Abstract
Microbial diversity and populations in a hydrothermal plume that was present inside the caldera of the Suiyo Seamount, a submarine volcano on the Izu-Bonin Arc, were investigated by performing a phylogenetic analysis of the 16S rRNA gene and by using fluorescence in situ hybridization (FISH). Corresponding to transmissivity, an indicator of turbidity, the vertical total cell count as determined by 4′,6′-diamidino-2-phenylindole (DAPI) staining varied from 5.6 × 104 to 1.1 × 105 cells ml−1, and the apparent plume layer was assessed to be at a depth of 1,050 to 1,200 m inside the caldera and to contain 1.0 × 105 to 1.1 × 105 cells ml−1. From microbial samples collected in the plume by an in situ filtration system, the following two major phylogenetic groups, which were closely related to sulfur-oxidizing microbes, were obtained: the SUP05 group belonging to the gamma subclass of the Proteobacteria (13 of 20 clones) and the SUP01 group belonging to the epsilon subclass of the Proteobacteria (5 of 20 clones). Specific oligonucleotide probes for these groups (SUP05-187 and SUP01-63) were designed and were used with various water samples obtained from the Suiyo Seamount. In the apparent plume layer, up to 66% of the total counts of microbial cells were estimated to be Bacteria cells that hybridized to EUB338, and few cells were identified by the archaeal probe ARCH915. Almost all Bacteria cells were hard to identify with the known group-specific probes, such as ALF19, GAM42a, and CF319, while 88 to 90% of the Bacteria cells hybridized with SUP05-187 and >98% of them were considered members of the SUP05 and SUP01 populations. In a low-temperature vent fluid emitted from a bivalve-colonized mound, the SUP05 cells accounted for >99% of the Bacteria cells, suggesting that a portion of the plume cells originated on the surface of the seafloor at a depth of about 1,380 m. From further analysis of cell morphology (i.e., cell size and cell elongation index) we inferred that the SUP05 cells were active in the plume layer at a depth of 1,050 to 1,200 m compared to the activity in a near-bottom layer, while many elongated cells were found between these layers. These findings suggest that the morphology and distribution of SUP05 cells have complex relationships with hydrothermal activities and water circulation. Although growth and production rates remain to be defined, we concluded that this Suiyo Seamount caldera has functioned as a natural continuous incubator for these two phylotypes of Bacteria in an aphotic deep-sea environment.
Hydrothermal circulation activities give rise to buoyant plumes in deep oceans (46). Compared with the concentrations in the surrounding ambient seawater, hydrothermal plumes contain several-orders-of-magnitude-higher concentrations of reduced chemicals, such as methane, hydrogen, hydrogen sulfide, iron, and manganese, some of which are derived directly from deep subsurface magma chambers. Elevated numbers of microbes have also been detected in hydrothermal plumes (28, 45), in which the reduced chemicals seem to support the growth of microbes (i.e., chemolithoautotrophic growth). In previous microbiological studies, the methane oxidation rate was calculated by performing incubation experiments with plume water (8), manganese-containing microbial cells were observed with an electron microscope (7), and sulfur-oxidizing microbes and their activities were detected (20, 32). Such microbial chemolithoautotrophic activities may play important roles in organic carbon production in deep oceans (20, 28) or may occasionally enhance zooplankton aggregation around a plume (40).
Generally, the culturable microbes represent only a limited proportion of the whole microbial population in a sample of seawater, both quantitatively (22) and qualitatively (12). This limited representation is attributable to the presence of viable but nonculturable cells in seawater (48), as well as to problems with cultivation techniques. Molecular and cellular detection techniques, such as direct cloning of environmental DNA and fluorescence in situ hybridization (FISH), are overcoming these problems and providing real images of microbial communities in nature (2). However, these culture-independent techniques have not been applied previously to communities in deep-sea hydrothermal plumes.
The Suiyo Seamount is one of the most active submarine volcanoes in the Izu-Bonin Arc. Its caldera, which is a horseshoe-shaped structure, contains many active hydrothermal vents, most of which are 5 to 15 cm high, in the center (16). Large amounts of reduced chemicals (e.g., 108 μM methane and 1.6 mM hydrogen sulfide) have been detected in hydrothermal fluids emitted from these vents (42). However, little is known about the local microbial communities. In this study, we aimed to clarify (i) the microbial diversity by phylogenetic analysis of DNA samples collected from the hydrothermal plume inside the Suiyo Seamount caldera, (ii) the composition and population of the microbial community in the plume by quantitative FISH analysis, and (iii) the vertical distribution of some representative Bacteria groups. This was the first quantitative study in which both molecular and cellular techniques were used to elucidate the structure of a whole microbial community in a deep-sea hydrothermal plume.
MATERIALS AND METHODS
Study site and sample collection.
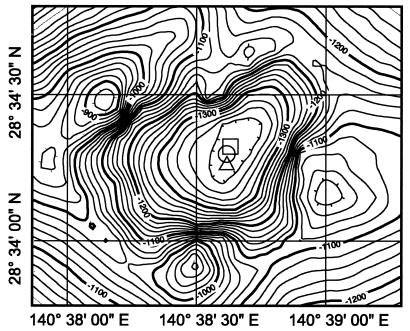
Microbial samples were collected in the Suiyo Seamount caldera (Fig. 1) during the NT-01-09 cruise (28 September to 26 October 2001) of RV Natsushima and Shinkai 2000 of the Japanese Marine Science and Technology Center (JAMSTEC, Yokosuka, Japan) and during the KR-01-15 cruise (8 to 28 December 2001) of RV Kairei (JAMSTEC). During the KR-01-15 cruise, hydrothermal plumes were detected by a conductivity, temperature, and depth (CTD) sensor system (911plus; Sea-Bird, Inc., Bellevue, Wash.) attached to a transmissometer (SeaTech Inc.). On this cruise, strong anomalies in transmissivity were detected at a depth of 1,050 to 1,200 m inside the caldera through on-board observation by towing the CTD system up and down (tow-yo) and traversing the caldera from the southwest to the northeast; these anomalies corresponded to elevated concentrations of methane and manganese (U. Tsunogai and K. Okamura, personal communication). Slight anomalies in both transmissivity and chemicals were found at depths above 1,000 m, and the values were general seawater values (i.e., less than 1/30 of the values in the apparent plume layer). In the tow-yo, water samples for microscopic analysis were collected at different water depths with 12-liter Niskin bottle samplers attached to the CTD from almost the center of the caldera (Fig. 1). Near the center, samples were also collected from a diffuse flow vent in a mound heavily colonized with some bivalve species (e.g., Bathymodiolus septemdierum) at a depth of about 1,380 m, as well as at a location 2 m above this bivalve colony, by using 2.7-liter Niskin bottles attached to a Shinkai 2000 submersible during the NT-01-09 cruise. Immediately after retrieval, 50 to 200 ml of each sample was fixed with neutralized 38% formalin (final formaldehyde concentration, 3.8%) at 4°C overnight. A 5- to 20-ml portion of each fixed sample was filtered onto a poly-l-lysine-coated 0.2-μm-pore-size polycarbonate Nuclepore filter (PLL filter) (30) by using a vacuum pressure of less than 0.03 MPa. Microbial cells immobilized on the PLL filter were dried for 2 h at room temperature, and the cells were then kept at −80°C until analysis. Microbial samples used for DNA extraction and phylogenetic analysis were collected during the KR-01-15 cruise at a depth of 1,200 m directly on aseptic 0.2-μm-pore-size polyethersulfone membrane filters (diameter, 142 mm; Pall Life Sciences, Ann Arbor, Mich.) equipped with an in situ filtration system (WTS 6-12-142FH; McLane Research Laboratories Inc., Falmouth, Mass.). More than 10 liters of the hydrothermal plume water was filtered, and the microbial DNA on the filters was stored at −80°C until analysis.
FIG. 1.
Location of Suiyo Seamount and sampling points. Seawater samples for microscopic analysis were collected at sites indicated by the triangle (28°34′31"N, 140°28′59"E) during the KR01-05 cruise and by the open circle (28°34′27"N, 140°38′61"E) during the NT01-09 cruise, while in situ filtration samples for microbial DNA extraction and phylogenetic analysis were obtained at the site indicated by the square (28°34′35"N, 140°38′64"E) during the KR-01-15 cruise.
Phylogenetic analysis of 16S rRNA gene sequences.
Each microbial sample was homogenized with sterile glass beads (diameter, ≤106 μm; Sigma, St. Louis, Mo.) by using 1.5 g in 1.5 ml of lysis buffer (30 mM Tris [pH 7.4], 100 mM NaCl, 5 mM EDTA, 1% sodium dodecyl sulfate, 0.01% proteinase K) and an MSK cell homogenizer (B. Braun Biotech International, Melsungen, Germany) operating at 2,000 rpm for 2 min at about 5°C (31). After the solution stood at 37°C for 60 min, it was combined with an equal amount of a chloroform-isoamyl alcohol (24/1) mixture and centrifuged at 1,500 × g for 15 min at 4°C. The supernatant was recovered, combined with an equal amount of a phenol-chloroform-isoamyl alcohol (25/24/1) mixture, and centrifuged as described above (37). The supernatant was then mixed with 10 ml of absolute ethanol and stored at −30°C overnight. After centrifugation at 10,300 × g for 30 min at 4°C, the precipitate was rinsed with 70% ethanol, dried at room temperature, resuspended in 100 μl of Tris-EDTA buffer, and stored at −30°C.
The procedures used to amplify the 16S rRNA gene, purify and sequence the amplified products, and construct a phylogenetic tree have been described previously (29). The bacterial 16S rRNA gene was amplified by PCR by using an oligonucleotide primer set consisting of 27f (AGAGTTTGATCATGGCTCAG) and 1492r (GGTTACCTTGTTACGACTT) (23). The amplified product was then purified with a spin column (Microspin TM S-400 HR column; Amersham Pharmacia Biotech, Piscataway, N.J.) and subcloned with a TA cloning kit (Invitrogen, Carlsbad, Calif.); both the column and the kit were used according to the manufacturers' instructions. The PCR clones were sequenced with an automated DNA sequencer (ABI PRISM 3700; Applied Biosystems, Tokyo, Japan) by performing cycle sequencing according to the manufacturer's instructions. After chimeric sequences were eliminated by using the Chimera-Check software available from Ribosomal Database Project II (http://rdp.cme.msu.edu/html/analyses.html), target sequences and sequences related to them, obtained from the database, were aligned with the Se-Al software (version 2.0) (http://evolve.zoo.ox.ac.uk/software/Se-Al/main.html), and we fixed a gap-free comparable sequence data set (length, 1,180 bp). A phylogenetic tree was then constructed by the neighbor-joining method by using ClustalW, version 1.8 (ftp://ftp.ebi.ac.uk/pub/software/unix/clustalw/).
Oligonucleotide probes.
Using 16S rRNA gene sequences obtained in this study, Probe-Check software from Ribosomal Database Project II, and the FASTA homology search program from the DNA Data Bank of Japan (http://www.ddbj.nig.ac.jp/), we designed two oligonucleotide probes, SUP05-187 (GGGCTCCTTTTCTCCATA) for specific detection of the SUP05 group in the gamma subclass of the Proteobacteria and SUP01-63 (TAAGACCCGTTCTCGTTCG) for detection of the SUP01 group in the epsilon subclass of the Proteobacteria. To estimate the composition of the microbial community, we also used general probes, such as EUB338, ARCH915, ALF19, GAM42a, CF319, MOALF142, MOGAM197, and NON338 (Table 1). These DNA probes were each labeled with fluorescein 5-isothiocyanate (FITC) or tetramethylrhodamine 5-(and 6-)-isothiocyanate (TRITC) by 5′-aminolink conjugation, while indodicarbocyanine (Cy5) labeling was performed by 5′-trifluoroacetic acid aminolink cyanoethyl phosphoramidite (PE Biosystems, Chiba, Japan) conjugation.
TABLE 1.
Oligonucleotide probes used in this study
| Probe | Target microbial group | Sequence (5′-3′) | Target site (rRNA positions)a | Formamide concn (%, vol/vol)b | Reference |
|---|---|---|---|---|---|
| EUB338 | Bacteria | GCTGCCTCCCGTAGGAGT | 16S (338-355) | 20 | 1 |
| ARCH915 | Archaea | GTGCTCCCCCGCCAATTCCT | 16S (915-934) | 30 | 15 |
| NON338 | Negative control | ACTCCTACGGGAGGCAGC | 20 | 44 | |
| GAM42a | Gamma subclass of Proteobacteria | GCCTTCCCACATCGTTT | 23S (1027-1043) | 35 | 26 |
| ALF19 | Alpha subclass of Proteobacteria | CGTTCG(C/T)TCTGAGCCAG | 16S (19-35) | 20 | 26 |
| CF319 | Cytophaga-Flavobacterium | TGGTCCGTGTCTCAGTAC | 16S (319-336) | 35 | 27 |
| MOALF142 | Serine pathway methanotroph | CCCTGAGTTATTCCGAAC | 16S (142-159) | 20 | 41 |
| MOGAM197 | RuMP pathway methanotroph | GGTCCGAAGATCCCCCGCTT | 16S (197-216) | 20 | 41 |
| SUP05-187 | SUP05 | GGGCTCCTTTTCTCCATA | 16S (187-205) | 20 | This study |
| SUP01-63 | SUP01 | TAAGACCCGTTCTCGTTCG | 16S (63-82) | 30 | This study |
Position in the 16S or 23S rRNA of Escherichia coli (4).
Concentration of formamide in in situ hybridization buffer.
FISH analysis.
The compositions of both the hybridization and wash buffers used have been described elsewhere (30). A fluorochrome-labeled probe was added to a hybridization buffer that contained the formamide concentration suitable for each probe (Table 1); the final concentration was 0.2 pmol μl−1. Then 50 μl of the processed hybridization solution was added to a PLL filter sample placed on a glass slide with three holes (diameter of each hole, 11 mm; Matsunami Glass, Osaka, Japan), and the glass slide was placed in a 50-ml plastic tube. After the tube was sealed tightly, the sample was incubated for hybridization at 42°C for 4.5 h without shaking. Cells on the PLL filter were then rinsed in the wash buffer at 44°C for 30 min. The hybridization and washing cycle described above was repeated for multiple labeling of cells with different probes at each appropriate formamide concentration. Cells on the PLL filter were then stained with 4′,6′-diamidino-2-phenylindole (DAPI) (final concentration, 5 μg ml−1) for 10 min at room temperature, rinsed with pure water, and mounted on a glass slide. After 1 drop of Prolong antifade solution (Molecular Probes, Eugene, Oreg.) was added, the filter sample was covered with a coverslip. For the newly developed probes, SUP05-187 and SUP01-63, appropriate formamide concentrations were determined by changing the formamide concentration in the hybridization buffer and using environmental microbes collected at a depth of 1,163 m in the plume.
Microscopy and image analysis.
Fluorescence microscopic images of cells were captured with a cooled charge-coupled device camera (MicroMax; Nippon Princeton Instruments, Chiba, Japan) by using a fluorescence microscope (Axioplan II; Carl Zeiss, Oberkochen, Germany) equipped with a 100× objective lens (Plan-Apochromat; Carl Zeiss). For the four different fluorochromes, the following filter sets with different excitation/emission wavelengths (in nanometers) were used: for DAPI, 360/460 (set 46001; Chroma Technology, Brattleboro, Vt.); for FITC, 480/535 (set 41001; Chroma); for TRITC, 546/565 (set 41003; Chroma); and for Cy5, 620/700 (set 41008; Chroma). Digital images of the fluorochrome-labeled cells were captured at 0.1 s for DAPI, at 7 s for FITC, at 5 s for TRITC, and at 10 s for Cy5. The images were then analyzed with the IP Lab spectrum software (version 3.1.2; Scanalytics, Fairfax, Va.), using a Macintosh G3 computer. To obtain quantitative reliability in the microbial population analysis, cell numbers were determined by counting the microbial cells in more than 30 microscopic fields per sample, and each sample contained at least 5,000 DAPI-stainable cells.
Based on the fluorescence intensities in the images captured, both cell size and morphology were analyzed. As an indicator of cell morphology, we used a cell elongation index, which was defined as the ratio of the major axis length of a cell to the minor axis length. In this study, a cellular particle with an index close to 1.0 was considered a coccoid cell, while a cell with an index greater than 1.5 was considered a rod or dividing cell.
Nucleotide sequence accession numbers.
The accession numbers of the sequences which we determined in this study are AB112446 to AB112450 for the SUP01 group, AB112451 to AB112463 for the SUP05 group, AB112464 for SUP24-24, and AB112465 for SUP27-27.
RESULTS AND DISCUSSION
Phylogenic positions and possible functions of microbes in the plume.
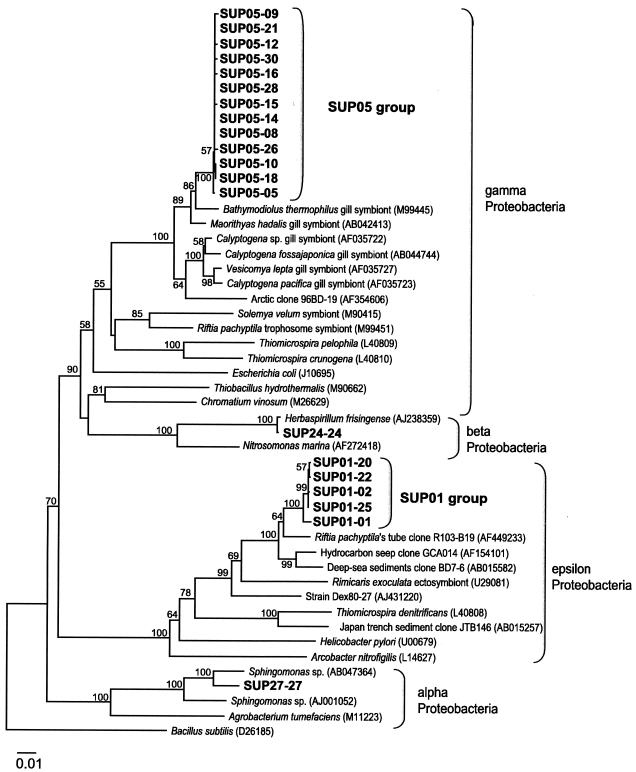
The 20 environmental clones prepared from the DNA sample collected at a depth of 1,200 m inside the caldera were divided into four phylogenetic groups of Proteobacteria (Fig. 2). The largest group (designated the SUP05 group; 13 clones) belonged to the gamma subclass of the Proteobacteria and was most closely related to an environmental clone (sequence accession no. AF469222; not shown in Fig. 2, since the sequence length [833 bp] was too short to obtain a reliable tree topology) that was obtained from a diffuse flow vent after a volcanic eruption at Juan de Fuca Ridge (19). Although only partial sequences were available for comparison, the levels of 16S rRNA gene similarity between members of the SUP05 group and the accession number AF469222 sequence were about 98%. The levels of similarity were about 97% for gill symbionts of bivalves, including Bathymodiolus thermophilus (accession no. M99445) and Maorithyas hadalis (accession no. AB042413), collected from deep-sea hydrothermal vent regions. The second major group (designated the SUP01 group; five clones) was located in the same clade as the environmental clones associated with deep-sea sediment (accession no. AB015582) (24) and with a tubeworm, Riftia pachyptila, from the East Pacific Rise at 9°N (accession no. AF4492330) (25) belonging to the epsilon subclass of the Proteobacteria; the levels of similarity were 96 to 98%. One of the other two groups was assigned to the beta subclass of the Proteobacteria, which is closely related to a member of the genus Herbaspirillum (accession no. AJ238357; a nitrogen fixer in C4 plants). The last group belonged to the alpha subclass of the Proteobacteria, which is closely related to Sphingomonas sp. (accession no. AB047364; a microbe from the oil degradation process).
FIG. 2.
Phylogenetic positions of microbial 16S rRNA gene clones obtained from the Suiyo Seamount hydrothermal plume at a depth of 1,200 m (i.e., SUP clones). In the SUP01 and SUP05 clone groups, the levels of sequence similarity were >99.7% for 1,467 and 1,446 bp, respectively. Bootstrap confidence values are expressed as percentages of 1,000 replications; the values at the nodes are the values that were greater than 50%. Bacillus subtilis was used as an outgroup. Scale bar = 0.01 nucleotide substitution per sequence position.
The SUP05 group belonging to the cluster of symbiotic microbes in the gamma subclass of the Proteobacteria was thought to be thioautotrophic, judging by the host habitats (10, 11, 13), and sulfur oxidizing, judging by the metabolic characteristics of the phylogenetically closest genus, Thiomicrospira, among cultivated microbes (3, 47). The SUP01 group was determined to be closely related to ectosymbiotic microbes in hydrothermal vent fields, including microbes associated with the hosts R. pachyptila (25) and Rimicaris exoculata (36), and it belonged to the epsilon subclass of the Proteobacteria (group F) (6). Culturable microbes belonging to the epsilon subclass of the Proteobacteria can be characterized as microaerophilic and sulfur metabolizing (43). In addition, all isolates in group F of the epsilon subclass of the Proteobacteria, as well as the isolates from a vent-related polychaete, Alvinella ponpesina, are known to utilize sulfur compounds (5, 38).
Hydrothermal fluids from the seafloor inside the Suiyo Seamount caldera are known to contain high concentrations of hydrogen sulfide (ca. 1 to 2 mM), while the water column above the seafloor is aerobic (1.5 to 2.0 ml of oxygen per liter). Therefore, we speculated that both the SUP01 and SUP05 groups of Bacteria are able to oxidize reduced sulfur compounds, such as hydrogen sulfide, elemental sulfur, and thiosulfate, and use them as energy sources. Actually, in an experiment in which a thiosulfate-containing medium was used, microbes closely related to the SUP01 group were enriched from seawater obtained from inside the caldera (K. Mori, personal communication). Although further isolation studies are needed, direct analysis of possible functional genes, such as soxB (35), may also be useful for determining their metabolic functions.
Total and domain-specific cell counts.
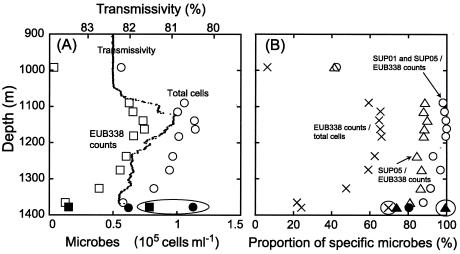
The vertical profile of total numbers of microbial cells, as determined by DAPI staining, almost coincided with the vertical profile of transmissivity (Fig. 3A). The number of cells increased from about 6.0 ×104 cells ml−1 near the seafloor (depth, ca. 1,366 to 1,380 m) to 9.7 ×104 cells ml−1 at a depth of 1,238 m. In an apparent plume layer that corresponded to anomalies in transmissivity, at a depth of ca. 1,050 to 1,200 m, the total number of cells was always high, 1.0 ×105 to 1.1 ×105 cells ml−1. In ambient seawater collected at a depth of 992 m, just above the top of the outer rim of the Suiyo Seamount caldera, the total number of cells was 5.6 × 104 cells ml−1. The highest level of cells, ca. 1.1 ×105 cells ml−1, was also obtained for a low-temperature vent fluid emitted from a bivalve-colonized mound located at almost the center of the caldera seafloor. Since the Suiyo Seamount has a horseshoe-shaped caldera enclosed on all sides (Fig. 1), the stagnant water conditions inside the caldera are partially responsible for the larger microbial populations found there.
FIG. 3.
(A) Vertical profiles of cell numbers and transmissivity. Circles, total cell counts obtained with DAPI; squares, EUB338 probe-hybridized cell counts; triangles, SUP05-187 probe-hybridized cell counts; open symbols, KR-01-15 cruise; solid symbols, NT-01-09 cruise. (B) Vertical profiles of relative abundance of specific microbial cells. Circles, SUP05-187 and SUP01-63 counts in EUB338 counts; multiplication signs, EUB338 counts in DAPI counts; triangles, SUP05-187 counts in EUB338 counts; open symbols, KR-01-15 cruise; solid symbols, NT-01-09 cruise; symbols in circles, samples from low-temperature hydrothermal fluid emitted from a mound heavily colonized with bivalves.
The concentrations of cells that hybridized with EUB338 (i.e., total Bacteria cells) were estimated to be 1.3 × 104 to 1.5 × 104 cells ml−1 near the seafloor, which corresponded to 21.8 to 24.3% of the total microbial cell counts. The concentration increased as the depth decreased, from 3.9 × 104 cells ml−1 (47.6% of the total cells) at 1,327 m to 6.0 × 104 cells ml−1 (62.4% of the total cells) at 1,238 m. In the apparent plume layer, the cell concentration was almost constant, ranging from 6.3 × 104 to 7.5 × 104 cells ml−1 (59.2 to 65.9% of the total cells). Only 6.2% of the total microbial cells, 3.5 × 103 cells ml−1, hybridized with EUB338 in ambient seawater obtained above the top of the outer rim of the caldera. In the low-temperature fluid from the bivalve colony, 69.7% of the total cells, 7.8 × 104 cells ml−1, were determined to be Bacteria cells. Few DAPI-stained cells were detected with the NON338 and ARCH915 probes.
The levels of microbial cells that were detected with DAPI but were not detected with the EUB338 and ARCH915 probes were estimated to be 3.5 × 104 to 4.7 × 104 cells ml−1 throughout the water column in the caldera. This range was very similar to the total concentration determined with DAPI in the ambient seawater above the top of the outer rim, which was 5.3 × 104 cells ml−1. Since the proportion of cells was relatively small and the cells were dim as determined by the DAPI method, the values were attributed to general deep-sea microbes in the seawater that flowed from outside the caldera. Therefore, it is possible that some of the organisms belonged to the Archaea, members of which are known to be present in the general deep-sea environment (21), but they probably belonged to some groups that are not involved with hydrothermal activities. The cells that were detected with EUB338 accounted for the excess cells in the hydrothermal plume water compared to the cells in the ambient seawater. These Bacteria cells accounted for 59 to 66% of the total cells in the hydrothermal plume. Without any treatment to enhance the fluorescence signals in the cells, including 2-hydroxy-3-naphthoic acid-2′-phenylanilide phosphate-Fast Red TR FISH (49), in situ PCR-FISH (39), and polynucleotide FISH (9), this range of values nearly corresponded to that obtained for near-shore surface seawater (12, 17, 34).
Group-specific cell counts.
When the Probe Check software available from the Ribosomal Database Project was used, only three environmental clones were selected with one mismatch compared with the DNA database, one (accession no. AF469222) for the SUP05-187 probe and two (accession no. AF468750 and AF468752) for the SUP01-63 probe. The former clone was located in the same phylogenetic clade as the SUP05 group belonging to the gamma subclass of the Proteobacteria, and the latter clones were located in the same phylogenetic clade as the SUP01 group belonging to the epsilon subclass of the Proteobacteria. Since no other clone or isolate with fewer than two mismatches compared with the database was selected, it appears that the SUP05-187 and SUP01-63 probes are highly specific for the SUP05 and SUP01 groups, respectively, and possibly for their sister groups, as has been found with samples from the Juan de Fuca Ridge (accession no. AF469222) (19).
The microbes that hybridized with these probes were predominant (>80% of the Bacteria cells that hybridized with EUB338) inside the caldera, especially in the apparent plume layer (98 to 100%) (Fig. 3B). The concentrations of cells that hybridized with SUP05-187 (i.e., SUP05 cells) ranged from 1.0 × 104 cells ml−1 (18% of the total cells) near the seafloor to 6.6 × 104 cells ml−1 (58% of the total cells) at a depth of 1,139 m in the plume. The vertical profile of the SUP05 cells was very similar to that of the Bacteria cells (Fig. 3A), and these cells accounted for 88 to 90% of the total Bacteria cells in the plume layer (Fig. 3B). Cells that hybridized with SUP01-63 (i.e., SUP01 cells) accounted for 5 to 8% of the total cells in the plume layer but only 1.5% of the total cells near the seafloor. In the low-temperature fluid emitted from the bivalve colony, almost all the Bacteria cells (99%) hybridized with the SUP05-187 probe (Fig. 3B), but only a limited portion (<1%) did hybridized with SUP01-63. These findings show that a portion of the SUP05 cells detected in the plume layer may have originated from the surface seafloor environment and circulated in the water column inside the caldera. In seawater and plume water above the seafloor, SUP01 cells were also estimated to be widespread and to account for 5 to 11.5% of the total cells. Therefore, we assume that there is somehow steady water circulation inside the caldera. Actually, during dive surveys on the NT-01-09 cruise, we frequently observed a very strong water current (ca. 0.5 knot) just above the seafloor. This current may have been caused by the inflow of seawater from outside the caldera.
Few cells in the plume water samples collected at a depth of 1,186 m hybridized with the ALF19, GAM42a, and CF319 probes, which have been widely used for identifying microbes in the general marine environment (12). Cells that hybridized with the GAM42a probe (i.e., members of the gamma subclass of the Proteobacteria) were observed, but the level was below the detection limit (<1% of the total cells). This showed that SUP05 cells could not be detected with this probe even though they phylogenetically belong to the gamma subclass of the Proteobacteria. The MOALF142 and MOGAM197 probes were also used with the 1,186-m water sample, but in both cases the number of hybridized cells was below the detection limit.
Since microbes from deep-sea hydrothermal plumes are usually difficult to visualize in FISH analysis compared to microbes from vent fluids, at least in samples from the southern East Pacific Rise (data not shown), it is notable that the microbial community in the Suiyo Seamount plume was easily characterized by FISH and that only two phylogenetic types of Bacteria were found to be predominant (>98% of the Bacteria cells) in the plume layer. These results are attributable not only to the sizes of the populations but also to the relatively high intercellular contents of the target 16S rRNA molecules in the plume microbes. Therefore, as it is sustained by the reduced sulfur compounds delivered from the seafloor, the Suiyo Seamount caldera seems to act as a natural continuous incubator for the SUP05 and SUP01 microbes. Actually, we always observed significant anomalies in turbidity at depths ranging from 1,100 to 1,250 m using a nephelometer in 18 dives of the submersible Shinkai 2000 during two cruises in October 2001 and September 2002 (data not shown). In three samples collected at a depth of 1,200 m during these dives, moreover, we detected 1.0 × 105 to 1.5 × 105 cells ml−1 and found that more than 50% of the cells were attributable to the SUP05 group. Therefore, we assume that these microbes are indigenous to and almost always predominant in the hydrothermal plume inside the caldera. To date, such remarkable predominance of a limited phylogenetic group of microbes has been reported only for sulfur-rich particle samples collected from hydrothermal fluids in the East Pacific Rise; in the previous study, dot blot hybridization revealed that 51.4% of microbial 16S rRNA was attributable to a group of R. exoculata ectosymbionts and their relatives (36).
Cell size and morphology.
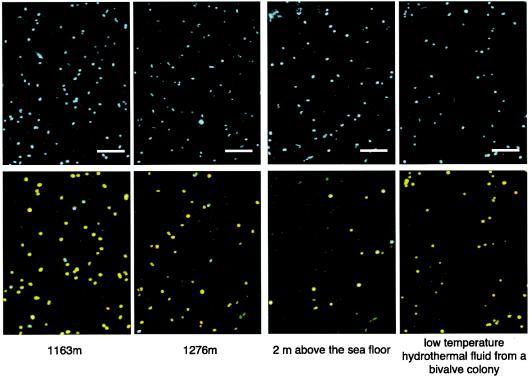
Fluorescence microscopic images of microbial cells stained with DAPI and various oligonucleotide probes are shown in Fig. 4. A significant portion of the DAPI-stained cells hybridized with both the EUB338 and SUP05-187 probes, and another portion hybridized with both the EUB338 and SUP01-63 probes (see Fig. 3 for exact numbers of cells). Most of the cells that hybridized with probes were quite bright under the fluorescence microscope, indicating that the intracellular rRNA contents were relatively high. However, the fluorescence intensity, size, and morphology of the cells that hybridized with SUP05-187 seemed to change slightly with water depth (Fig. 4).
FIG. 4.
Digital images of fluorescently labeled microbial cells. The samples are from different depths (1,163 m, 1,276 m, and 2 m above the seafloor) in the water column inside the Suiyo Seamount caldera and from low-temperature vent fluid from a bivalve colony. Total cells were stained with DAPI (upper panels), and specific cells were detected by FISH analysis (lower panels, which are synthesized digital images) in the same fields for the same samples. The colors indicate microbial cells that hybridized with both the EUB338 and SUP05-187 probes (yellow), with both the EUB338 and SUP01-63 probes (cyan), and with only the EUB338 probe (green). Bars = 10 μm.
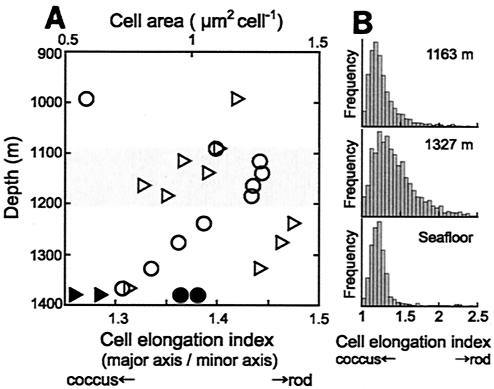
To clarify differences in cell size and morphology among the samples, further digital imaging analysis was carried out by using fluorescence microscope images of cells that hybridized with SUP05-187. Cells that had an area of about 1 μm2 were detected in the water and in fluid samples obtained from just above the seafloor, while the microbes were smaller in a higher water layer about 15 m above the seafloor (i.e., 1,366 m) (Fig. 5). As the water depth decreased, the average cell size increased again, from 0.7 μm2 at 1,366 m to 1.1 μm2 at 1,238 m, and the maximum average cell size was 1.2 to 1.3 μm2 in the apparent plume layer at a depth of 1,050 to 1,200 m. The cell size decreased again, to 0.6 μm2, in seawater above the plume layer, at a depth of 992 m.
FIG. 5.
(A) Vertical profiles of morphological features of SUP05 cells in the water column inside the caldera. Cell size (area) (circles) and the cell elongation index (ratio of the major axis length to the minor axis length of a cell) (triangle) were determined for samples from the KR-01-15 cruise (open symbols) and the NT-01-09 cruise (solid symbols). Each value is the average for more than 200 cells measured by digital imaging analysis. (B) Histograms of the cell elongation indices for SUP05 cells from a depth of 1,163 m, a depth of 1,327m, and the seafloor.
The mean cell elongation index was 1.27 near the seafloor and 1.31 at 1,366 m. The index values were almost constant, ranging from 1.44 to 1.48, in the layer at a depth of 1,327 to 1,238 m. In the layer at a depth of 1,186 to 1,163 m, which corresponded to the bottom of the apparent plume layer, the elongation index decreased to 1.32 to 1.39. In ambient seawater above the somma, at a depth of 992 m, the index increased again, to 1.42. In histograms of the elongation index, most of the SUP05 cells detected near the seafloor, as well as most of those in the apparent plume layer, gave a sharp peak (Fig. 5B), indicating that these cells were mostly coccoid. A portion of the remaining SUP05 cells in the plume water from a depth of 1,163 m, as well as the remaining SUP05 cells near the seafloor, was determined to be short rods or dividing cells. In contrast, the sample from 1,327 m produced a broad peak in the histogram. The frequency of dividing cells (18) was almost constant through the water column inside the caldera (8 to 11% of the SUP05 cells). These results indicate that most of the SUP05 cells in the bottom layer of the hydrothermal plume, as well as most of the SUP05 cells near the seafloor, were coccoid, while rod-shaped cells were abundant in the layer at a depth of 1,327 to 1,238 m inside the caldera.
The elongation index for the SUP05 cells showed that there were obvious changes between the depths of 1,186 and 1,238 m, corresponding to the changes in cell size. On the basis of these morphological features, we divided the water column inside the caldera into three layers: 1,050 to 1,200 m, 1,200 to 1,360 m, and near-bottom (ca. 1,360 to 1,380 m). The layer at a depth of 1,050 to 1,200 m corresponded to the apparent hydrothermal plume layer, which was characterized by relatively high concentrations of particulate material (low transmissivity) and of reduced chemicals involved in hydrothermal activities. Since this layer was also characterized by elevated numbers of large coccoid SUP05 cells with strong fluorescence intensities, we assumed that these cells had relatively high levels of metabolic activity.
The SUP05 cells in the near-bottom layer were smaller than those in the upper layers, and there were fewer of them. Since microbial cells are known to become smaller and spherical under starvation conditions (33), the levels of activity of the SUP05 cells seemed lowest near the seafloor inside the caldera. Interestingly, however, one of the largest populations of SUP05 cells in the water column inside the caldera was found in the vent fluid on the seafloor. Since an intermittent strong water current was observed near the seafloor, we assumed that this inflow diluted reduced chemicals and microbial cells from hydrothermal venting and also reduced the levels of activity.
In the layer at a depth of 1,200 to 1,360 m, the number and size of SUP05 cells increased as the water depth decreased. Considering the cell morphology histogram and the relatively constant frequencies of dividing cells throughout the water column, we supposed that SUP05 cells obviously elongated in this layer; that is, the SUP05 cells had higher ratios of the major axis to the minor axis (1.5 to 1.9) than the cells in the other layers (Fig. 5). It is known that microbial cell elongation is triggered by changes in environmental conditions, such as starvation (14) and high pressure (50). Although we have only limited data to explain why elongation occurred in the middle layer, the apparent elongation of SUP05 cells suggests that the environmental conditions are not stable in this layer.
In conclusion, up to 66% of the total microbial cells in the hydrothermal plume layer of the Suiyo Seamount were SUP01 and SUP05 cells. The phylogenetic analysis suggested that these phylotypes of bacteria utilize reduced sulfur compounds for growth. As determined by the FISH analysis, these cells were larger and brighter in the apparent hydrothermal plume layer, at a depth of ca. 1,050 to 1,200 m, than they were in the other layers. The caldera of the Suiyo Seamount has a structure that protects the water column inside the caldera. This may allow these microbes to grow or maintain their populations and activities inside the caldera at much higher levels than the levels that occur in ridge-type hydrothermal plumes. Our findings indicate that the Suiyo Seamount caldera has acted as a suitable natural incubator for these sulfur oxidizer-like microbes, which should be very important primary producers in the Suiyo Seamount hydrothermal ecosystem.
Acknowledgments
This research was funded by the Ministry of Education, Culture, Science and Technology (MEXT), Japan, through a special coordination fund (Archaean Park Project: International Research Project on Interaction between Sub-Vent Biosphere and Geo-Environments).
We thank the crews of RV Natsushima and RV Kairei (JAMSTEC) and the Shinkai 2000 operation team for their support in sample collection.
REFERENCES
- 1.Amann, R. I., L. Krumholz, and D. Stahl. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I., W. Ludwig, and K.-H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkhoff, T., S. M. Severt, J. Kuever, and G. Muyzer. 1999. Distribution and diversity of sulfur-oxidizing Thiomicrospira spp. at a shallow-water hydrothermal vent in the Aegean Sea (Milos, Greece). Appl. Environ. Microbiol. 65:3843-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosius, J., T. J. Dull, D. D. Sleeter, and H. F. Noller. 1981. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J. Mol. Biol. 148:107-127. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, B. J., C. Jeanthon, J. E. Kostka, G. W. Luther III, and S. C. Cary. 2001. Growth and phylogenetic properties of novel bacteria belonging to the epsilon subdivision of the Proteobacteria enriched from Alvinella pompejana and deep-sea hydrothermal vents. Appl. Environ. Microbiol. 67:4566-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corre, E., A.-L. Reysenbach, and D. Prieur. 2001. Epsilon-proteobacterial diversity from a deep-sea hydrothermal vent on the Mid-Atlantic Ridge. FEMS Microbiol. Lett. 205:329-335. [DOI] [PubMed] [Google Scholar]
- 7.Cowen, J. P., G. J. Massoth, and E. T. Baker. 1986. Bacterial scavenging of Mn and Fe in a mid-field to far-field hydrothermal particle plume. Nature 322:169-171. [Google Scholar]
- 8.DeAngelis, M. A., M. D. Lilley, E. J. Olsen, and J. A. Baross. 1993. Methane oxidation in deep-sea hydrothermal plumes of the Endeavour Segment of the Juan de Fuca Ridge. Deep-Sea Res. I 40:1169-1186. [Google Scholar]
- 9.DeLong, E. F., L. T. Taylor, T. L. Marsh, and C. M. Preston. 1999. Visualization and enumeration of marine planktonic archaea and bacteria by using polyribonucleotide probes and fluorescent in situ hybridization. Appl. Environ. Microbiol. 65:5554-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Distel, D. L., D. J. Lane, G. J. Olsen, S. J. Giovannoni, B. Pace, N. R. Pace, D. A. Stahl, and H. Felbeck. 1988. Sulfur-oxidizing bacterial endosymbionts: analysis of phylogeny and specificity by 16S rRNA sequences. J. Bacteriol. 170:2506-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Distel, D. L., H. K.-W. Lee, and C. M. Cavanaugh. 1995. Intracellular coexistence of methano- and thioautotrophic bacteria in a hydrothermal vent mussel. Proc. Natl. Acad. Sci. USA 92:9598-9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eilers, H., J. Pernthaler, F. O. Glöckner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujiwara, Y., C. Kato, N. Masui, K. Fujikura, and S. Kojima. 2001. Dual symbiosis in the cold-seep thyasirid clam Maorithyas hadalis from the hadal zone in the Japan Trench, western Pacific. Mar. Ecol. Prog. Ser. 214:151-159. [Google Scholar]
- 14.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast Saccharomyces cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 15.Giovannoni, S. J., E. F. DeLong, G. J. Olsen, and N. R. Pace. 1988. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 170:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasby, G. P., K. Iizasa, M. Yuasa, and A. Usui. 2000. Submarine hydrothermal mineralization on the Izu-Bonin Arc, south of Japan: an overview. Mar. Geores. Geotechnol. 18:141-176. [Google Scholar]
- 17.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagström, A., U. Larsson, P. Horstedt, and S. Normark. 1979. Frequency of dividing cells, a new approach to the determination of bacterial growth rates in aquatic environments. Appl. Environ. Microbiol. 37:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huber, J. A., D. A. Butterfield, and J. A. Baross. 2003. Bacterial diversity in a subseafloor habitat following a deep-sea volcanic eruption. FEMS Microbiol. Ecol. 43:393-409. [DOI] [PubMed] [Google Scholar]
- 20.Jannasch, H. W., and M. J. Mottl. 1985. Geomicrobiology of deep-sea hydrothermal vents. Science 229:717-725. [DOI] [PubMed] [Google Scholar]
- 21.Karner, M. B., E. F. DeLong, and D. M. Karl. 2001. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507-510. [DOI] [PubMed] [Google Scholar]
- 22.Kogure, K., U. Shimidu, and N. Taga. 1979. A tentative direct microscopic method for counting living marine bacteria. Can. J. Microbiol. 25:415-420. [DOI] [PubMed] [Google Scholar]
- 23.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 24.Li, L., C. Kato, and K. Horikoshi. 1999. Bacterial diversity in deep-sea sediments from different depths. Biodivers. Conserv. 8:659-677. [Google Scholar]
- 25.López-Garcia, P., F. Gaill, and D. Moreira. 2002. Wide bacterial diversity associated with tubes of the vent worm Riftia pachyptila. Environ. Microbiol. 4:204-215. [DOI] [PubMed] [Google Scholar]
- 26.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 27.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K.-H. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama, A., T. Urabe, J. Ishibashi, R. A. Feely, and E. T. Baker. 1998. Global hydrothermal primary production rate estimated from the southern East Pacific Rise. Cah. Biol. Mar. 39:249-252. [Google Scholar]
- 29.Maruyama, A., D. Honda, H. Yamamoto, K. Kitamura, and T. Higashihara. 2000. Phylogenetic analysis of psychrophilic bacteria isolated from the Japan Trench, including a description of the deep-sea species Psychrobacter pacificensis sp. nov. Int. J. Syst. E vol. Microbiol. 50:835-846. [DOI] [PubMed] [Google Scholar]
- 30.Maruyama, A., and M. Sunamura. 2000. Simultaneous direct counting of total and specific microbial cells in seawater, using a deep-sea microbe as a target. Appl. Environ. Microbiol. 66:2211-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moré, M. I., J. B. Herrick, M. C. Silva, W. C. Ghiorse, and E. L. Madsen. 1994. Quantitative cell lysis of indigenous microorganisms and rapid extraction of microbial DNA from sediment. Appl. Environ. Microbiol. 60:1572-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naganuma, T., A. Otsuki, and H. Seki. 1989. Abundance and growth-rate of bacterioplankton community in hydrothermal vent plumes of the North Fiji Basin. Deep-Sea Res. Part A Oceanogr. Res. Pap. 36:1379-1390. [Google Scholar]
- 33.Oliver, J. D., L. Nilsson, and S. Kjelleberg. 1991. Formation of nonculturable Vibrio vulnificus cells and its relationship to the starvation state. Appl. Environ. Microbiol. 57:2640-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouverney, C. C., and J. A. Fuhrman. 1997. Increase in fluorescence intensity of 16S rRNA in situ hybridization in natural samples treated with chloramphenicol. Appl. Environ. Microbiol. 63:2735-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri, R., L. Podgorsek, and J. F. Imhoff. 2001. Phylogeny and distribution of the soxB gene among thiosulfate-oxidizing bacteria. FEMS Microbiol. Lett. 197:171-178. [DOI] [PubMed] [Google Scholar]
- 36.Polz, M. F., and C. M. Cavanaugh. 1995. Dominance of one bacterial phylotype at a Mid-Atlantic Ridge hydrothermal vent site. Proc. Natl. Acad. Sci. USA 92:7232-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt, T. M., E. F. DeLong, and N. R. Pace. 1991. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takai, K., F. Inagaki, S. Nakagawa, H. Hirayama, T. Nunoura, Y. Sako, K. H. Nealson, and K. Horikoshi. 2003. Isolation and phylogenetic diversity of members of previously uncultivated epsilon-Proteobacteria in deep-sea hydrothermal fluids. FEMS Microbiol. Lett. 218:167-174. [DOI] [PubMed] [Google Scholar]
- 39.Tani, K., K. Kurosawa, and M. Nasu. 1998. Development of a direct in situ PCR method for detection of specific bacteria in natural environments. Appl. Environ. Microbiol. 64:1536-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomson, R. E., B. J. Burd, A. G. Dolling, R. L. Gordon, and G. S. Jamieson. 1992. The deep scattering layer associated with the Endeavour Ridge hydrothermal plume. Deep-Sea Res. Part A Oceanogr. Res. Pap. 39:55-73. [Google Scholar]
- 41.Tsien, H. C., B. J. Bratina, K. Tsuji, and R. S. Hanson. 1990. Use of oligodeoxynucleotide signature probes for identification of physiological groups of methylotrophic bacteria. Appl. Environ. Microbiol. 56:2858-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsunogai, U., J. Ishibashi, H. Wakita, T. Gamo, K. Watanabe, T. Kajimura, S. Kanayama, and H. Sakai. 1994. Peculiar features of Suiyo Seamount hydrothermal fluids, Izu-Bonin Arc: differences from subaerial volcanism. Earth Planet. Sci. Lett. 126:289-301. [Google Scholar]
- 43.Vandamme, P., E. Falsen, R. Rossau, B. Hoste, P. Segers, R. Tytgat, and J. DeLey. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 41:88-103. [DOI] [PubMed] [Google Scholar]
- 44.Wallner, G., R. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganism. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 45.Winn, C. D., D. M. Karl, and G. J. Massoth. 1986. Microorganisms in deep-sea hydrothermal plumes. Nature 320:744-746. [Google Scholar]
- 46.Winn, C. D., J. P. Cowen, and D. M. Karl. 1995. Microbes in deep-sea hydrothermal plumes, p. 255-273. In D. M. Karl (ed.), The microbiology of deep-sea hydrothermal vents. CRC Press, Inc., Boca Raton, Fla.
- 47.Wirsen, C. O., T. Brinkhoff, J. Kuever, G. Muyzer, S. Molyneaux, and H. W. Jannasch. 1998. Comparison of a new Thiomicrospira strain from the Mid-Atlantic Ridge with known hydrothermal vent isolates. Appl. Environ. Microbiol. 64:4057-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu, H. S., N. Roberts, F. L. Singleton, R. W. Attwell, D. J. Grimes, and R. R. Colwell. 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine-environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi, N., S. Inaoka, K. Tani, T. Kenzaka, and M. Nasu. 1996. Detection of specific bacterial cells with 2-hydroxy-3-naphthoic acid-2′-phenylanilide phosphate and Fast Red TR in situ hybridization. Appl. Environ. Microbiol. 62:275-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ZoBell, C. E., and C. H. Oppenheimer. 1950. Some effects of hydrostatic pressure on the multiplication and morphology of marine bacteria. J. Bacteriol. 60:771-781. [DOI] [PMC free article] [PubMed] [Google Scholar]