Abstract
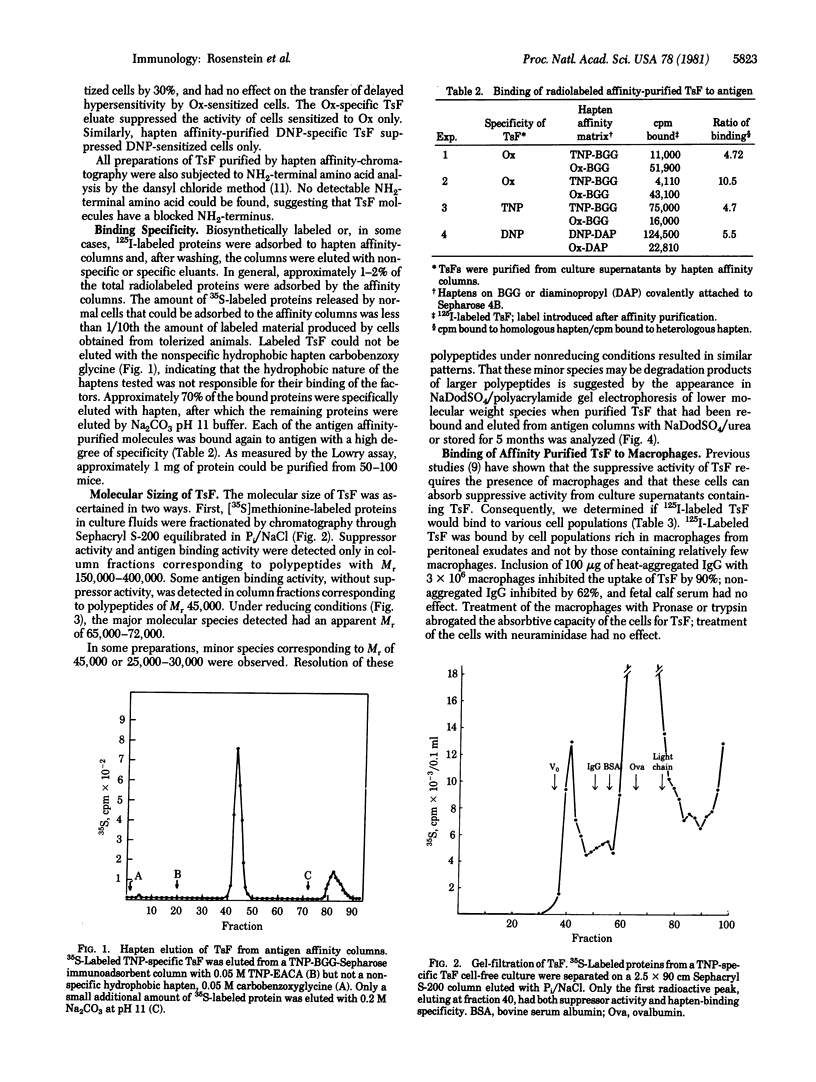
Antigen-specific factors associated with immunosuppressive activity, released by cultured T cells from mice tolerant to the haptens trinitrophenyl, dinitrophenyl and oxazolone, were purified by hapten affinity chromatography. Their binding specificity for antigens paralleled their immunoregulatory activity. Like some immunoglobulin molecules, these factors had blocked NH2 termini and could be bound to Fc-like receptors on macrophages. However, neither immunoglobulin constant region determinants (isotypes) nor antigens encoded by the major histocompatibility complex were detected on the suppressive factors. The purified factors occurred as 68,000-dalton proteins and non-covalently linked dimers. No associated immunoglobulin light chain molecules were detected. The factors showed a marked propensity toward degradation with major breakdown products of 45,000-50,000 and 25,000-30,000 daltons. These results suggest that these molecules are the T-cell products analogous to B-cell immunoglobulin (equivalent to heavy chains) and that they may be the antigen-specific components which act in conjunction with major histocompatibility-controlled gene products to perform antigen-specific suppression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Binz H., Wigzell H. Antigen-binding, idiotypic T-lymphocyte receptors. Contemp Top Immunobiol. 1977;7:113–177. doi: 10.1007/978-1-4684-3054-7_4. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Brown W. C. Isolation of membrane associated immunoglobulins from T lymphocytes by non-ionic detergents. Immunochemistry. 1976 Jul;13(7):571–579. doi: 10.1016/0019-2791(76)90168-3. [DOI] [PubMed] [Google Scholar]
- Cone R. E., Feldmann M., Marchalonis J. J., Nossal G. J. Cytophilic properties of surface immunoglobulin of thymus-derived lymphocytes. Immunology. 1974 Jan;26(1):49–60. [PMC free article] [PubMed] [Google Scholar]
- Eichmann K. Expression and function of idiotypes of lymphocytes. Adv Immunol. 1978;26:195–254. doi: 10.1016/s0065-2776(08)60231-x. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Murphy D. B., Cone R. E. Ia antigen turnover. II. The kinetics of biosynthesis and release of Ia alpha- and beta-chains by murine spleen cells in culture. J Immunol. 1980 Jul;125(1):406–410. [PubMed] [Google Scholar]
- Fresno M., McVay-Boudreau L., Nabel G., Cantor H. Antigen-specific T lymphocyte clones. II. Purification and biological characterization of an antigen-specific suppressive protein synthesized by cloned T cells. J Exp Med. 1981 May 1;153(5):1260–1274. doi: 10.1084/jem.153.5.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Eardley D. D., Durum S., Green D. R., Shen F. W., Yamauchi K., Cantor H., Murphy D. B. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981 Jun 1;153(6):1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Pierres A., Dorf M. E., Benacerraf B. The I-J subregion codes for determinats on suppressor factor(s) which limit the contact sensitivity response to picryl chloride. J Exp Med. 1977 Jul 1;146(1):293–296. doi: 10.1084/jem.146.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead J. W. Soluble factors in tolerance and contact sensitivity to 2,4-dinitrofluorobenzene in mice. I. Suppression of contact sensitivity by soluble suppressor factor released in vitro by lymph node cell populations containing specific suppressor cells. J Immunol. 1977 Jul;119(1):315–321. [PubMed] [Google Scholar]
- Ptak W., Rózycka D., Rewicka M. Induction of suppressor cells and cells producing antigen-specific suppressor factors by haptens bound to self carriers. Immunobiology. 1980 Jan;156(4-5):400–409. doi: 10.1016/S0171-2985(80)80073-8. [DOI] [PubMed] [Google Scholar]
- Ptak W., Zembala M., Gershon R. K. Intermediary role of macrophages in the passage of suppressor signals between T-cell subsets. J Exp Med. 1978 Aug 1;148(2):424–434. doi: 10.1084/jem.148.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S. The H-2 major histocompatibility complex and the I immune response region: genetic variation, function, and organization. Adv Immunol. 1975;20:125–195. doi: 10.1016/s0065-2776(08)60208-4. [DOI] [PubMed] [Google Scholar]
- Tada T., Okumura K. The role of antigen-specific T cell factors in the immune response. Adv Immunol. 1979;28:1–87. doi: 10.1016/s0065-2776(08)60799-3. [DOI] [PubMed] [Google Scholar]
- Taniguchi M. Two distinct molecules that compose an antigen-specific suppressor factor. Transplant Proc. 1980 Sep;12(3):423–426. [PubMed] [Google Scholar]
- Taussig M. J., Holliman A. Structure of an antigen-specific suppressor factor produced by a hybrid T-cell line. Nature. 1979 Jan 25;277(5694):308–310. doi: 10.1038/277308a0. [DOI] [PubMed] [Google Scholar]


