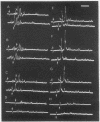
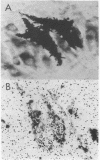
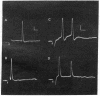
Abstract
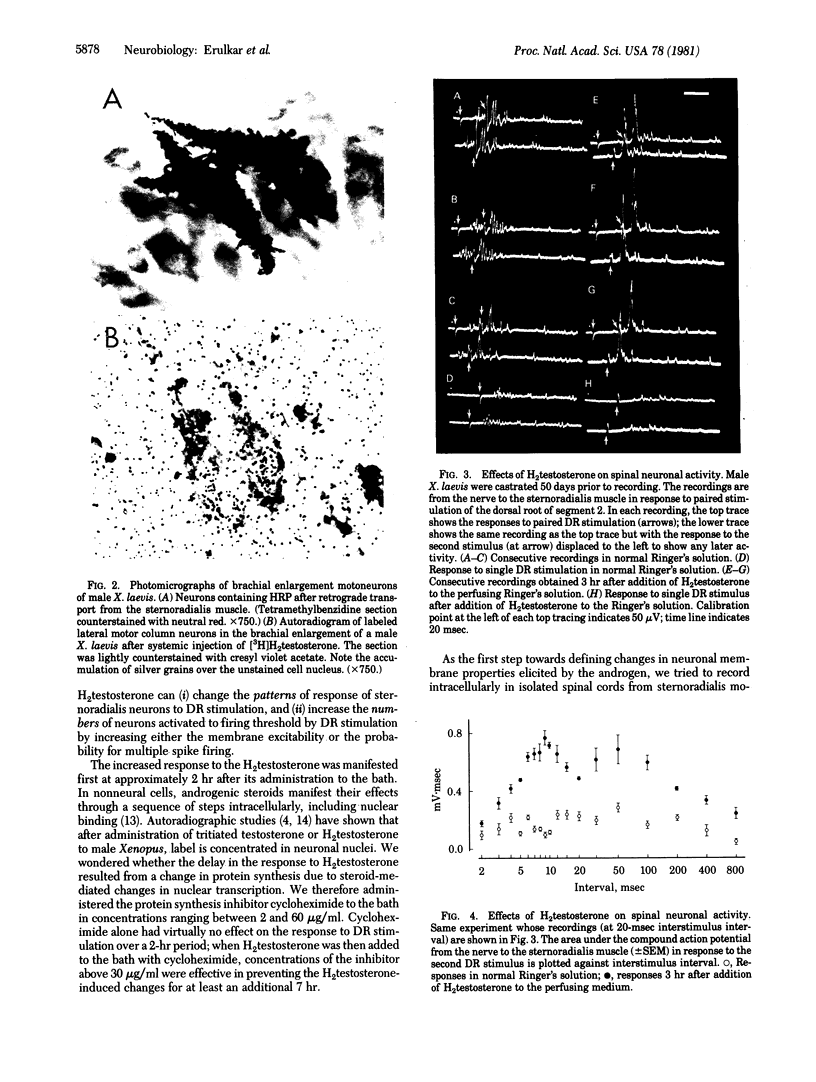
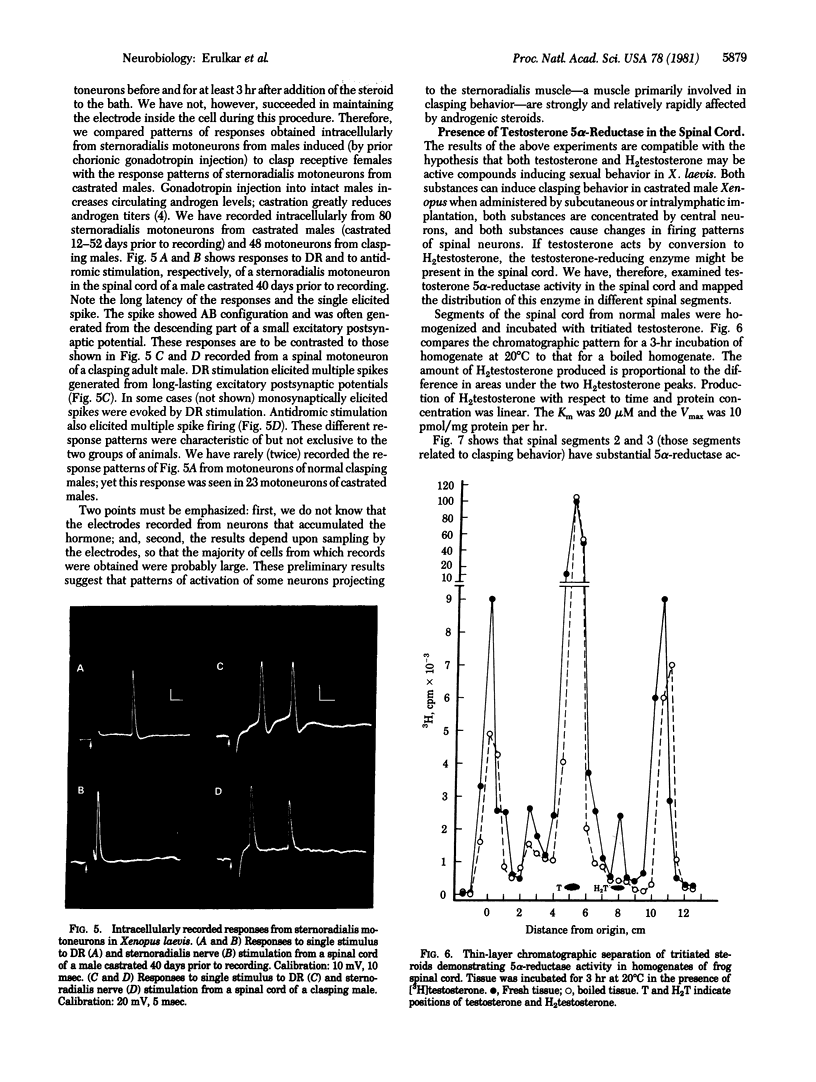
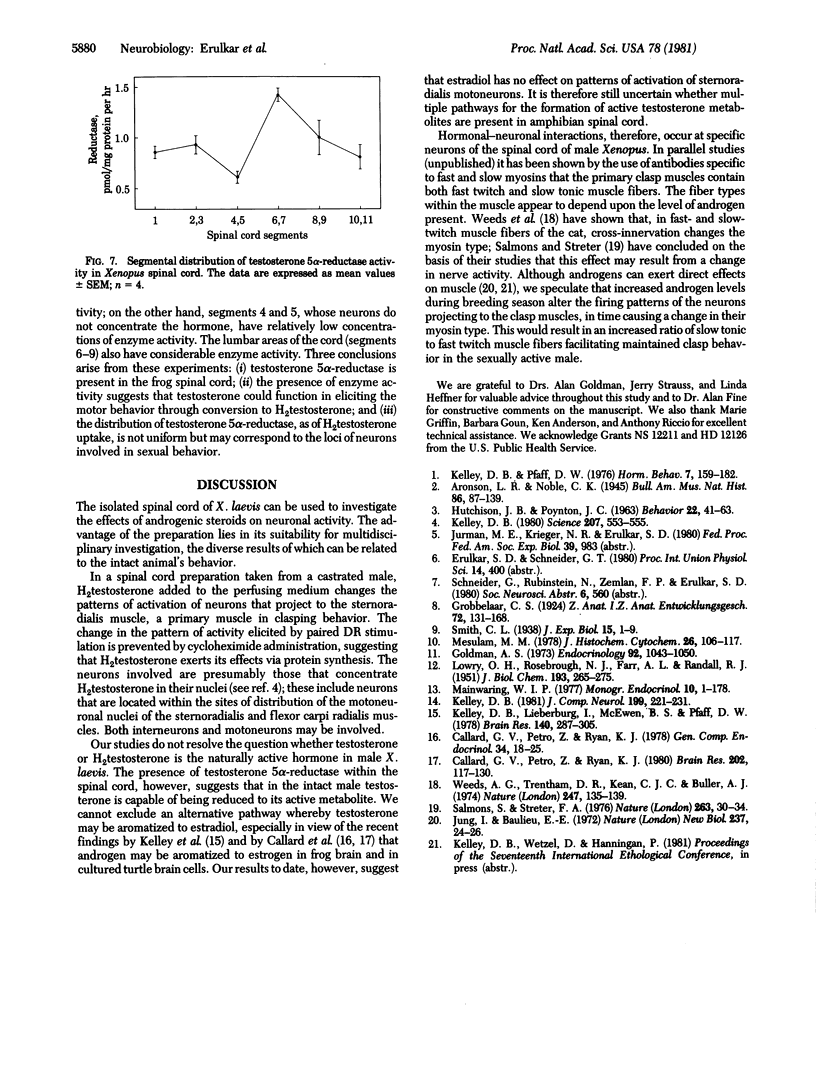
The neural control of the clasp reflex in male Xenopus laevis has been studied by using anatomical, electrophysiological, and biochemical techniques. Neurons in spinal segment 2 of castrated males accumulate label after injection of [3H]-dihydrotestosterone; these neurons are distributed within the rostral portions of the motoneuronal pools of the sternoradialis and flexor carpi radialis muscles. In vitro recordings from the nerve to the sternoradialis muscle in the isolated spinal cord preparation from castrated male Xenopus showed increased activation to paired dorsal root stimulation after addition of dihydrotestosterone to the bath. This increase could be prevented by prior administration of cycloheximide. The reducing enzyme testosterone 5 alpha-reductase is present and is selectively distributed in male Xenopus spinal cord. It is speculated that the androgens may alter patterns of neuronal activity leading to the "clasp" muscles and thereby influence myosin types within these muscles.
Full text
PDF
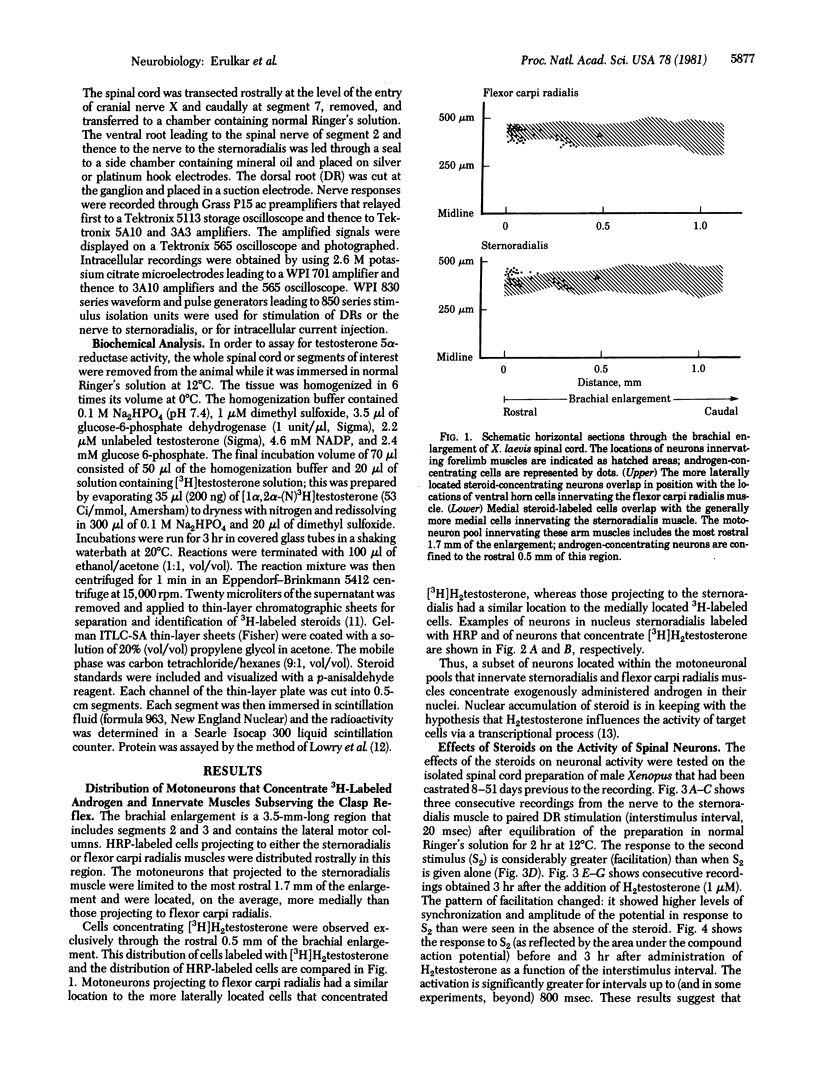
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Callard G. V., Petro Z., Ryan K. J. Androgen metabolism in the brain and non-neural tissues of the bullfrog Rana catesbeiana. Gen Comp Endocrinol. 1978 Jan;34(1):18–25. doi: 10.1016/0016-6480(78)90239-3. [DOI] [PubMed] [Google Scholar]
- Callard G. V., Petro Z., Ryan K. J. Aromatization of androgen to estrogen by cultured turtle brain cells. Brain Res. 1980 Nov 24;202(1):117–130. [PubMed] [Google Scholar]
- Goldman A. S. Rat fetal target organ delta-5, 3 beta-hydroxysteroid dehydrogenase: effect of cyanoketone and cyproterone acetate. Endocrinology. 1973 Apr;92(4):1043–1050. doi: 10.1210/endo-92-4-1043. [DOI] [PubMed] [Google Scholar]
- Jung I., Baulieu E. E. Testosterone cytosol "receptor" in the rat levator ani muscle. Nat New Biol. 1972 May 3;237(70):24–26. doi: 10.1038/newbio237024a0. [DOI] [PubMed] [Google Scholar]
- Kelley D. B. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980 Feb 1;207(4430):553–555. doi: 10.1126/science.7352269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. B., Lieberburg I., McEwen B. S., Pfaff D. W. Autoradiographic and biochemical studies of steroid hormone-concentrating cells in the brain of Rana pipiens. Brain Res. 1978 Jan 27;140(2):287–305. doi: 10.1016/0006-8993(78)90461-4. [DOI] [PubMed] [Google Scholar]
- Kelley D. B. Locations of androgen-concentrating cells in the brain of Xenopus laevis: autoradiography with 3H-dihydrotestosterone. J Comp Neurol. 1981 Jun 20;199(2):221–231. doi: 10.1002/cne.901990206. [DOI] [PubMed] [Google Scholar]
- Kelley D. B., Pfaff D. W. Hormone effects on male sex behavior in adult South African clawed frogs, Xenopus laevis. Horm Behav. 1976 Jun;7(2):159–182. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mainwaring W. I. The mechanism of action of androgens. Monogr Endocrinol. 1977;10:1–178. [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]