Abstract
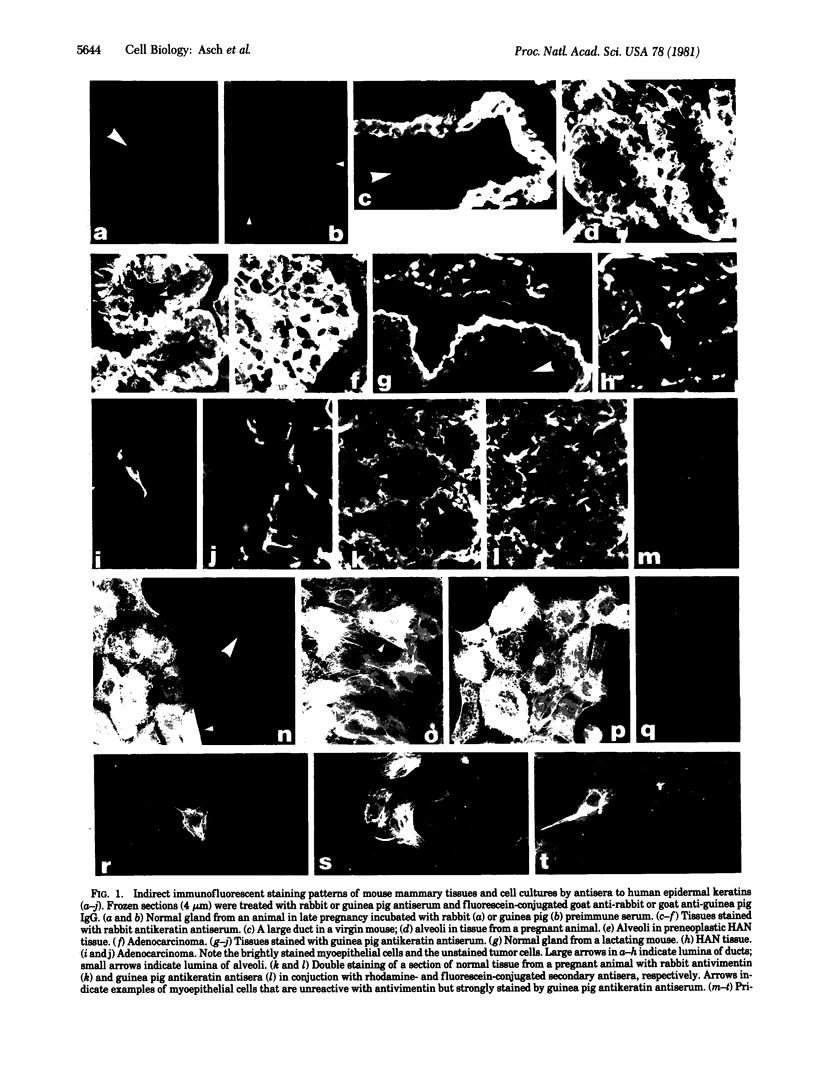
Few markers are available to identify the three types of mammary epithelial cells--ductal, alveolar, and myoepithelial--especially in pathological conditions and in cell cultures. We have used antisera to human keratins in immunofluorescence to facilitate the identification of the three mouse mammary epithelial cell types. In frozen tissue sections and primary cell cultures, a rabbit antikeratin antiserum specifically stained cytoplasmic filaments in all three types of epithelial cells. A guinea pig antiserum against the same keratin preparation, however, reacted preferentially with filaments in myoepithelial cells and readily detected this cell type in normal, dysplastic, and malignant mammary tissues and cell cultures. Neither antisera reacted with fibroblasts or any other mesenchymal cells. The combined use of the two antikeratin antisera thereby permits rapid surveys of tissue sections and cultures for the localization of not only all epithelial cells but also the subpopulation of myoepithelial cells. Moreover, when mammary cultures established from late-pregnant or lactating mice were stained simultaneously with guinea pig antikeratin and rabbit anticasein antisera, three populations of epithelial cells were mutually exclusive: those stained by anticasein antiserum, those stained by guinea pig antikeratin antiserum, and those stained by neither, consistent with properties of alveolar, myoepithelial, and ductal cells, respectively. These antisera thus offer a tool for studying different epithelial cell types during mammary development, tumorigenesis, and malignant progression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch B. B., Kamat B. R., Burstein N. A. Interactions of normal, dysplastic, and malignant mammary epithelial cells with fibronectin in vivo and in vitro. Cancer Res. 1981 Jun;41(6):2115–2125. [PubMed] [Google Scholar]
- Asch B. B., Medina D. Concanavalin A-induced agglutinability of normal, preneoplastic, and neoplastic mouse mammary cells. J Natl Cancer Inst. 1978 Dec;61(6):1423–1430. [PubMed] [Google Scholar]
- Bannasch P., Zerban H., Schmid E., Franke W. W. Liver tumors distinguished by immunofluorescence microscopy with antibodies to proteins of intermediate-sized filaments. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4948–4952. doi: 10.1073/pnas.77.8.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale B. A., Stern I. B., Rabin M., Huang L. The identification of fibrous proteins in fetal rat epidermis by electrophoretic and immunologic techniques. J Invest Dermatol. 1976 Apr;66(4):230–235. doi: 10.1111/1523-1747.ep12482148. [DOI] [PubMed] [Google Scholar]
- Doran T. I., Vidrich A., Sun T. T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980 Nov;22(1 Pt 1):17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- Feldman M. K., Deome K. B. Localization of casein-rich, fat-rich and DNA-synthesizing cells in monolayer cultures of mid-pregnant mouse mammary epithelium. Histochem J. 1975 Sep;7(5):411–418. doi: 10.1007/BF01003878. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Herbert D. C., Burke R. E., McGuire W. L. Casein and alpha-lactalbumin detection in breast cancer cells by immunocytochemistry. Cancer Res. 1978 Aug;38(8):2221–2223. [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Baden H. P., Kunilus J., Fleming B. F. Immunology of epidermal fibrous proteins. J Invest Dermatol. 1976 Oct;67(4):521–525. doi: 10.1111/1523-1747.ep12664546. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Kubilus J., Baden H. P. Intraspecies heterogeneity of epidermal keratins isolated from bovine hoof and snout. Biochem J. 1979 Jan 1;177(1):187–196. doi: 10.1042/bj1770187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The permeability of mammary ducts. J Physiol. 1971 Aug;216(3):701–716. doi: 10.1113/jphysiol.1971.sp009548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Effects of various oncogenic agents on tumor-producing capabilities of series D BALB-c mammary nodule outgrowth lines. J Natl Cancer Inst. 1970 Aug;45(2):353–363. [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starger J. M., Goldman R. D. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner L. A., Eisen H. N. Symposium on in vitro studies of the immune response. I. Variations in the immune response to a simple determinant. Bacteriol Rev. 1966 Jun;30(2):383–396. doi: 10.1128/br.30.2.383-396.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. Postsynthetic modifications of mammalian epidermal alpha-keratin. Biochemistry. 1979 Dec 11;18(25):5664–5669. doi: 10.1021/bi00592a022. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. The polypeptide composition of bovine epidermal alpha-keratin. Biochem J. 1975 Dec;151(3):603–614. doi: 10.1042/bj1510603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Freedberg I. M. Epidermal structural proteins. II. Isolation and purification of tonofilaments of the newborn rat. Biochim Biophys Acta. 1972 Apr 15;263(2):382–396. [PubMed] [Google Scholar]