Background: The involvement of inositol phosphates produced by the yeast inositol-polyphosphate multikinase, Arg82, in transcription is controversial.
Results: Catalytically inactive Arg82 restores the regulation of arginine-dependent genes in an ARG82 knock-out.
Conclusion: Inositol phosphates do not regulate arginine-dependent gene expression.
Significance: Independently of its enzymatic activity Arg82 controls arginine-responsive genes.
Keywords: Cell Signaling, Inositol Phosphates, Phosphatidylinositol 3-Kinase, Phosphatidylinositol Signaling, Signaling
Abstract
Inositol phosphates are key signaling molecules affecting a large variety of cellular processes. Inositol-polyphosphate multikinase (IPMK) is a central component of the inositol phosphate biosynthetic routes, playing essential roles during development. IPMK phosphorylates inositol 1,4,5-trisphosphate to inositol tetrakisphosphate and subsequently to inositol pentakisphosphate and has also been described to function as a lipid kinase. Recently, a catalytically inactive mammalian IPMK was reported to be involved in nutrient signaling by way of mammalian target of rapamycin and AMP-activated protein kinase. In yeast, the IPMK homologue, Arg82, is the sole inositol-trisphosphate kinase. Arg82 has been extensively studied as part of the transcriptional complex regulating nitrogen sensing, in particular arginine metabolism. Whether this role requires Arg82 catalytic activity has long been a matter of contention. In this study, we developed a novel method for the real time study of promoter strength in vivo and used it to demonstrate that catalytically inactive Arg82 fully restored the arginine-dependent transcriptional response. We also showed that expression in yeast of catalytically active, but structurally very different, mammalian or plant IPMK homologue failed to restore arginine regulation. Our work indicates that inositol phosphates do not regulate arginine-dependent gene expression.
Introduction
Inositol phosphates constitute a family of soluble molecules which play a central role in cell signaling (1–3). The large variety of inositol phosphate species stems from the attachment of one or more phosphate groups to the six carbon myo-inositol ring. Inositol hexakisphosphate (also known as phytic acid) presents orthophosphate groups in all six carbons. More complex forms of inositol phosphates also exist, e.g. inositol pyrophosphates where one or several carbons hold pyrophosphate groups (4). The array of soluble inositol polyphosphate molecules depends on the activity of several inositol kinases and phosphatases (5). The many inositol phosphate species present in the cytosol and nucleus of eukaryotic cells constitute a metabolically interconnected grid, regulating almost every aspect of cell physiology. The foremost member of such network is the second messenger inositol 1,4,5-trisphosphate, which is involved in the release of calcium from intracellular stores. This mechanism provides one of the best characterized examples of signal transduction (6).
The phosphorylation of inositol 1,4,5-trisphosphate into more complex forms is achieved by inositol kinases of which inositol-polyphosphate multikinase (IPMK)2 is one of the most relevant. IPMK phosphorylates at positions 3 and 6, converting inositol 1,4,5-trisphosphate to inositol tetrakisphosphate and then inositol tetrakisphosphate to inositol pentakisphosphate (7). The function of IPMK is highly conserved in all eukaryotes, and its importance is underlined by the embryonic lethality observed in homozygous knock-out mice (8). It has also been demonstrated that IPMK acts as a phosphatidylinositol 3-kinase (PI3K) in vivo, converting phosphatidylinositol 4,5-bisphosphate to phosphatidylinositol 3,4,5-trisphosphate (9, 10). In yeast, the IPMK homologue Arg82 (or Ipk2) is the sole inositol-trisphosphate kinase (11). It was originally identified in a screen for mutants unable to grow on alternative nitrogen sources (arginine/ornithine) (12). Arg82 participates in the regulation of genes involved in arginine synthesis and degradation. It does so by stabilizing the trimeric complex, Arg80-Arg81-Mcm1, which binds to specific promoters possessing the consensus sequences designated “arginine boxes” (13). A long running controversy surrounds the requirement for Arg82 catalytic activity in regulating arginine metabolism, particularly the role of putative inositol phosphates produced by Arg82 (14, 15).
Very little is known about how IPMK itself is regulated. A recent publication indicates that regulation of IPMK may take place by altering its nuclear/cytoplasmic localization (16). In addition, it has recently been discovered to interact with two chief nutrient regulators in mammalian cells. First, IPMK binds to AMP-activated protein kinase (17), which works as a gauge monitoring the energy status of the cell and coordinates the response to nutrient scarcity (18). Second, IPMK interacts with target of rapamycin (TOR) (19), which monitors nitrogen quality and availability and is therefore essential for cell growth (20). In both cases, IPMK activity was not required to establish a physical association. In this work, we revisited the putative requirement for Arg82 activity in gene regulation. To do so, we developed a novel transcriptional assay that allows real time measurement of arginine-regulated promoters in vivo.
EXPERIMENTAL PROCEDURES
Strains
Saccharomyces cerevisiae strains used in this study are isogenic to BY4741 and described in Table 1. Knock-out mutants arg82Δ, plc1Δ, kcs1Δ, and arg82Δ plc1Δ have been described earlier (10, 21); arg82Δ kcs1Δ was generated for this study by crossing kcs1Δ and arg82Δ strains.
TABLE 1.
S. cerevisiae strains used in this study
Plasmids
ARG82 (YDR173C) was amplified from S. cerevisiae BY4741 genomic DNA with SpeI/HindIII restriction sites and ligated into the T Easy vector (Promega). Subsequently, it was used as a template to generate ARG82-DK/AA (D131A,K133A) and ARG82-SDST/ADAA (S95A,S97A,T98A) mutations via QuikChange site-directed mutagenesis (Stratagene). Arabidopsis thaliana Ipk2β was cloned from genomic cDNA (a gift from C. Azevedo) with BamHI/PstI restriction sites into a T Easy vector and used as a template to generate AtIpk2β-D/A (D100A) (QuikChange). The constructs were subcloned into p425-MET25, a vector with a methionine-regulated promoter (22). Human IPMK and a kinase-dead variant, IPMK-DK (D144A,K146A), were subcloned from human vectors (a gift from A. Resnick) with BamHI/SalI restriction sites into p425-MET25. Point mutations were confirmed by sequencing, and plasmid presence was confirmed by complementation of the leucine auxotrophy. pYES-HSV1-TK plasmid was generated by cloning the herpes simplex virus 1 thymidine kinase (HSV1-TK) gene obtained from pKHTK (23) into a pYES2 plasmid (Invitrogen) with HindIII/BamHI sites. A SpeI site was introduced upstream of HindIII. The construction of ARG3 or ARG5,6 promoters fused to HSV1 thymidine kinase was achieved by PCR amplifying the 1-kb sequence upstream of the start codon of ARG3 and ARG5,6 from genomic DNA with restriction sites SpeI/HindIII. PCR products were inserted into the pYES-HSV1-TK plasmid in-frame with HSV1-TK. Cloning was confirmed by sequencing and by complementation of the uracil auxotrophy.
Growth Tests in Solid Medium
Strains were precultured overnight in synthetic complete medium (yeast nitrogen base without amino acids; Formedium) with the addition of auxotrophic amino acids and 110 mm glucose. The next day, cells were washed three times with water to remove traces of ammonium sulfate and then diluted to an optical density (OD) of 1. This dilution was used to make 6 × 10-fold dilutions, 5 μl of which were spotted on agar plates containing synthetic complete medium with ammonium, arginine, or ornithine as the sole nitrogen source. Plates were incubated at 30 °C for 48 h.
Growth Tests in Liquid Medium
Strains were cultivated overnight as described above. The next day, fresh medium was added to each overnight culture, which was then grown for 4 h. Cells were harvested, washed three times with minimal synthetic medium (yeast nitrogen base without amino acids or ammonium sulfate; Formedium), diluted to an OD of 0.05, and inoculated into minimal synthetic medium plus 110 mm glucose and either 38 mm ammonium (5 g/liter), 5.7 mm arginine (1 g/liter), or 15 mm ornithine (2 g/liter) as the sole nitrogen source. Cultivations were performed in a 384-well plate with a volume of 20 μl/well. The plate was shaken for 1 min every 2 min, and cell density was measured every 30 min using a plate reader with temperature control set at 30 °C (Infinite F200, Tecan). Cultivations were carried out for 25–70 h depending on the experimental conditions.
Growth Rate Quantification
To calculate the maximum growth rate, the raw OD was treated as described by Warringer et al. (24, 25). First, OD was normalized, and the initial value at time 0 was subtracted from all of the following values. Second, to account for a nonlinear correlation between OD and cell density at high cell densities, OD values were calibrated according to the following formula: Calibrated OD = OD + 0.8324 × OD3. Third, growth curves were smoothed to reduce contributions from noise by averaging (nonweighted averaging) over three consecutive measurements (no averaging of initial value). Fourth, artifacts arising from nonbiological events were avoided by removing measurements yielding negative slopes. The growth rate was calculated by e(log) transforming smoothed growth curves. Slopes were calculated between every pair of values spaced 90 min apart along the curve. No slopes were calculated from the eight initial time points to filter for digitization effects. Of the seven highest slopes, the top two were discarded, and a mean was calculated from the remaining five.
Calculation of Differences in Promoter Expression
To compare the influence of promoter expression on different nitrogen sources using the HSV1-TK assay, we developed the following method. The maximum slope of the growth curve for either ammonium or arginine was calculated using the above described method (25). The tangents to the slopes were drawn, and the corresponding intersection point was determined (y1 = y2) (supplemental Fig. S1). The area of the triangle formed was calculated using the following formula: Area = ½(h × b) where h (height) corresponds to the x axis distance between the points at which the lines intersected (x1) with the last time point in the experiment (x2) and b (base) corresponds to the y axis absorbance segment (y2 − y1) obtained at the last time point (x2). Because arg82Δ showed a natural decrease in growth in arginine with respect to ammonium, we introduced a correction in the calculations. The area of the triangle formed by ammonium and arginine growth in DMSO was added to that calculated after growth on fialuridine (FIAU): Area (arg82Δ + FIAU) = Area (ammonium-arginine in FIAU) + Area (ammonium-arginine in DMSO). Every experimental condition was studied using six replicas. The corresponding S.E. is displayed as an error bar. Growth profiles show representative cultivations.
HSV1-TK Activity
Cells expressing the viral kinase gene under the control of the ADH1 promoter were grown in 6-well plates (volume per well, 2 ml). Cells were harvested at exponential growth rate. Proteins were extracted upon vortexing with glass beads (Genie 2T, Scientific Industries) in lysis buffer (10 mm Tris, pH 7.5, 1 mm NaF). The activity of the protein extract was assayed according to Yaghoubi and Gambhir (26). The activity measured as the percentage of conversion of [3H]penciclovir/min/mg of protein was normalized to samples treated with DMSO. Experiments were performed in triplicates, and growth profiles show representative cultivations.
Real Time Quantitative PCR
The relative amounts of ARG3 and ARG5,6 mRNA were quantified by RT-quantitative PCR. Yeast cells were grown to exponential phase in 6-well plates as indicated above. Cells were then harvested and washed once with cold water, and the mRNA was extracted using the Qiagen RNeasy kit following the manufacturer's recommendations. PCRs (20 μl) contained 10 μl of MESA BLUE quantitative PCR SYBR Green mixture (Eurogentec) and 0.3 μm primers. All reactions were performed in triplicate with an Opticon 2 System (MJ Research, Cambridge, MA), and each experiment included a standard curve and a no-template control. Standard template consisted of a known concentration of ACT1 cDNA, and each standard curve consisted of five serial dilutions of template. At the end of 40 cycles of amplification, a dissociation curve was performed in which SYBR Green was measured at 1 °C intervals between 50 and 100 °C to generate a melting curve. Results were normalized to ACT1 and expressed as -fold changes over samples grown with ammonium as a nitrogen source.
RESULTS
ARG82 Knock-out Grows Well in Ammonium
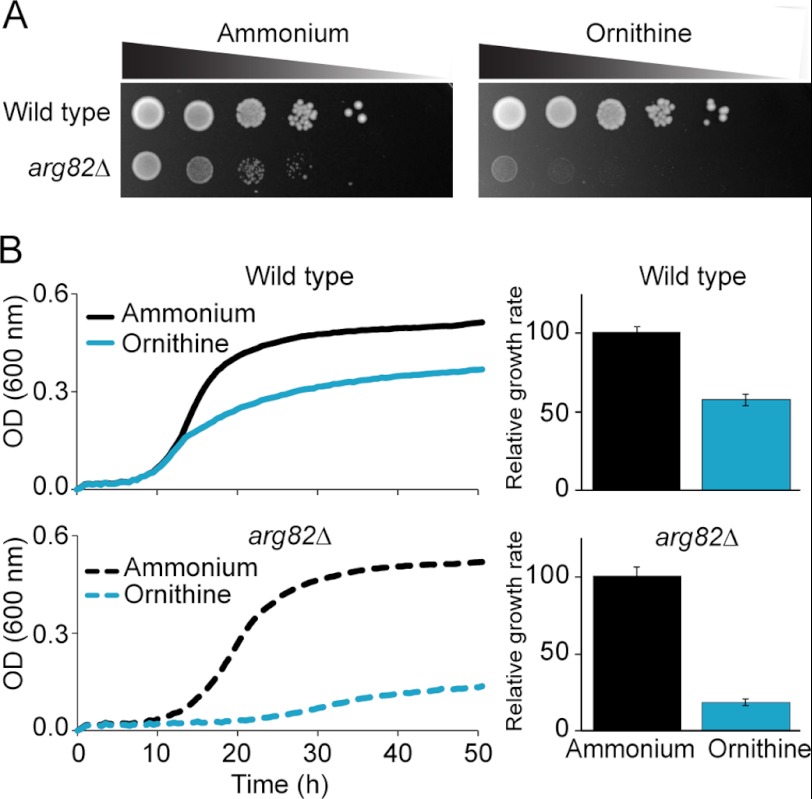
Growth on solid medium has been the primary readout of Arg82 activity as a transcriptional regulator. However, such assay has previously produced opposite results: York and co-workers (15) claimed a key role for Arg82 enzymatic activity, whereas Dubois and co-workers (14) argued the contrary. To test these contrasting hypotheses, we first repeated the growth assays in question. The wild type BY4741 strain grew well in solid medium supplemented with ammonium or ornithine as the sole nitrogen source. The ARG82 knock-out presented a growth defect with respect to the wild type in ammonia that was more pronounced during growth in ornithine (Fig. 1A) as reported previously (14). Given the difficulty of quantifying growth differences in solid medium, we turned instead to growth in liquid medium where the continuous monitoring of optical density provided a more reliable readout. We measured the growth rate of arg82Δ with respect to wild type and found a modest 19% reduction in ammonium (supplemental Fig. S2) and a more robust 75% drop in ornithine (Fig. 1B). We also noted that the wild type strain displayed reduced growth during cultivation in ornithine that could not be appreciated in solid medium (Fig. 1B).
FIGURE 1.
Comparison of growth of wild type and arg82Δ in ammonium and ornithine. A, growth assay in solid medium. 10-Fold serial dilutions of cells were spotted on agar plates containing minimal medium supplemented with either ammonium or ornithine as the sole nitrogen source and incubated at 30 °C for 48 h. B, growth assay in liquid minimal medium. The growth rate was quantified as the average of the highest slopes of six growth curves. Error bars correspond to S.D.
ARG82 Activity Plays a Minor Role during Growth in Ornithine
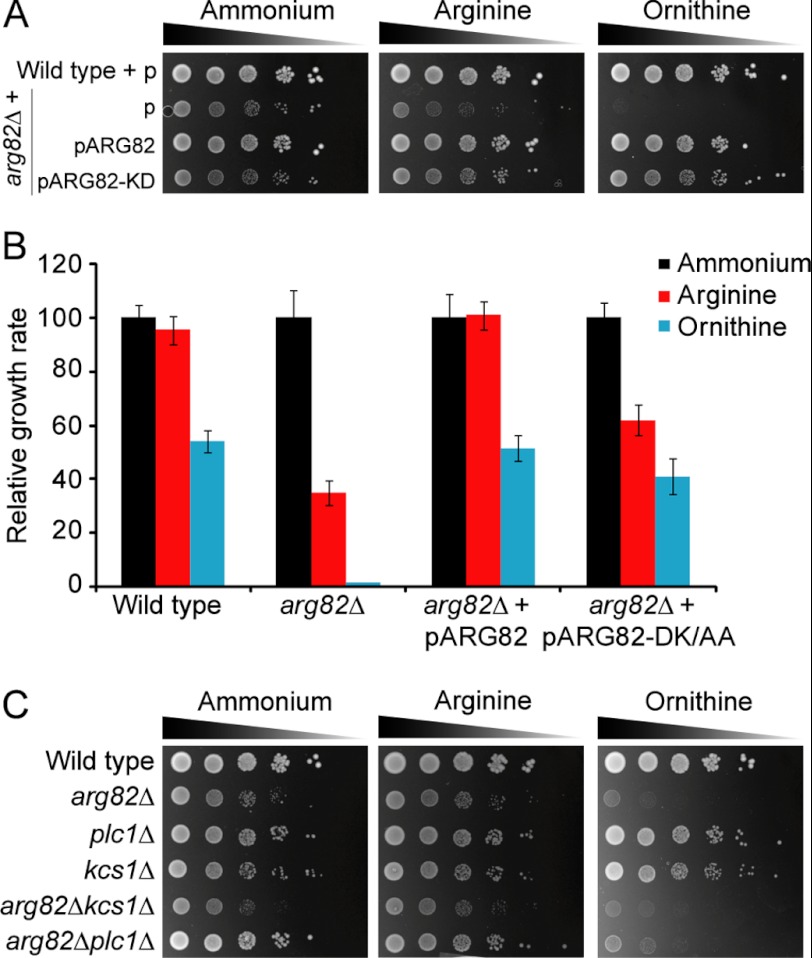
We took advantage of the ability of arg82Δ to grow relatively well in ammonium but poorly in ornithine to test whether its catalytic activity played a role in nitrogen regulation. We transformed arg82Δ with a plasmid expressing a version of Arg82 devoid of enzymatic activity (two point mutations turned aspartate 131 and lysine 133 to alanines, therefore disrupting the inositol binding domain) (14) and tested solid and liquid growth in ammonium, arginine, and ornithine. As shown in Fig. 2, A and B, catalytically inactive Arg82 was able to substantially restore growth in arginine and ornithine. Note, however, that it did not fully complement growth on arginine. This suggested that Arg82 devoid of its enzymatic activity plays a role in the adaptation of the cell to alternative nitrogen sources. The expression of plasmids was generally confirmed by growth in medium lacking the corresponding amino acid marker. We further confirmed that Arg82 was expressed in the cells by detecting a tagged version by means of Western blot (data not shown).
FIGURE 2.
Growth of yeast strains in different nitrogen sources. Growth in minimal synthetic medium containing ammonium, arginine, or ornithine as the sole nitrogen source is shown. A, yeast strains transformed with a either an empty plasmid (p) or expressing catalytically active or inactive (inositol phosphate binding domain-disrupted) Arg82. Growth assays were performed in solid medium as indicated in Fig. 1A. B, quantification of the growth rate in arginine and ornithine with respect to the maximum achieved in ammonium for the indicated strains. The growth rate was quantified as the average of the highest slopes of five growth curves. Error bars correspond to S.D. C, yeast strains bearing deletions of genes encoding either inositol-polyphosphate multikinase (Arg82), phospholipase C (Plc1), inositol-hexakis- and -heptakisphosphate 6-kinase (Kcs1), or a combination of the above. Growth assays were performed in solid medium as indicated in Fig. 1A.
We tested whether the lack of soluble inositol phosphates may play a role during growth in alternative nitrogen sources. In budding yeast, knock-out of the only phospholipase C (Plc1) prevents the generation of soluble inositol phosphate species. We tested the growth of plc1Δ in solid medium and observed a growth reduction similar to that for the wild type when switching from ammonium to ornithine medium (Fig. 2C and supplemental Fig. S3). Deletion of KCS1, which blocks synthesis of most inositol pyrophosphates (diphosphoinositol pentakisphosphate and bisdiphosphoinositol tetrakisphosphate), did not affect the growth in ornithine. Finally, we tested the growth ability of double knock-outs arg82Δ plc1Δ and arg82Δ kcs1Δ and found that growth corresponded to the arg82Δ phenotype.
Arg82 Homologues Expressed in arg82Δ Restore Growth in Ammonium but Not in Ornithine
It has been shown that mammalian and plant IPMK homologues are able to restore growth of arg82Δ in rich medium (27, 28). This is probably achieved through their catalytic activity because their sequence identities with respect to Arg82 are very low. Human IPMK has been shown to phosphorylate both soluble and lipid inositols, whereas its plant counterpart only metabolizes inositol phosphates. We tested whether any of these homologues would be able to restore growth in arginine or ornithine. As seen in Fig. 3, both rescued growth in ammonium and did so partially in arginine. However, they were unable to complement the arg82Δ growth phenotype in ornithine. This was not due to a defect in general growth because plant and particularly human IPMKs were able to restore normal growth in ammonium in liquid culture (supplemental Fig. S4). Instead, these results suggest that Arg82 structure is more important than its catalytic activity for regulating growth in ornithine.
FIGURE 3.
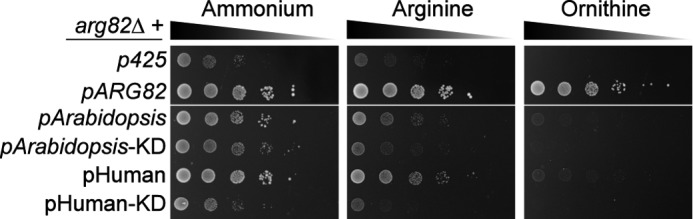
Ability of IPMK homologues to rescue arg82Δ growth defects. Growth assays were performed in solid medium as indicated in Fig. 1A. arg82Δ strains were transformed with a plasmid bearing either ARG82, an Arabidopsis IPMK (AtIpk2β) or its kinase-dead (KD) version, or the human IPMK or a kinase-dead version.
A Novel Method to Measure Real Time Promoter Strength
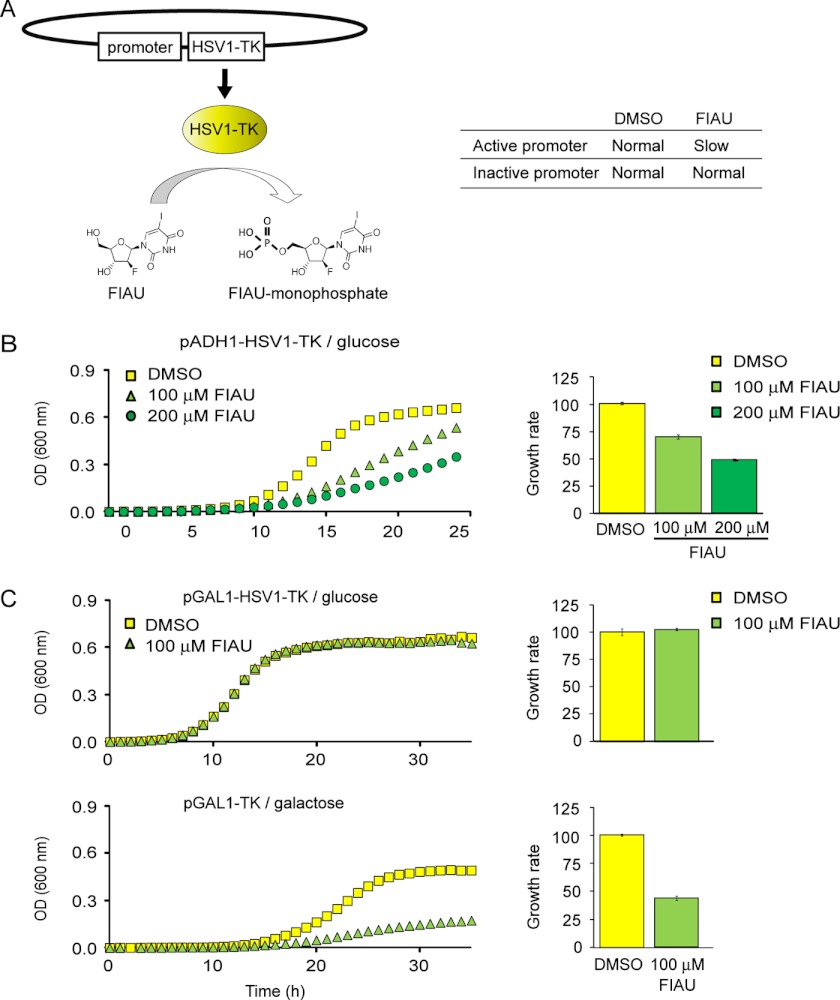
The growth tests presented so far recapitulated the overall defects of arg82Δ without making a distinction between its general effect on well being or its specific role as transcriptional regulator. To understand the specific role that Arg82 catalytic activity might play in stabilizing the Arg80-Mcm1-Arg81 complex, we looked at the regulation of promoters containing arginine boxes (supplemental Fig. S5). We developed a new technique that allows measurements of promoter strength in vivo by assaying cell growth. To that end, we constructed a plasmid bearing the promoter of interest fused to the gene encoding HSV1-TK. HSV1-TK has greater affinity than the eukaryotic thymidine kinase for nucleoside analogues like acyclovir or fialuridine, which are incorporated into DNA strands upon phosphorylation, blocking further extension (Fig. 4A). The growth rate of budding yeast will thus be inversely proportional to the strength of the promoter controlling HSV1-TK expression. FIAU was chosen as the nucleoside analog for its efficiency and relatively low cost.
FIGURE 4.
A novel method to measure promoter strength applied to the study of arginine-regulated genes. A, the promoter of the gene of interest was fused to the HSV1-TK and expressed in a high copy plasmid. FIAU is phosphorylated by HSV1-TK and becomes a toxic compound once incorporated into DNA, reducing the growth rate. The table indicates the expected influence of FIAU on the growth rate of cells transformed with HSV1-TK fused to an active or inactive promoter. The rate of growth inhibition is proportional to the strength of the promoter studied. Yeast strains expressing HSV1-TK gene under the control of either ADH1 (B) or GAL1 (C) promoters are shown. ADH1 is active in the presence of glucose. GAL1 promoter is down-regulated in the presence of glucose and strongly up-regulated when galactose is the sole carbon source. Growth profiles in minimal medium containing ammonium as unique nitrogen source are shown. Error bars correspond to S.E.
The overall growth of a yeast strain carrying a plasmid with HSV1-TK under the control of a strong promoter, ADH1, is shown in Fig. 4B. The decrease in growth rate correlated well with the concentration of FIAU. To prove that FIAU per se is not toxic to yeast, we fused HSV1-TK to the GAL1 promoter, which is repressed in glucose medium but induced in galactose medium. As shown in Fig. 4C, 100 μm FIAU did not decrease the growth rate of yeast expressing GAL1-HSV1-TK on glucose but led to a 50% drop when galactose was the only carbon source.
ARG3 and ARG5,6 Promoters Are Deregulated in arg82Δ
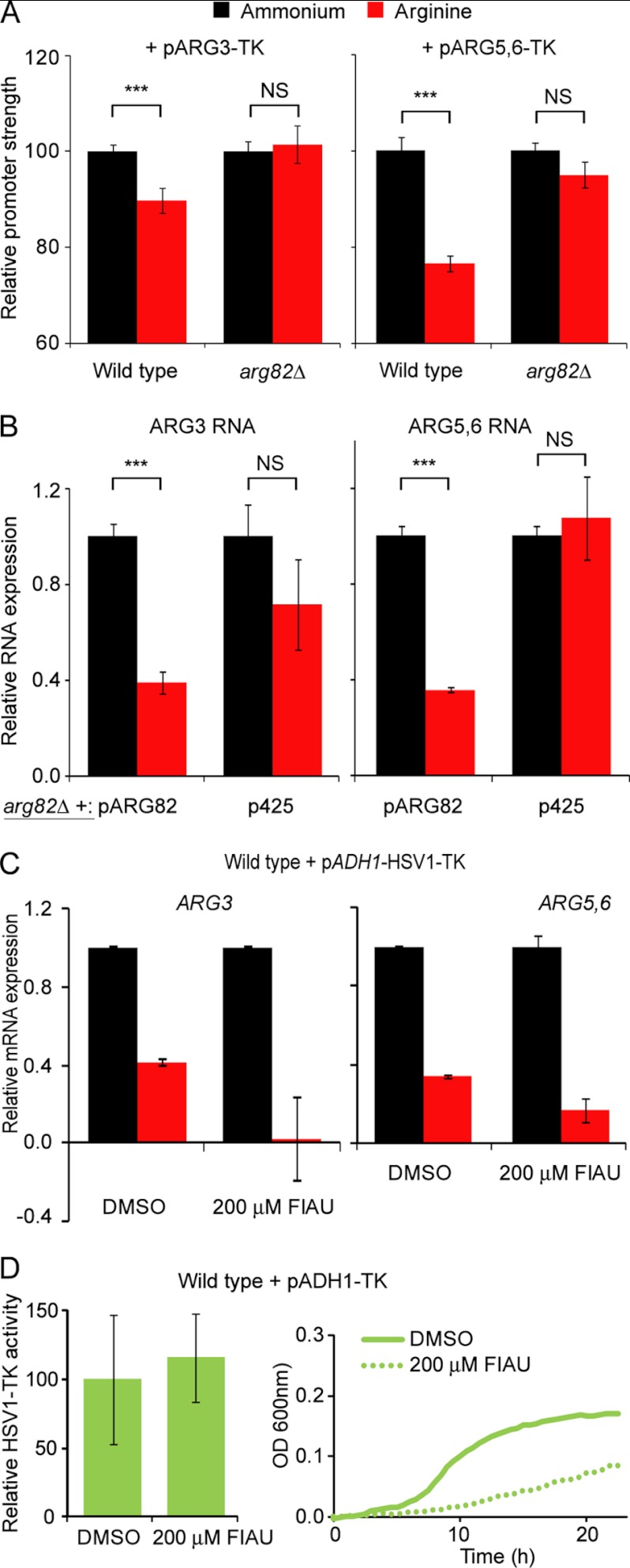
We studied two promoters to genes of the arginine biosynthetic pathway, ARG3 and ARG5,6, encoding for ornithine carbamoyltransferase and acetylglutamate kinase, respectively, fused to HSV1-TK (29, 30). Because growth of arg82Δ in ornithine was minimal, and thus no differences could be appreciated before and after addition of FIAU (data not shown), we chose to study the response of the promoters in arginine where the effect on growth was less dramatic (Figs. 2 and 3). Expression of ARG3 and ARG5,6 increases during growth on ammonium and decreases on arginine. This was confirmed in our system: addition of FIAU to the medium led to a slower growth rate in the presence of ammonium with respect to arginine (supplemental Fig. S6, A and B). We quantified the differences between growth rates by calculating the area of the triangles generated by the tangents to the growth curves (see “Experimental Procedures”) and presented them in a column chart (Fig. 5A). The arg82Δ strain cannot properly regulate ARG3 and ARG5,6 promoters because it fails to repress them during growth in arginine. Our system reflected this behavior by displaying a similar promoter response during growth in ammonium and arginine.
FIGURE 5.
Transcriptional response of ARG3 and ARG5,6 promoters and HSV1-TK activity. A, the down-regulation of ARG3 and ARG5,6 promoters was calculated as the decrease in growth rate in the presence of FIAU with respect to DMSO (see “Experimental Procedures”). The difference in promoter expression in arginine medium is depicted in relation to ammonium, which received an arbitrary value of 100. Error bars correspond to S.E. The statistical analysis was performed using a two-tailed and homoscedastic t test (***, p < 0.001; NS, not significant). B, comparison of mRNA expression of ARG3 and ARG5,6 from arg82Δ bearing either an empty plasmid or one containing ARG82. The relative mRNA level was calculated using as a reference the growth in ammonium, which received an arbitrary value of 1. The error bars correspond to S.D. Statistical analysis was performed as above. C, comparison of mRNA expression of ARG3 and ARG5,6 from cells growing in the presence or absence of FIAU. The strain corresponded to the wild type expressing TK under the control of ADH1. The relative mRNA level was calculated using as a reference the growth in ammonium. The error bars correspond to S.D. D, HSV1-TK activity from wild type cells expressing TK under the control of ADH1. The error bars correspond to S.D.
We compared the response of the TK system during growth in ammonium and arginine with the change in mRNA levels of ARG3 and ARG5,6. We observed a similar regulation, although the growth reduction upon ARG3-TK expression was less dramatic than the corresponding drop in mRNA levels (Fig. 5B). We think that the portion of ARG3 promoter fused to HSV1-TK may not be fully responsive to arginine regulation. Thus, we concentrated on ARG5,6 promoter to study the rescue of arg82Δ by different IPMK forms.
Another caveat of the TK system is that the decrease in growth associated with using a nucleoside analog may alter the regulation of arginine-dependent genes. We address this by looking into the levels of ARG3 and ARG5,6 mRNA levels after treatment with 200 μm FIAU. As can be observed in Fig. 5C, gene regulation appeared in line with the untreated condition (DMSO). Also, HSV1-TK activity did not seem affected by the reduction in growth rate caused by FIAU (Fig. 5D).
ARG5,6 Promoter Regulation Is Not Dependent on the Catalytic Activity of Arg82
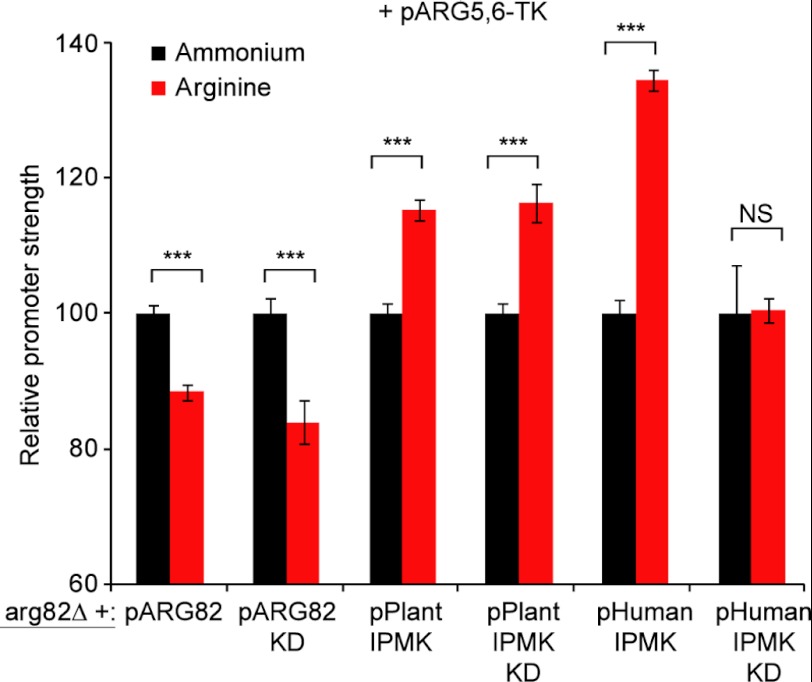
We looked into whether ARG5,6 regulation in arginine could be restored in an arg82Δ mutant by expressing the active or inactive Arg82. As shown in Fig. 6 and supplemental Fig. S7A, both constructs restored correct ARG5,6 promoter expression, indicating that the structure of Arg82 and not its activity is needed to stabilize the complex.
FIGURE 6.
Transcriptional response of ARG3 and ARG5,6 promoters in an arg82Δ strain rescued with IPMK homologues. Quantification of ARG5,6 promoter strength was determined as indicated in Fig. 5A. arg82Δ was rescued with an Arabidopsis IPMK homologue (AtIpk2β), its kinase-dead (KD) variant (AtIpk2β-D/A), the human IPMK homologue (hIPMK), or a kinase-dead version (hIPMK-DK/AA). Error bars correspond to S.E. The statistical analysis was performed using a two-tailed and homoscedastic t test (***, p < 0.001; NS, not significant).
Arg82 Homologues Are Unable to Restore Nitrogen Regulation to arg82Δ
We also studied whether misregulation of the ARG5,6 promoter in arg82Δ could be rescued by expressing catalytically active, but structurally diverse, plant AtIpk2β and human IPMK homologues. Both Arg82 homologues were unable to restore regulation of ARG5,6 promoter (Fig. 6 and supplemental Fig. S7B). Instead, ARG5,6 promoter expression even seemed to be up-regulated, which could be due to off-target effects of expressing these IPMK homologues.
DISCUSSION
Different studies have shown that IPMK accumulates preferentially in the nucleus (10, 31, 32). This is a strong indication that some of its inositol phosphate products may be involved in regulating gene expression. In yeast, the role of Arg82 in transcription is well established (12, 13, 30); however, whether its catalytic activity plays a substantial role has long been contended (14, 15). Recent discoveries showing that enzymatically inactive mammalian IPMK participates in nutrient signaling (17, 19) prompted us to revisit this debate. Crucially, we assayed growth in liquid culture, which reflected a more accurate assessment of growth differences than in previous reports. First, we looked at the ability of arg82Δ to grow in liquid minimal medium supplemented with ammonium or ornithine as the sole nitrogen source. We observed a 20% reduction in growth with respect to the wild type strain in ammonium and an 80% drop in ornithine. This contrasted with a stronger effect observed previously in solid medium (14, 15). We tested the ability of an Arg82 kinase-dead mutant to rescue the arg82Δ phenotype and observed a partial restoration of growth in arginine and ornithine of 60 and 40%, respectively. These results support the idea championed by the Messenguy/Dubois laboratory whereby Arg82 kinase activity is not required for the control of arginine-regulated growth.
The lack of a role for Arg82 activity may indicate that inositol phosphate intermediates are not involved in nitrogen regulation. To test this hypothesis, we looked at mutants unable to synthesize pools of inositol phosphates: plc1Δ in which production of all higher phosphorylated forms is abolished and kcs1Δ in which the synthesis of inositol pyrophosphates is substantially decreased. The mild growth defect observed in both mutants when cultured in media containing arginine and ornithine as compared with ammonium was similar to that displayed by the wild type strain. Hence, abolishing inositol phosphate synthesis did not affect growth in demanding nitrogen sources. It has been proposed that the transcriptional role of Arg82 could depend on its PI3K activity. This would be independent of PLC activity and hence would not be affected in the plc1Δ mutant (10). We tested this hypothesis by expressing in arg82Δ the plant and human IPMK homologues. Expression of the plant AtIpk2β, which lacks PI3K activity, and the human IPMK, which hosts both lipid and soluble inositol enzymatic activities, restored normal growth in ammonium but not in ornithine. Therefore, Arg82 is able to control arginine-regulated transcription independently of its activity, perhaps due to its scaffolding properties (13). It should be noted that the interaction between rat IPMK and AMP-activated protein kinase depends on the phosphorylation of a tyrosine in position 174, which is also conserved in yeast. On the contrary, interaction with TOR depends on the N-terminal region (amino acids 1–60), which is particularly variable among eukaryotes.
Several high throughput screens have indicated that the N-terminal end of Arg82 is phosphorylated at positions in close proximity (33–35). We tested whether these modifications have any implication for cell growth or transcriptional regulation of arginine-regulated promoters in vivo. The expression of a form of Arg82 that cannot be phosphorylated (ARG82-SDST/ADAA) in the arg82Δ mutant was able to complement growth in ornithine and restored nitrogen transcriptional regulation of ARG5,6 promoter (data not shown). Thus, we conclude that Arg82 phosphorylation does not play a relevant role in vivo.
A number of publications have shown that expression of IPMK homologues (human, fly, plant (Arabidopsis, potato, rice, and salt cress), and fission yeast) in arg82Δ complement some of its growth defects (28, 36–42). This has been interpreted as a proof that the IPMK catalytic activity is involved in transcriptional regulation. Most of these experiments, however, addressed only growth in ammonium on solid medium. Given that total growth is a blunt measure of phenotypes caused by ARG82 deletion, we decided to look into transcriptional readouts. Available methods, such as Northern blot, RT-PCR, and enzymatic activity of genes under Arg80-Arg81-Mcm1 control, currently do not resolve the regulation of promoter expression in real time and in vivo. Instead, we developed a new technology to study promoter strength. We took advantage of the specificity of viral thymidine kinases toward nucleoside analogues, which once metabolized are toxic to the cell (43, 44). We constructed a plasmid bearing the promoter of interest fused to HSV1-TK and transformed it together with plasmids carrying IPMK homologues and Arg82 mutants into the arg82Δ strain. The promoter of ARG8, encoding acetylornithine aminotransferase, has been used to determine the capacity of Arg80-Arg81-Mcm1 to bind and regulate arginine boxes by means of RT-PCR (10). As the full extent of the promoter region is not known, our efforts to clone it in full failed. Instead, we studied the promoters of two other genes strongly down-regulated in the presence of arginine, ARG3 and ARG5,6. Both promoters behaved as expected using HSV1-TK and RT-quantitative PCR with ARG5,6 being more responsive. Hence, the system was used to study the effect of a kinase-dead Arg82. The results of ARG5,6 promoter regulation showed that Arg82 kinase activity is to a large extent dispensable. In addition, expression of plant or human IPMK was unable to restore wild type promoter functionality, indicating that enzymatic activity alone was insufficient. In conclusion, Arg82 kinase activity plays a minor role, if any, in the control of arginine-responsive promoters.
The novel strategy presented to measure promoter strength (fusion to HSV1-TK) offers the possibility of unlimited real time data collection by monitoring growth rate. Its use, combined with high density microtiter plates, may speed up the study of conditions altering gene expression. It also presents drawbacks associated with growth in plates (i.e. poor aeration makes it unsuitable for growth in non-fermentative substrates). Also, mutants with poor growth or conditions that dramatically reduce fitness or cell shape may not be used with this approach.
Indirect evidence indicates that Arg82 activity plays a relevant role in other aspects of nitrogen metabolism. For instance, arginine represents the largest nitrogen storage reserve in yeast, accumulating in vast quantities inside vacuoles (45) where it counteracts negatively charged polyphosphate polymers (46, 47). These are long inorganic phosphate polymers that constitute the main phosphate repository in yeast (48). Polyphosphate polymer metabolism is linked to inositol pyrophosphate synthesis of which Arg82 activity is a necessary step (49). Lysine is an alternative nitrogen storage amino acid in the absence of arginine. arg82Δ has been shown to display higher lysine levels than wild type (36). Another link between Arg82 and nitrogen metabolism is via the TOR signaling pathway. The yeast TOR pathway comprises two branches, and at least one of them is dedicated to monitoring nitrogen availability (20), although the amino acid-sensing module remains largely unknown (50). arg82Δ, like TOR complex 1, is hypersensitive to rapamycin, suggesting a common regulation (51, 52). It becomes clear that Arg82 influences multiple phenotypic traits; hence, its catalytic activity may affect transcriptionally independent aspects of amino acid metabolism.
The importance of Arg82 enzymatic activity for growth is highlighted by the near complete rescue seen by expressing the plant or human IPMK homologue. However, the lack of a requirement for Arg82 enzymatic activity to regulate arginine-dependent transcription suggests that this evolutionarily conserved enzyme has also acquired non-catalytic functions, such as the stabilization of the Arg80-Arg81-Mcm1 transcriptional complex (13). The non-enzymatic role of Arg82 can be compared with that played by pseudokinases. About 10% of the human kinome is constituted by kinases that have lost their protein phosphorylation ability (53); however, many pseudokinases keep their signaling functions often as scaffolds or chaperones toward proteins that likely were their original kinase substrate (54, 55).
The importance of Arg82 to non-enzymatic regulated transcription of arginine boxes might be interpreted as an adaptation of an ancient more fundamental role played by this enzyme and by their enzymatic products in controlling transcription. These considerations are supported by the discovery that histone deacetylase polypeptides from Tetrahymena and Paramecium possess an inositol-polyphosphate kinase domain similar to Arg82 (56). In addition, inositol tetrakisphosphate (a product of IPMK) has recently been shown to aid in the interaction of histone deacetylase 3 with a transcriptional corepressor (NCOR2) (57). This opens up the possibility that the catalytic activity of IPMK could play a subsidiary role in protein-protein interaction influencing gene transcription. Finally, a recent report showed that IPMK phosphorylated phosphatidylinositol 4,5-bisphosphate bound to a nuclear protein, pointing to a new mechanism of gene regulation (58). It is likely that novel IPMK partners will emerge in the future, shedding light on the important roles that this enzyme plays in the nucleus.
Supplementary Material
Acknowledgments
We are grateful to Emiliana Borreli, Adam C. Resnick, and Cristina Azevedo for providing materials and Janos Kriston-Vizi, Jonas Warringer, and Miranda Wilson for data analysis advice. Also, we thank the members of the Saiardi laboratory for fruitful discussions.
This work was supported by Medical Research Council funding of the Cell Biology Unit.

This article contains supplemental Figs. S1–S7.
- IPMK
- Arg82, and Ipk2, inositol-polyphosphate multikinase
- Kcs1
- inositol-hexakisphosphate and inositol-heptakisphosphate kinase
- PLC and Plc1
- phospholipase C
- TOR
- target of rapamycin
- Arg3
- ornithine carbamoyltransferase
- Arg5,6
- acetylglutamate kinase
- HSV1-TK
- herpes simplex virus 1 thymidine kinase
- FIAU
- fialuridine.
REFERENCES
- 1. Alcázar-Román A. R., Wente S. R. (2008) Inositol polyphosphates: a new frontier for regulating gene expression. Chromosoma 117, 1–13 [DOI] [PubMed] [Google Scholar]
- 2. Monserrate J. P., York J. D. (2010) Inositol phosphate synthesis and the nuclear processes they affect. Curr. Opin. Cell Biol. 22, 365–373 [DOI] [PubMed] [Google Scholar]
- 3. Resnick A. C., Saiardi A. (2009) in Wiley Encyclopedia of Chemical Biology (Begley T. P., ed) Vol. 2, pp. 349–359, John Wiley & Sons, Inc., New York [Google Scholar]
- 4. Burton A., Hu X., Saiardi A. (2009) Are inositol pyrophosphates signalling molecules? J. Cell. Physiol. 220, 8–15 [DOI] [PubMed] [Google Scholar]
- 5. Irvine R. F., Schell M. J. (2001) Back in the water: the return of the inositol phosphates. Nat. Rev. 2, 327–338 [DOI] [PubMed] [Google Scholar]
- 6. Michell R. H., Kirk C. J., Jones L. M., Downes C. P., Creba J. A. (1981) The stimulation of inositol lipid metabolism that accompanies calcium mobilization in stimulated cells: defined characteristics and unanswered questions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 296, 123–138 [DOI] [PubMed] [Google Scholar]
- 7. Resnick A. C., Saiardi A. (2008) Inositol polyphosphate multikinase: metabolic architect of nuclear inositides. Front. Biosci. 13, 856–866 [DOI] [PubMed] [Google Scholar]
- 8. Frederick J. P., Mattiske D., Wofford J. A., Megosh L. C., Drake L. Y., Chiou S. T., Hogan B. L., York J. D. (2005) An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc. Natl. Acad. Sci. U.S.A. 102, 8454–8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Maag D., Maxwell M. J., Hardesty D. A., Boucher K. L., Choudhari N., Hanno A. G., Ma J. F., Snowman A. S., Pietropaoli J. W., Xu R., Storm P. B., Saiardi A., Snyder S. H., Resnick A. C. (2011) Inositol polyphosphate multikinase is a physiologic PI3-kinase that activates Akt/PKB. Proc. Natl. Acad. Sci. U.S.A. 108, 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Resnick A. C., Snowman A. M., Kang B. N., Hurt K. J., Snyder S. H., Saiardi A. (2005) Inositol polyphosphate multikinase is a nuclear PI3-kinase with transcriptional regulatory activity. Proc. Natl. Acad. Sci. U.S.A. 102, 12783–12788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saiardi A., Erdjument-Bromage H., Snowman A. M., Tempst P., Snyder S. H. (1999) Synthesis of diphosphoinositol pentakisphosphate by a newly identified family of higher inositol polyphosphate kinases. Curr. Biol. 9, 1323–1326 [DOI] [PubMed] [Google Scholar]
- 12. Bercy J., Dubois E., Messenguy F. (1987) Regulation of arginine metabolism in Saccharomyces cerevisiae: expression of the three ARGR regulatory genes and cellular localization of their products. Gene 55, 277–285 [DOI] [PubMed] [Google Scholar]
- 13. Messenguy F., Dubois E. (1993) Genetic evidence for a role for MCM1 in the regulation of arginine metabolism in Saccharomyces cerevisiae. Mol. Cell. Biol. 13, 2586–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dubois E., Dewaste V., Erneux C., Messenguy F. (2000) Inositol polyphosphate kinase activity of Arg82/ArgRIII is not required for the regulation of the arginine metabolism in yeast. FEBS Lett. 486, 300–304 [DOI] [PubMed] [Google Scholar]
- 15. Odom A. R., Stahlberg A., Wente S. R., York J. D. (2000) A role for nuclear inositol 1,4,5-trisphosphate kinase in transcriptional control. Science 287, 2026–2029 [DOI] [PubMed] [Google Scholar]
- 16. Meyer R., Nalaskowski M. M., Ehm P., Schröder C., Naj X., Brehm M. A., Mayr G. W. (2012) Nucleocytoplasmic shuttling of human inositol phosphate multikinase is influenced by CK2 phosphorylation. Biol. Chem. 393, 149–160 [DOI] [PubMed] [Google Scholar]
- 17. Bang S., Kim S., Dailey M. J., Chen Y., Moran T. H., Snyder S. H., Kim S. F. (2012) AMP-activated protein kinase is physiologically regulated by inositol polyphosphate multikinase. Proc. Natl. Acad. Sci. U.S.A. 109, 616–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carling D., Mayer F. V., Sanders M. J., Gamblin S. J. (2011) AMP-activated protein kinase: nature's energy sensor. Nat. Chem. Biol. 7, 512–518 [DOI] [PubMed] [Google Scholar]
- 19. Kim S., Kim S. F., Maag D., Maxwell M. J., Resnick A. C., Juluri K. R., Chakraborty A., Koldobskiy M. A., Cha S. H., Barrow R., Snowman A. M., Snyder S. H. (2011) Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab. 13, 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Loewith R., Hall M. N. (2011) Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189, 1177–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saiardi A., Sciambi C., McCaffery J. M., Wendland B., Snyder S. H. (2002) Inositol pyrophosphates regulate endocytic trafficking. Proc. Natl. Acad. Sci. U.S.A. 99, 14206–14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mumberg D., Müller R., Funk M. (1994) Regulable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic Acids Res. 22, 5767–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borrelli E., Heyman R., Hsi M., Evans R. M. (1988) Targeting of an inducible toxic phenotype in animal cells. Proc. Natl. Acad. Sci. U.S.A. 85, 7572–7576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warringer J., Blomberg A. (2003) Automated screening in environmental arrays allows analysis of quantitative phenotypic profiles in Saccharomyces cerevisiae. Yeast 20, 53–67 [DOI] [PubMed] [Google Scholar]
- 25. Warringer J., Ericson E., Fernandez L., Nerman O., Blomberg A. (2003) High-resolution yeast phenomics resolves different physiological features in the saline response. Proc. Natl. Acad. Sci. U.S.A. 100, 15724–15729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yaghoubi S. S., Gambhir S. S. (2006) Measuring herpes simplex virus thymidine kinase reporter gene expression in vitro. Nat. Protoc. 1, 2137–2142 [DOI] [PubMed] [Google Scholar]
- 27. Chang S. C., Miller A. L., Feng Y., Wente S. R., Majerus P. W. (2002) The human homolog of the rat inositol phosphate multikinase is an inositol 1,3,4,6-tetrakisphosphate 5-kinase. J. Biol. Chem. 277, 43836–43843 [DOI] [PubMed] [Google Scholar]
- 28. Stevenson-Paulik J., Odom A. R., York J. D. (2002) Molecular and biochemical characterization of two plant inositol polyphosphate 6-/3-/5-kinases. J. Biol. Chem. 277, 42711–42718 [DOI] [PubMed] [Google Scholar]
- 29. Bechet J., Greenson M., Wiame J. M. (1970) Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur. J. Biochem. 12, 31–39 [DOI] [PubMed] [Google Scholar]
- 30. Dubois E., Messenguy F. (1991) In vitro studies of the binding of the ARGR proteins to the ARG5,6 promoter. Mol. Cell. Biol. 11, 2162–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El Bakkoury M., Dubois E., Messenguy F. (2000) Recruitment of the yeast MADS-box proteins, ArgRI and Mcm1 by the pleiotropic factor ArgRIII is required for their stability. Mol. Microbiol. 35, 15–31 [DOI] [PubMed] [Google Scholar]
- 32. Nalaskowski M. M., Deschermeier C., Fanick W., Mayr G. W. (2002) The human homologue of yeast ArgRIII protein is an inositol phosphate multikinase with predominantly nuclear localization. Biochem. J. 366, 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smolka M. B., Albuquerque C. P., Chen S. H., Zhou H. (2007) Proteome-wide identification of in vivo targets of DNA damage checkpoint kinases. Proc. Natl. Acad. Sci. U.S.A. 104, 10364–10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Albuquerque C. P., Smolka M. B., Payne S. H., Bafna V., Eng J., Zhou H. (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell. Proteomics 7, 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu R., Haas W., Dephoure N., Huttlin E. L., Zhai B., Sowa M. E., Gygi S. P. (2011) A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat. Methods 8, 677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caddick S. E., Harrison C. J., Stavridou I., Johnson S., Brearley C. A. (2007) A lysine accumulation phenotype of ScIpk2Δ mutant yeast is rescued by Solanum tuberosum inositol phosphate multikinase. Biochem. J. 403, 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El Alami M., Messenguy F., Scherens B., Dubois E. (2003) Arg82p is a bifunctional protein whose inositol polyphosphate kinase activity is essential for nitrogen and PHO gene expression but not for Mcm1p chaperoning in yeast. Mol. Microbiol. 49, 457–468 [DOI] [PubMed] [Google Scholar]
- 38. Seeds A. M., Bastidas R. J., York J. D. (2005) Molecular definition of a novel inositol polyphosphate metabolic pathway initiated by inositol 1,4,5-trisphosphate 3-kinase activity in Saccharomyces cerevisiae. J. Biol. Chem. 280, 27654–27661 [DOI] [PubMed] [Google Scholar]
- 39. Suzuki M., Tanaka K., Kuwano M., Yoshida K. T. (2007) Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): implications for the phytic acid biosynthetic pathway. Gene 405, 55–64 [DOI] [PubMed] [Google Scholar]
- 40. Xia H. J., Brearley C., Elge S., Kaplan B., Fromm H., Mueller-Roeber B. (2003) Arabidopsis inositol polyphosphate 6-/3-kinase is a nuclear protein that complements a yeast mutant lacking a functional ArgR-Mcm1 transcription complex. Plant Cell 15, 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang L., Tang R., Zhu J., Liu H., Mueller-Roeber B., Xia H., Zhang H. (2008) Enhancement of stress tolerance in transgenic tobacco plants constitutively expressing AtIpk2β, an inositol polyphosphate 6-/3-kinase from Arabidopsis thaliana. Plant Mol. Biol. 66, 329–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu J. Q., Zhang J. T., Tang R. J., Lv Q. D., Wang Q. Q., Yang L., Zhang H. X. (2009) Molecular characterization of ThIPK2, an inositol polyphosphate kinase gene homolog from Thellungiella halophila, and its heterologous expression to improve abiotic stress tolerance in Brassica napus. Physiol. Plant. 136, 407–425 [DOI] [PubMed] [Google Scholar]
- 43. Hsieh C. H., Liu R. S., Wang H. E., Hwang J. J., Deng W. P., Chen J. C., Chen F. D. (2006) In vitro evaluation of herpes simplex virus type 1 thymidine kinase reporter system in dynamic studies of transcriptional gene regulation. Nucl. Med. Biol. 33, 653–660 [DOI] [PubMed] [Google Scholar]
- 44. Jonsson J. J., McIvor R. S. (1991) Herpes simplex virus thymidine kinase enzymatic assay in transient transfection experiments using thymidine kinase-deficient cells. Anal. Biochem. 199, 232–237 [DOI] [PubMed] [Google Scholar]
- 45. Davis R. H. (1986) Compartmental and regulatory mechanisms in the arginine pathways of Neurospora crassa and Saccharomyces cerevisiae. Microbiol. Rev. 50, 280–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dürr M., Urech K., Boller T., Wiemken A., Schwencke J., Nagy M. (1979) Sequestration of arginine by polyphosphate in vacuoles of yeast (Saccharomyces cerevisiae). Arch. Microbiol. 121, 169–175 [DOI] [PubMed] [Google Scholar]
- 47. Saiardi A. (2012) How inositol pyrophosphates control cellular phosphate homeostasis? Adv. Biol. Regul. 52, 351–359 [DOI] [PubMed] [Google Scholar]
- 48. Rao N. N., Gómez-García M. R., Kornberg A. (2009) Inorganic polyphosphate: essential for growth and survival. Annu. Rev. Biochem. 78, 605–647 [DOI] [PubMed] [Google Scholar]
- 49. Lonetti A., Szijgyarto Z., Bosch D., Loss O., Azevedo C., Saiardi A. (2011) Identification of an evolutionary conserved family of inorganic polyphosphate endopolyphosphatases. J. Biol. Chem. 286, 31966–31974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. De Virgilio C., Loewith R. (2006) Cell growth control: little eukaryotes make big contributions. Oncogene 25, 6392–6415 [DOI] [PubMed] [Google Scholar]
- 51. Chan T. F., Carvalho J., Riles L., Zheng X. F. (2000) A chemical genomics approach toward understanding the global functions of the target of rapamycin protein (TOR). Proc. Natl. Acad. Sci. U.S.A. 97, 13227–13232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ikai N., Nakazawa N., Hayashi T., Yanagida M. (2011) The reverse, but coordinated, roles of Tor2 (TORC1) and Tor1 (TORC2) kinases for growth, cell cycle and separase-mediated mitosis in Schizosaccharomyces pombe. Open Biol. 1, 110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taylor S. S., Kornev A. P. (2011) Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem. Sci. 36, 65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scheeff E. D., Eswaran J., Bunkoczi G., Knapp S., Manning G. (2009) Structure of the pseudokinase VRK3 reveals a degraded catalytic site, a highly conserved kinase fold, and a putative regulatory binding site. Structure 17, 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fukuda K., Gupta S., Chen K., Wu C., Qin J. (2009) The pseudoactive site of ILK is essential for its binding to α-parvin and localization to focal adhesions. Mol. Cell 36, 819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Smith J. J., Torigoe S. E., Maxson J., Fish L. C., Wiley E. A. (2008) A class II histone deacetylase acts on newly synthesized histones in Tetrahymena. Eukaryot. Cell 7, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Watson P. J., Fairall L., Santos G. M., Schwabe J. W. (2012) Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature 481, 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blind R. D., Suzawa M., Ingraham H. A. (2012) Direct modification and activation of a nuclear receptor-PIP2 complex by the inositol lipid kinase IPMK. Sci. Signal. 5, ra44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.