Abstract
A method of analysis is presented whereby the structure of multilocus associations among and within several populations can be partitioned into its components. The components are measured by their contributions to the variance in the number of heterozygous loci in two randomly chosen gametes. The singlelocus components are the average and the variation among populations in gene diversity and the variance among populations in allele frequency. The two-locus components include the mean and variance of disequilibria, the covariance of allele frequencies over populations, and various interactions. When applied to allozyme data from populations of wild (Hordeum spontaneum) and cultivated barley (H. vulgare), the analysis highlighted the repetitive pattern of the multilocus associations in the composite crosses whereas it emphasized the regionally localized and geographically variable pattern present in the natural populations of the wild species. The analysis is flexible and applicable to multilocus gametic data from any set of populations, without regard to the number of alleles per locus or the reproductive method of the organism.
Keywords: barley, enzyme polymorphisms, gametic disequilibrium, coadaptation, subdivided populations
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allard R. W., Babbel G. R., Clegg M. T., Kahler A. L. Evidence for coadaptation in Avena barbata. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3043–3048. doi: 10.1073/pnas.69.10.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
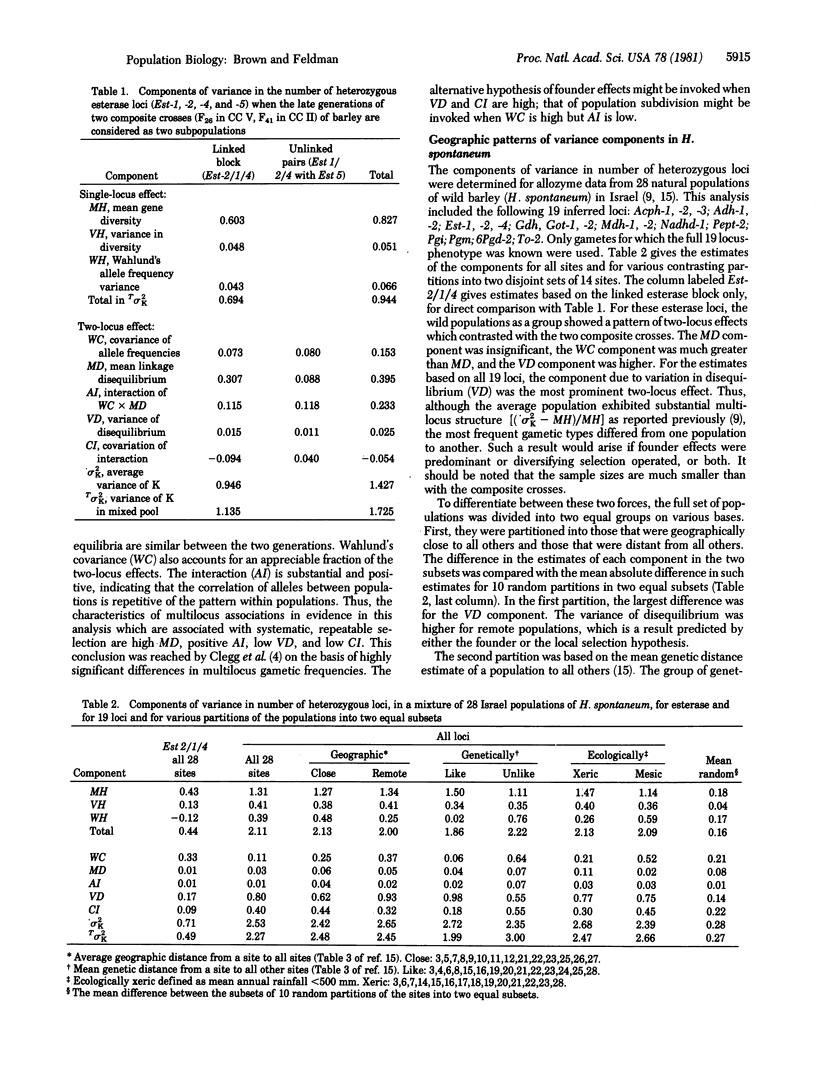
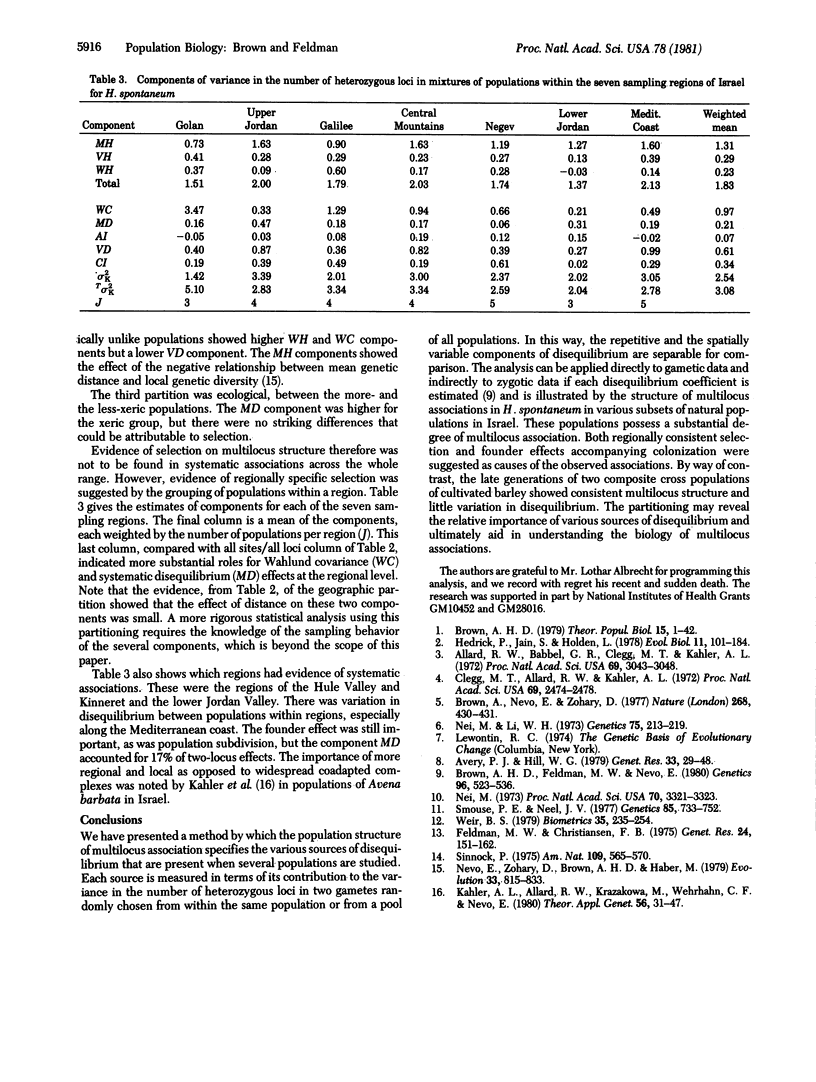
- Brown A. H., Feldman M. W., Nevo E. Multilocus Structure of Natural Populations of HORDEUM SPONTANEUM. Genetics. 1980 Oct;96(2):523–536. doi: 10.1093/genetics/96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg M. T., Allard R. W., Kahler A. L. Is the gene the unit of selection? Evidence from two experimental plant populations. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2474–2478. doi: 10.1073/pnas.69.9.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M. W., Christiansen F. B. The effect of population subdivision on two loci without selection. Genet Res. 1974 Oct;24(2):151–162. doi: 10.1017/s0016672300015184. [DOI] [PubMed] [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Linkage disequilibrium in subdivided populations. Genetics. 1973 Sep;75(1):213–219. doi: 10.1093/genetics/75.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smouse P. E., Neel J. V. Multivariate analysis of gametic disequilibrium in the Yanomama. Genetics. 1977 Apr;85(4):733–752. doi: 10.1093/genetics/85.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir B. S. Inferences about linkage disequilibrium. Biometrics. 1979 Mar;35(1):235–254. [PubMed] [Google Scholar]


