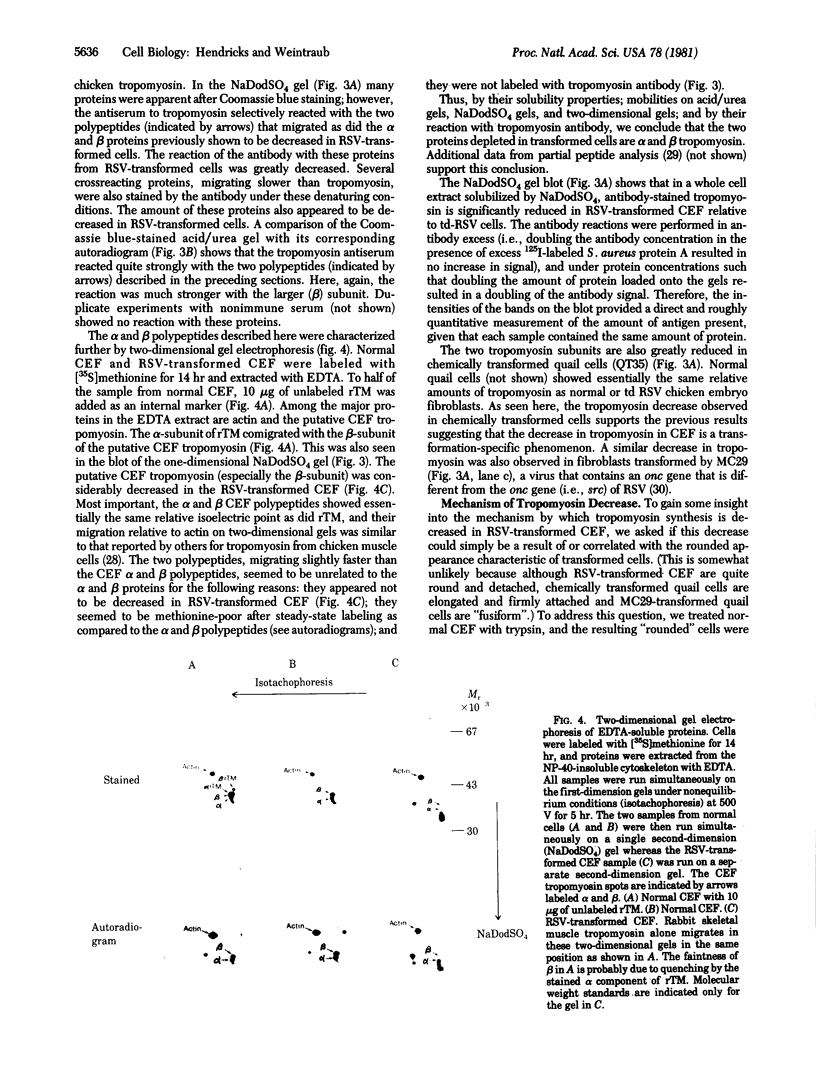

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., West M. H., Stedman J. D. Two-dimensional gel analysis of histones in acid extracts of nuclei, cells, and tissues. Eur J Biochem. 1980 Aug;109(1):17–23. doi: 10.1111/j.1432-1033.1980.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Cohen C., Longley W. Tropomyosin: crystal structure, polymorphism and molecular interactions. J Mol Biol. 1969 Apr 14;41(1):87–107. doi: 10.1016/0022-2836(69)90128-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. B., Sobel M. E., Yamada K. M., de Crombrugghe B., Pastan I. Effects of transformation on fibronectin gene expression using cloned fibronectin cDNA. J Biol Chem. 1981 Jan 10;256(1):520–525. [PubMed] [Google Scholar]
- Goudswaard J., van der Donk J. A., Noordzij A., van Dam R. H., Vaerman J. P. Protein A reactivity of various mammalian immunoglobulins. Scand J Immunol. 1978;8(1):21–28. doi: 10.1111/j.1365-3083.1978.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Das S., Neiman P., Weintraub H. Regulation of expression and chromosomal subunit conformation of avian retrovirus genomes. Cell. 1978 Aug;14(4):865–878. doi: 10.1016/0092-8674(78)90342-2. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of cellular genes by avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5351–5354. doi: 10.1073/pnas.77.9.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Kirschner M. W. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980 Jul;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. H., Adams S. L., Sobel M. E., Pastan I., de Crombrugghe B. Decreased levels of collagen mRNA in rous sarcoma virus-transformed chick embryo fibroblasts. J Biol Chem. 1978 Aug 25;253(16):5869–5874. [PubMed] [Google Scholar]
- Izant J. G., Lazarides E. Invariance and heterogeneity in the major structural and regulatory proteins of chick muscle cells revealed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1450–1454. doi: 10.1073/pnas.74.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. SDS microslab linear gradient polyacrylamide gel electrophoresis. Anal Biochem. 1978 Jul 1;87(2):386–396. doi: 10.1016/0003-2697(78)90688-7. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Paulin D., Perreau J., Jakob H., Jacob F., Yaniv M. Tropomyosin synthesis accompanies formation of actin filaments in embryonal carcinoma cells induced to differentiate by hexamethylene bisacetamide. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1891–1895. doi: 10.1073/pnas.76.4.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Sandmeyer S., Bornstein P. Declining procollagen mRNA sequences in chick embryo fibroblasts infected with rous sarcoma virus. Correlation with procollagen synthesis. J Biol Chem. 1979 Jun 25;254(12):4950–4953. [PubMed] [Google Scholar]
- Stéhelin D., Graf T. Avian myelocytomatosis and erythroblastosis viruses lack the transforming gene src of avian sarcoma viruses. Cell. 1978 Apr;13(4):745–750. doi: 10.1016/0092-8674(78)90224-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Rathke P. C., Osborn M., Franke W. W. Distribution of actin and tubulin in cells and in glycerinated cell models after treatment with cytochalasin B (CB). Exp Cell Res. 1976 Oct 15;102(2):285–297. doi: 10.1016/0014-4827(76)90044-6. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Henderson D., Osborn M., Weber K. Three-dimensional electron microscopical visualization of the cytoskeleton of animal cells: immunoferritin identification of actin- and tubulin-containing structures. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5511–5515. doi: 10.1073/pnas.75.11.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]