Abstract
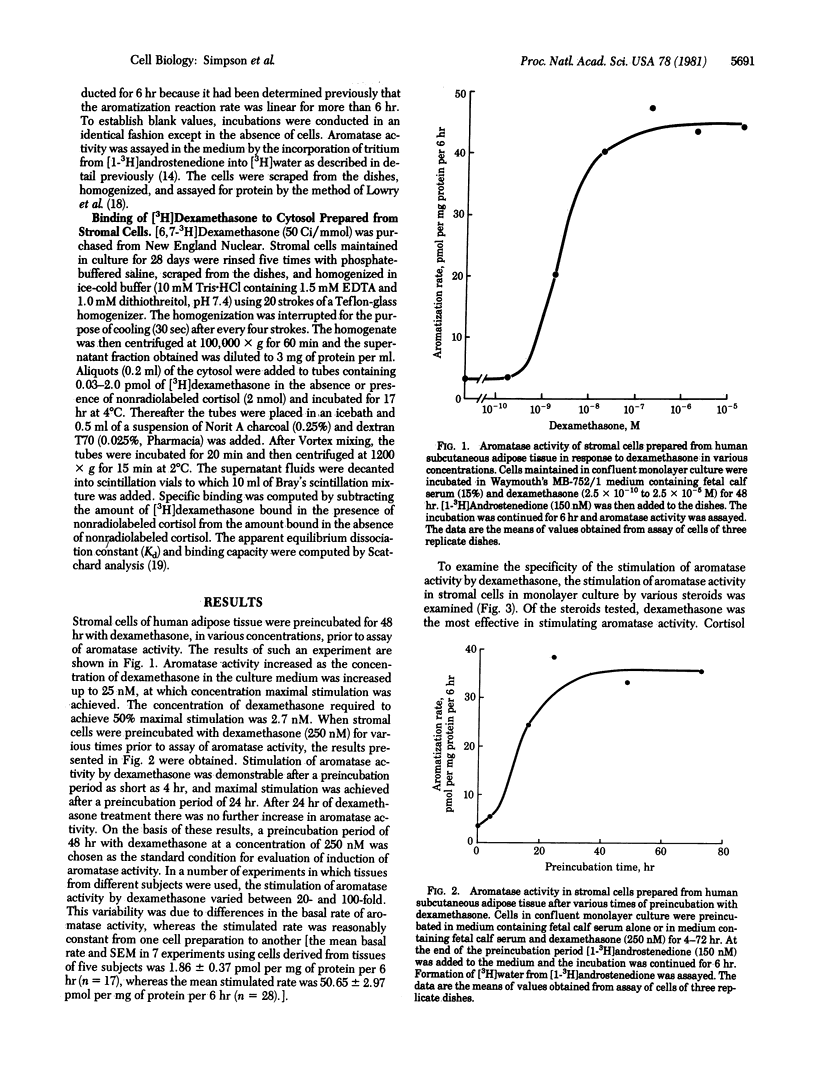
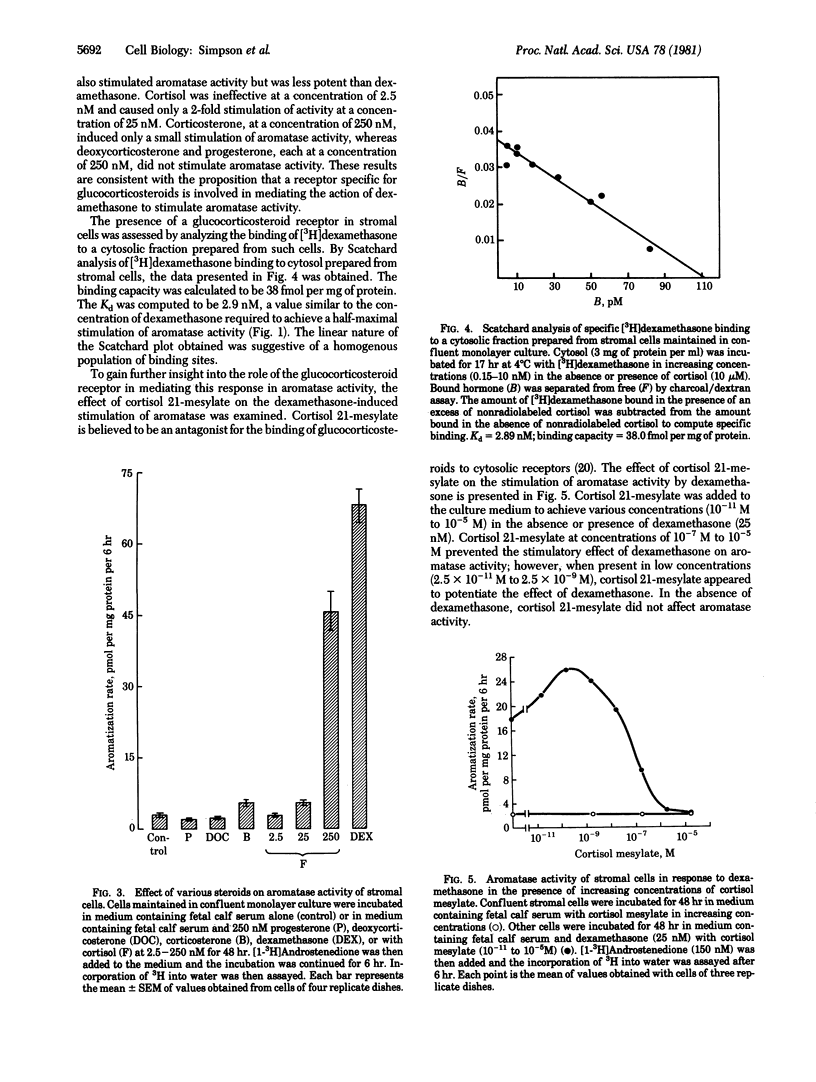
Stromal cells prepared from adipose tissue of women were maintained in monolayer culture to study the regulation of aromatase activity by hormones. Aromatase activity was stimulated 20- to 100-fold by dexamethasone at a concentration of 250 nM. Half-maximal stimulation of aromatase activity was attained at a dexamethasone concentration of 2.7 nM. The stimulatory effect of dexamethasone was apparent after a preincubation time of 4 hr, and stimulation was maximal after 24 hr of preincubation. The stimulatory effect of dexamethasone was observed only when fetal calf serum also was present in the culture medium. Of the various steroids tested, dexamethasone was the most potent in stimulating aromatase activity. Cortisol was less effective than dexamethasone, whereas corticosterone, at a concentration of 250 nM, caused only a small stimulation of aromatase activity. Progesterone and deoxycorticosterone (250 nM) did not affect aromatase activity. Cytosolic fractions prepared from stromal cells that had been maintained in monolayer culture were found to contain a homogenous population of sites that specifically bound [3H]dexamethasone with relatively high affinity (Kd = 2.9 nM) and low capacity (38 fmol per mg of protein). The stimulatory effect of dexamethasone on aromatase activity was prevented by simultaneous incubation with cortisol 21-mesylate (0.1-10 microM), a compound known to block the binding of glucocorticosteroids to cytoplasmic receptors. The stimulatory effect of dexamethasone also was prevented by incubation of the cells with cycloheximide or actinomycin D. These findings are suggestive that glucocorticosteroids act to increase aromatase activity in stromal cells by inducing the synthesis of new enzyme protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman G. E., Smith M. E., Mendelson C. R., MacDonald P. C., Simpson E. R. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab. 1981 Aug;53(2):412–417. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- Bolt H. M., Göbel P. Formation of estrogens from androgens by human subcutaneous adipose tissue in vitro. Horm Metab Res. 1972 Jul;4(4):312–313. doi: 10.1055/s-0028-1097099. [DOI] [PubMed] [Google Scholar]
- Dixon-Shanies D., Knittle J. L. Effect of hormones on cyclic AMP levels in cultured human cells. Biochem Biophys Res Commun. 1976 Feb 9;68(3):982–987. doi: 10.1016/0006-291x(76)91242-0. [DOI] [PubMed] [Google Scholar]
- Dorrington J. H., Armstrong D. T. Follicle-stimulating hormone stimulates estradiol-17beta synthesis in cultured Sertoli cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2677–2681. doi: 10.1073/pnas.72.7.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington J. H., Moon Y. S., Armstrong D. T. Estradiol-17beta biosynthesis in cultured granulosa cells from hypophysectomized immature rats; stimulation by follicle-stimulating hormone. Endocrinology. 1975 Nov;97(5):1328–1331. doi: 10.1210/endo-97-5-1328. [DOI] [PubMed] [Google Scholar]
- Dunn L. J., Bradbury J. T. Endocrine factors in endometrial carcinoma. A preliminary report. Am J Obstet Gynecol. 1967 Feb 15;97(4):465–471. doi: 10.1016/0002-9378(67)90558-3. [DOI] [PubMed] [Google Scholar]
- Edman C. D., MacDonald P. C. Effect of obesity on conversion of plasma androstenedione to estrone in ovulatory and anovulator young women. Am J Obstet Gynecol. 1978 Feb 15;130(4):456–461. doi: 10.1016/0002-9378(78)90288-0. [DOI] [PubMed] [Google Scholar]
- Erickson G. F., Hsueh A. J. Stimulation of aromatase activity by follicle stimulating hormone in rat granulosa cells in vivo and in vitro. Endocrinology. 1978 Apr;102(4):1275–1282. doi: 10.1210/endo-102-4-1275. [DOI] [PubMed] [Google Scholar]
- Erickson G. F., Ryan K. J. The effect of LH/FSH, dibutyryl cyclic AMP, and prostaglandins on the production of estrogens by rabbit granulosa cells in vitro. Endocrinology. 1975 Jul;97(1):108–113. doi: 10.1210/endo-97-1-108. [DOI] [PubMed] [Google Scholar]
- Fritz I. B., Griswold M. D., Louis B. G., Dorrington J. H. Similarity of responses of cultured Sertoli cells to cholera toxin and FSH. Mol Cell Endocrinol. 1976 Aug-Sep;5(3-4):289–294. doi: 10.1016/0303-7207(76)90090-3. [DOI] [PubMed] [Google Scholar]
- Frost P. G., Reed M. J., James V. H. The aromatization of androstenedione by human adipose and liver tissue. J Steroid Biochem. 1980 Dec;13(12):1427–1431. doi: 10.1016/0022-4731(80)90055-2. [DOI] [PubMed] [Google Scholar]
- Gordon G. G., Olivo J., Rafil F., Southren A. L. Conversion of androgens to estrogens in cirrhosis of the liver. J Clin Endocrinol Metab. 1975 Jun;40(6):1018–1026. doi: 10.1210/jcem-40-6-1018. [DOI] [PubMed] [Google Scholar]
- Gore-Langton R., McKeracher H., Dorrington J. An alternative method for the study of follicle-stimulating hormone effects on aromatase activity in Sertoli cell cultures. Endocrinology. 1980 Aug;107(2):464–471. doi: 10.1210/endo-107-2-464. [DOI] [PubMed] [Google Scholar]
- Hemsell D. L., Grodin J. M., Brenner P. F., Siiteri P. K., MacDonald P. C. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. J Clin Endocrinol Metab. 1974 Mar;38(3):476–479. doi: 10.1210/jcem-38-3-476. [DOI] [PubMed] [Google Scholar]
- Hsueh A. J., Erickson G. F. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids. 1978 Dec;32(5):639–648. doi: 10.1016/0039-128x(78)90074-0. [DOI] [PubMed] [Google Scholar]
- Knab D. R. Estrogen and endometrial carcinoma. Obstet Gynecol Surv. 1977 May;32(5):267–281. doi: 10.1097/00006254-197705000-00001. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MLYNARYK P., GILLIES R. R., MURPHY B., PATTEE C. J. Cortisol production rates in obesity. J Clin Endocrinol Metab. 1962 Jun;22:587–591. doi: 10.1210/jcem-22-6-587. [DOI] [PubMed] [Google Scholar]
- MacDonald P. C., Edman C. D., Hemsell D. L., Porter J. C., Siiteri P. K. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am J Obstet Gynecol. 1978 Feb 15;130(4):448–455. doi: 10.1016/0002-9378(78)90287-9. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Davies I. J., Reddy V. V., Flores F., Petro Z., Kuhn M., White R. J., Takaoka Y., Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nimrod A., Ryan K. J. Aromatization of androgens by human abdominal and breast fat tissue. J Clin Endocrinol Metab. 1975 Mar;40(3):367–372. doi: 10.1210/jcem-40-3-367. [DOI] [PubMed] [Google Scholar]
- Perel E., Killinger D. W. The interconversion and aromatization of androgens by human adipose tissue. J Steroid Biochem. 1979 Jun;10(6):623–627. doi: 10.1016/0022-4731(79)90514-4. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- RYAN K. J. Metabolism of C-16-oxygenated steroids by human placenta: the formation of estriol. J Biol Chem. 1959 Aug;234(8):2006–2008. [PubMed] [Google Scholar]
- Schindler A. E., Ebert A., Friedrich E. Conversion of androstenedione to estrone by human tissue. J Clin Endocrinol Metab. 1972 Oct;35(4):627–630. doi: 10.1210/jcem-35-4-627. [DOI] [PubMed] [Google Scholar]
- Schweikert H. U., Milewich L., Wilson J. D. Aromatization of androstenedione by cultured human fibroblasts. J Clin Endocrinol Metab. 1976 Oct;43(4):785–795. doi: 10.1210/jcem-43-4-785. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B., Johnson D. F. Unique long-acting antiglucocorticoid in whole and broken cell systems. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5167–5171. doi: 10.1073/pnas.77.9.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M. The stereochemistry of actinomycin binding to DNA and its implications in molecular biology. Prog Nucleic Acid Res Mol Biol. 1973;13:153–190. doi: 10.1016/s0079-6603(08)60103-8. [DOI] [PubMed] [Google Scholar]
- Southren A. L., Olivo J., Gordon G. G., Vittek J., Brener J., Rafii F. The conversion of androgens to estrogens in hyperthyroidism. J Clin Endocrinol Metab. 1974 Feb;38(2):207–214. doi: 10.1210/jcem-38-2-207. [DOI] [PubMed] [Google Scholar]
- Valladares L. E., Payne A. H. Induction of testicular aromatization by luteinizing hormone in mature rats. Endocrinology. 1979 Aug;105(2):431–436. doi: 10.1210/endo-105-2-431. [DOI] [PubMed] [Google Scholar]
- Van R. L., Bayliss C. E., Roncari D. A. Cytological and enzymological characterization of adult human adipocyte precursors in culture. J Clin Invest. 1976 Sep;58(3):699–704. doi: 10.1172/JCI108516. [DOI] [PMC free article] [PubMed] [Google Scholar]


