Abstract
The goal of this study was to determine the mechanism of lubiprostone activation of epithelial chloride transport. Lubiprostone is a bicyclic fatty acid approved for the treatment of constipation [1]. There is uncertainty, however, as to how lubiprostone increases epithelial chloride transport. Direct stimulation of ClC-2 and CFTR chloride channels as well as stimulation of these channels via the EP4 receptor has been described [2; 3; 4; 5]. To better define this mechanism, two-electrode voltage clamp was used to assay Xenopus oocytes expressing ClC-2, with or without co-expression of the EP4 receptor or β adrenergic receptor (βAR), for changes in conductance elicited by lubiprostone. Oocytes co-expressing CFTR and either βAR or the EP4 receptor were also studied. In oocytes co-expressing ClC-2 and βAR conductance was stimulated by hyperpolarization and acidic pH (pH=6), but there was no response to the β adrenergic agonist, isoproterenol. Oocytes expressing ClC-2 only or co-expressing ClC-2 and EP4 did not respond to the presence of 0.1, 1, or 10 µM lubiprostone in the superperfusate. Oocytes co-expressing CFTR and βAR did not respond to hyperpolarization, acidic pH, or 1µM lubiprostone. However, conductance was elevated by isoproterenol and inhibited by CFTRinh172. Co-expression of CFTR and EP4 resulted in lubiprostone-stimulated conductance, which was also sensitive to CFTRinh172. The EC50 for lubiprostone mediated CFTR activation was ~ 10 nM. These results demonstrate no direct action of lubiprostone on either ClC-2 or CFTR channels expressed in oocytes. However, the results confirm that CFTR can be activated by lubiprostone via the EP4 receptor in oocytes.
Keywords: chloride channel, lubiprostone, CFTR, ClC-2, prostaglandin
INTRODUCTION
Apical anion channels are a key element in salt and water secretion by epithelia [6]. ClC-2 is a voltage-gated, pH sensitive epithelial chloride channel implicated in the regulation of cell volume [7; 8]. ClC-2 is of particular interest as a CFTR bypass channel in Cystic Fibrosis because of reports of localization in the apical membrane of respiratory epithelia [9; 10; 11]. Lubiprostone, a bicyclic fatty acid derived from prostaglandin E1, has been reported to stimulate apical epithelial ion transport via activation of the ClC-2 chloride channel [2; 3], and the compound has been approved by the FDA for the treatment of constipation in humans [1]. However, some studies have reported that lubiprostone effects epithelial ion transport via activation of the CFTR channels rather than ClC-2 [4; 5]. The first report of ClC-2 activation by lubiprostone employed ClC-2 heterologously expressed in HEK293 cells which otherwise exhibit minimal, native chloride conductance. Using whole cell patch clamp techniques, it was found that HEK293 cells expressing ClC-2 exhibited increased current in the presence of lubiprostone compared to control [2]. The activation of ClC-2 was found not to be pKA dependent. No direct or indirect action of lubiprostone on the conductance of HEK293 cells expressing CFTR was detected [2]. In contrast, a study of amphibian A6 renal epithelial cells via cell-attached patch clamp reported that lubiprostone activated two separate distinct conductances, one with properties consistent with ClC-2 and a second consistent with CFTR [3]. These authors reported no increase in cytosolic cyclic AMP in response to lubiprostone. Although both studies reported lubiprostone activation of the ClC-2 channel, lubiprostone’s actions on CFTR were less clear. Neither report suggested an increase in cAMP or pKA in response to lubiprostone.
Lubiprostone stimulation gastric muscle contraction suggested the compound may possess additional activity [12]. The observed contraction was inhibited by pre-treatment with an EP prostaglandin receptor antagonist selective for subtype 4 (EP4). EP4 is one of four prostaglandin E2 (PGE2) receptors and is a G-protein coupled receptor whose activation is associated with extensive second messaging via cAMP/protein kinase A (pKA) dependent pathways and phosphatidylinositol 3-kinase (PI3K) dependent pathways [13].
Later work found lubiprostone very selectively targets the EP4 prostanoid receptor [4; 14], resulting in increased cellular cAMP and CFTR dependent activity in gut cells and tissue [4]. To date, no studies have investigated possible lubiprostone-mediated ClC-2 conductance when specifically co-expressed with the EP4 receptor. Here we report the results of experiments designed to assay for possible activation of ClC-2 and CFTR by lubiprostone using an unambiguous model of heterologous channel and receptor expression in the Xenopus oocyte. We used two-electrode voltage clamp electrophysiology to determine if lubiprostone acts alone on ClC-2 or CFTR or if co-expression of the EP4 receptor is required for channel activation.
METHODS
Ethical Approval
Approval for harvesting of Xenopus Laevis oocytes was granted by the Animal Care and Use Committee of the Oregon Health and Sciences University.
Preparation and Microinjection of Oocytes
Female Xenopus Laevis, were anesthetized by immersion in cold water containing Tricaine, 3mg/ml (Sigma Chemical Co., St. Louis, MO). The oocytes were removed through a small abdominal incision which was then closed by 4.0 nylon suture. Frogs were then recovered in their tanks. The follicular membranes were removed by mechanical agitation (1–2 h) in a Ca2+-free solution containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES (pH 7.5), and 0.2 Wünsch units/mL Liberase Blendzyme TM (Roche Molecular Biochemicals, Indianapolis, IN). We selected Stage V and VI defolliculated oocytes which were then washed and incubated at 18° C in a modified Barth’s solution (MBSH) containing 88 mM NaCl, 1 mM KCl, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 2.4 mM NaHCO3, 10 mM HEPES-Hemi-Na, and 250 mg/L Amikacin with 150 mg/L Gentamicin (pH 7.5) until injection the next day. Oocytes were injected with 0.1–15 ng of cRNA (50nl volume) of human CFTR or rabbit ClC-2 cRNA (gift of N. McCarty) in conjunction with cRNA encoding the human β2-adrenergic receptor (βAR) or the human EP4 (PTGER4) receptor (Missouri University Science and Technology Resource Center, Rolla MO) using a microinjector (Drummond Scientific Co., Broomhall, PA). Injected oocytes were incubated at 18° C in 12-well plates containing MBSH. Injection pipettes were pulled from filamented glass capillary tubes (Sutter Instrument, Novato CA) on a P-97 Flaming – Brown micropipette puller (Sutter Instrument, Novato CA). Typically, CFTR oocytes were used 3 days after injection, while ClC-2 oocytes were used 5 days after injection.
Whole Cell Recordings
Individual oocytes were placed in a 200 µL RC-1Z recording chamber (Warner Instruments, Hamden CT) and continuously perfused with Frog Ringer’s solution (1.5mL/min) via a syringe pump. The Ringer’s solution contained 98 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES (hemi-Na) (pH 7.4). For those experiments in which the bath pH was modified, HEPES was replaced with MES (2-(N-morpholino) ethanesulfonic acid) which buffered the pH to 6.0. Oocytes were perfused continuously with the experimental solutions. Oocytes were initially maintained in the experimental chamber under open circuit conditions and experiments began when the transmembrane voltage was between −25mV and −40 mV. The membrane potential was held at −160 mV to +40 mV with steps at 25mV intervals for a period of 9 s. Three replicates of the voltage step protocol were performed for each of the perfusing solutions per oocyte. βAR receptors were activated by 10 µM isoproterenol (a β-adrenergic agonist) (Sigma Chemical Co., St. Louis, MO) and a phosphodiesterase inhibitor 1 mM 3-isobutyl-1-methylxanthine [IBMX] (Sigma Chemical Co., St. Louis, MO) [referred to as I+I in the figures]. Experiments utilizing EP4 receptor expression were initially performed with 1µm of lubiprostone (a kind gift of Sucampo Pharmaceuticals, Bethesda, MD) with 0.1% DMSO final concentration in the perfusate. A subset of ClC-2 – EP4 expressing oocytes were subjected to 0.1 µM and 10 µM lubiprostone containing perfusing solutions. In later CFTR - EP4 oocycte experiments, the membrane potential was ramped from −120 to +60 mV over a period of 1.8 s to construct whole-cell I –V plots while increasing concentrations of lubiprostone in the superperfusate from 1 pico molar to 1µ molar to determine dose response with 5 minutes exposure followed by 2 minutes of washout with Ringer’s solution. Conductance was then calculated from the slope of the I–V plot at the reversal potential (Vm = Erev) using a voltage range from Vm = Erev = 10 mV to Vm = Erev + 10 mV. The logarithm of the concentration was then plotted against normalized conductance and the EC50 was determined.
Membrane currents were recorded from oocytes with a two-electrode voltage clamp using an amplifier (TEV-200; Dagan, Minneapolis, MN) at a room temperature of ~ 22°C. Current-injecting and potential-measuring electrodes had resistances of ~0.5–2.0 and ~1.0–3.0 MΩ, respectively, when filled with 3 M KCl. The bath solution was connected to the ground via a low-resistance agarose bridge containing 2% agarose in 3 M KCl. A second reference electrode was used to avoid polarization errors. Current measurements were low-pass filtered at 0.5 kHz. Data acquisition and analysis were done on a Pentium-based microcomputer using pCLAMP software and an analog-to-digital converter (Axon Instruments, Foster City, CA). Comparison of mean current at the −110 mV membrane potential was performed by paired t-test.
RESULTS
ClC-2 and CFTR expressed in Xenopus oocytes are activated by hyperpolarization and cyclic AMP respectively
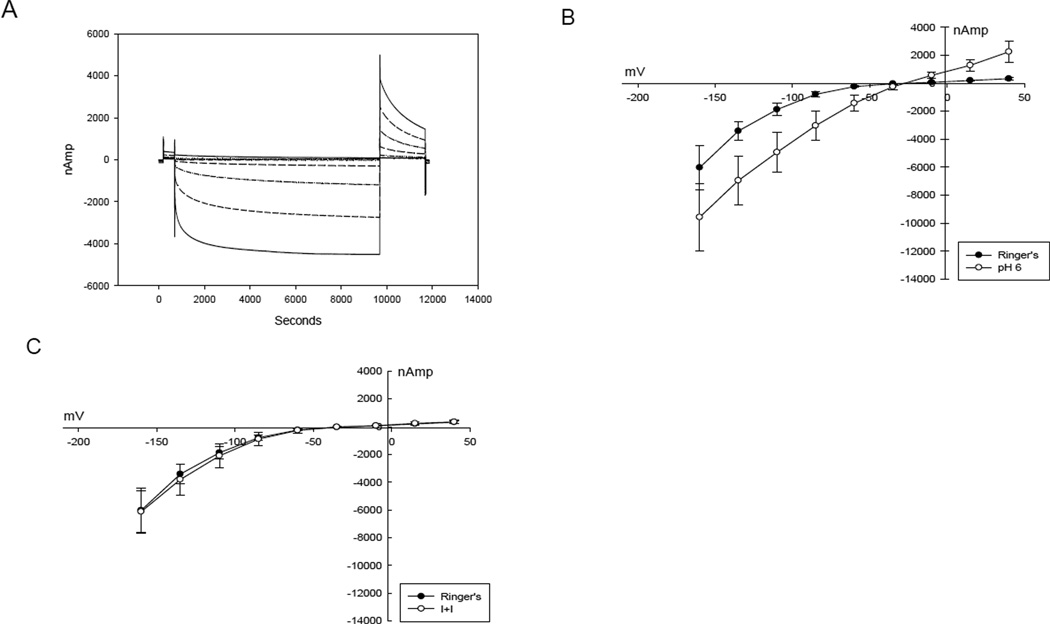
Figure 1A illustrates the response of oocytes co-expressing ClC-2 and βAR (n=6) to step changes in membrane potential, revealing voltage- and time-dependence at hyperpolarized potentials characteristic of ClC-2 channels [15; 16]. ClC-2 activation was enhanced by reducing the pH of the perfusate to 6.0 (Figure 1B) (p=0.0006). Exposure of the oocyte to isoproterenol and IBMX (I+I) in order to raise cystosolic cAMP did not alter ClC-2 activation (Figure 1C, p=0.3).
Figure 1.
A. Voltage step protocol demonstrating activation of current at each increasingly negative voltage step in a ClC-2 expressing oocyte. B. I – V plot of ClC-2 - βAR expressing oocyte showing enhanced activation with pH 6.0 superperfusate. C. I – V plot of ClC-2 and βAR expressing oocyte demonstrating no response to isoproterenol and IBMX.
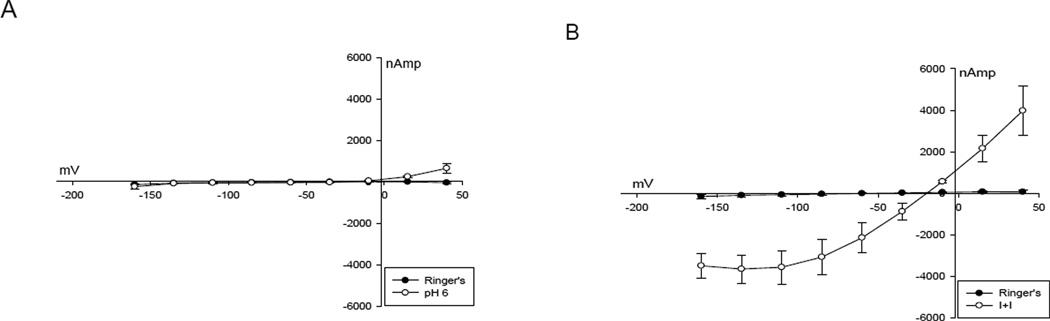
Oocytes expressing CFTR and βAR (n=4) failed to demonstrate significant voltage dependent activation or response to pH 6 perfusate (p=0.32) at negative membrane potentials (Figure 2A). In contrast, exposure of the oocyte to isoproterenol and IBMX (I+I) produced an increase in conductance and previously characterized voltage-dependent inhibition was seen at values of Vm exceeding 100 mV (Figure 2B)[17]. Un-injected oocytes (n=3) did not exhibit significant responses to the voltage step protocol, acidic pH, I+I, or 1 µm lubiprostone (data not shown).
Figure 2.
A. I – V plot of CFTR - βAR expressing oocyte demonstrating no activation by membrane hyperpolarization or pH 6.0 perfusate. B. I – V plot of CFTR – βAR expressing oocyte demonstrating activation by isoproterenol and IBMX. Note the inhibition as the transmembrane voltage becomes more negative.
Lubiprostone activates CFTR in oocytes co-expressing the EP4 receptor
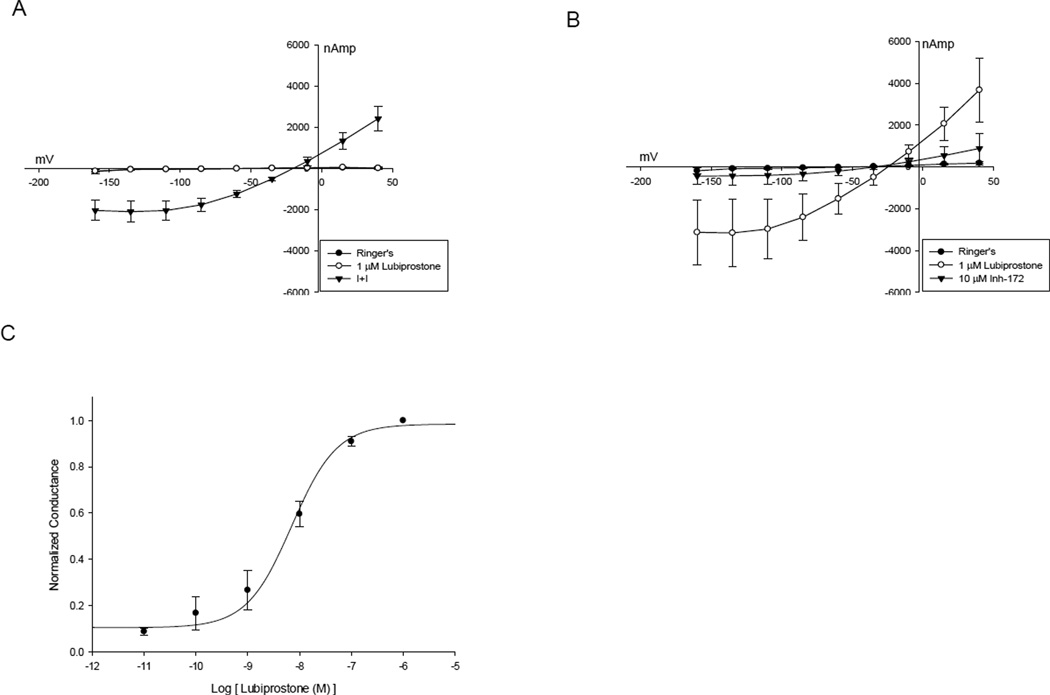
The conductance of oocytes co-expressing CFTR and βAR was not altered by exposure to 1 µm lubiprostone (Figure 3A). However, in oocytes co-expressing CFTR and EP4 receptor, exposure to lubiprostone elicited rapid increases in conductance (n=4) (p=0.03), similar to those evoked by I+I. This current was promptly inhibited by addition of the CFTR-specific inhibitor, CFTRinh-172 (10 µM, Figure 3B) to the perfusate, consistent with activation of CFTR-mediated chloride channels. The half maximal concentration (EC50) ~ 10 nM for lubiprostone activation of CFTR channels was estimated by plotting the change in normalized conductance (µS) versus concentration (Figure 3C). CFTR/EP4 co-expressing oocytes exhibited responses from 10 picomolar to a maximal effect at 1 µM.
Figure 3.
A. I –V plot of CFTR - βAR expressing oocyte demonstrating no activation by 1 µm lubiprostone with subsequent activation by isoproterenol and IBMX. B. I – V plot of CFTR - EP4 expressing oocyte activated by lubiprostone and then inhibited by CFTRinh- 172. C. Plot of conductance versus concentration of lubiprostone used to determine EC50 of lubiprostone activation of CFTR – EP4 oocyctes.
Lubiprostone does not activate conductance in ClC-2 expressing oocytes
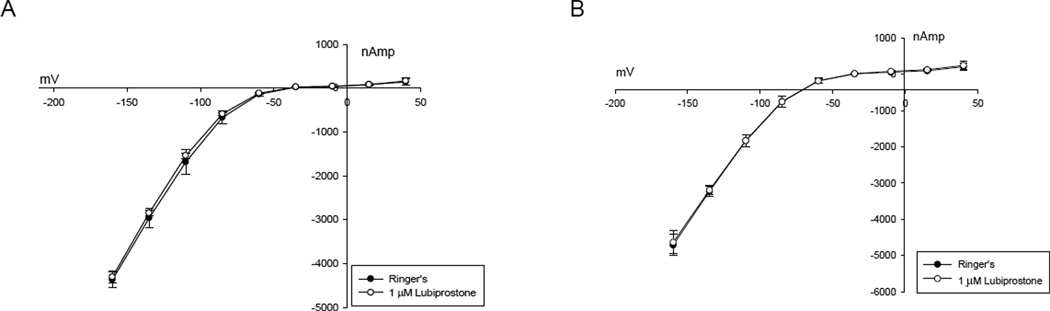
There was no change in conductance in ClC-2 only or ClC-2 and EP4 co-expressing oocytes exposed to 1 µm lubiprostone (Figures 4A and 4B) when compared to Ringers perfusate at each voltage step. Because a previous report suggested ClC-2 activation at lubiprostone doses lower than that for seen CFTR [3], we also examined current with 0.1 µM as well as 10 µM concentrations of lubiprostone, however, no change in conductance was noted. (data not shown).
Figure 4.
A. ClC-2 expressing oocyte with typical voltage activation followed by 1 µm lubiprostone added to the superperfusate showing no change. B. ClC-2 – EP4 oocyte showing voltage activation remains unchanged after 1 µm lubiprostone added to the superperfusate.
DISCUSSION
The goal of this study was to determine if a direct action of lubiprostone on ClC-2 and/or CFTR channels could be detected using a relatively simple, heterologous expression system, the Xenopus oocyte. A second goal was to use this expression system to test for a role of the EP4 receptor in previously reported ClC-2 channel activation [2; 3] by lubiprostone. Although our results clearly demonstrate the voltage and pH dependent activation expected for ClC-2 channels expressed in oocytes [7; 16; 18], no action of lubiprostone, either alone or in the context of the co-expressed receptors was detectable. In contrast, oocytes expressing CFTR and co-expressing either the βAR or EP4 receptor exhibited conductance increases in response to isoproterenol or lubiprostone, respectively, consistent with cAMP-dependent activation [19].
Previous reports suggest modification of ClC-2 channel conductance by pKA phosphorylation [20; 21], however, others report no change in channel activity despite ClC-2 phosphorylation [22]. While we did not measure cAMP or pKA directly, activation of CFTR by both I+I via βAR and lubiprostone via EP4, suggest a robust elevation of cAMP and increased pKA activity was achieved in oocytes. There was no change in current in ClC-2 – expressing oocytes in response to either βAR or EP4 receptor stimulation, consistent with the observation that c-AMP-dependent phosphorylation of ClC-2 does not activate the channel.
Lubiprostone application of 0.1µM, 1 µM, or 10 µM did not activate any ClC-2 current in oocytes expressing the channel. However, ClC-2-expressing oocytes did respond to hyperpolarization and mildly acidic pH, as expected. Reported lubiprostone activation of HEK293 cells expressing ClC-2 was assayed by whole cell patch clamp [2; 3]. While direct comparison is difficult, our model appears to generate greater current at each hyperpolarizing voltage step and is consistent with other published data on ClC-2 in oocytes [16; 23]. Jentsch [24], in a comprehensive review of ClC chloride channels, indicated that the I-V plots published for lubiprostone activation of ClC-2 were not consistent with previously demonstrated channel properties. To our knowledge, no data examining lubiprostone activation of ClC-2 expressed in oocytes has been published. Joo et al [25] describe personal observations of lubiprostone activating ClC-2 expressing oocytes, however, we did not achieve those results.
One report of presumed lubiprostone activation of ClC-2 utilized short circuit current determinations in T84 cells [2] where ClC-2 was reported to be expressed in the apical membrane. However, the membrane localization of ClC-2 in this cell line is highly controversial [4]. Our experiments remove the uncertainty of ClC-2 localization and expression and demonstrate a lack of ClC-2-mediated conductance by lubiprostone in a simplified physiological system.
We did not find evidence for activation of CFTR by lubiprostone alone. This differs from the findings of Bao et al [3] who reported lubiprostone activated CFTR channels in A6 cells absent an increase in cytosolic cAMP. We did detect vigorous activation of CFTR, however, when EP4 was co-expressed and lubiprostone was applied. This confirms other reports of CFTR activation by lubiprostone via EP4 [4; 5; 14].
There are several studies suggesting that non-CFTR-mediated anion conductance is activated by lubiprostone. In airway cells, lubiprostone activated currents despite CFTR knockdown by siRNA and the presence of a CFTR specific inhibitor [26]. In excised sheep tracheas, lubiprostone was reported to increase short circuit current, and the current was sensitive to Cd++, a non specific ClC-2 channel inhibitor [25]. MacDonald et al demonstrated small increases in transepithelial voltage in the nares of wild type and transgenic CFTR knockout mice in response to large 40 µm doses of lubiprostone delivered by continuous perfusion [27]. Oral dosing of lubiprostone in CFTR knockout mice ameliorated the intestinal phenotype by increasing intestinal transit time and mucus secretion [28]. A clinical case series and pilot study [29; 30], suggested lubiprostone was effective in treating constipation of Cystic Fibrosis patients. These reports suggest CFTR channel function is not required for the proposed increased epithelial secretion associated with lubiprostone although the molecular identity of the ion transport was not ascertained.
Lubiprostone activates CFTR expressing oocytes when the EP4 receptor is coexpressed, but in the absence of the receptor lubiprostone was without effect. Lubiprostone caused no change in conductance in ClC-2 - expressing oocytes either alone or when the EP4 receptor was co-expressed. Our data suggest ClC-2 may not be the anion conductance activated by lubiprostone when CFTR is absent or inhibited.
Highlights.
Lubiprostone chloride transport was examined in Xenopus oocytes
We studied ClC-2 and CFTR chloride channels
We examined ClC-2 co-expressed with prostaglandin receptor sub-type 4
We demonstrate lack of ClC-2 activation by lubiprostone in oocytes
ACKNOWLEGEMENT
The authors gratefully acknowledge David C Dawson and William R Skach for their mentorship. Supported by Parker B Frances Pulmonary Fellowship (KDM), OHSU Child Health Research Center (5 K12 HD057588 02), National Institute for Diabetes, Digestive and Kidney Diseases (DK45880), and the Cystic Fibrosis Foundation (DAWSON08G0).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lacy BE, Levy LC. Lubiprostone: a chloride channel activator. J Clin Gastroenterol. 2007;41:345–351. doi: 10.1097/01.mcg.0000225665.68920.df. [DOI] [PubMed] [Google Scholar]
- 2.Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G234–G251. doi: 10.1152/ajpgi.00366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijvelds MJ, Bot AG, Escher JC, De Jonge HR. Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–985. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci. 2011;56:339–351. doi: 10.1007/s10620-010-1495-8. [DOI] [PubMed] [Google Scholar]
- 6.Pilewski JM, Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79:S215–S255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 7.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356:57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 8.Jentsch TJ, Gunther W, Pusch M, Schwappach B. Properties of voltage-gated chloride channels of the ClC gene family. J Physiol. 1995;482:19S–25S. doi: 10.1113/jphysiol.1995.sp020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipecka J, Bali M, Thomas A, Fanen P, Edelman A, Fritsch J. Distribution of ClC-2 chloride channel in rat and human epithelial tissues. Am J Physiol Cell Physiol. 2002;282:C805–C816. doi: 10.1152/ajpcell.00291.2001. [DOI] [PubMed] [Google Scholar]
- 10.Murray CB, Morales MM, Flotte TR, McGrath-Morrow SA, Guggino WB, Zeitlin PL. CIC-2: a developmentally dependent chloride channel expressed in the fetal lung and downregulated after birth. Am J Respir Cell Mol Biol. 1995;12:597–604. doi: 10.1165/ajrcmb.12.6.7766424. [DOI] [PubMed] [Google Scholar]
- 11.Schwiebert EM, Cid-Soto LP, Stafford D, Carter M, Blaisdell CJ, Zeitlin PL, Guggino WB, Cutting GR. Analysis of ClC-2 channels as an alternative pathway for chloride conduction in cystic fibrosis airway cells. Proc Natl Acad Sci U S A. 1998;95:3879–3884. doi: 10.1073/pnas.95.7.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126–135. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sciences. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 14.Cuthbert AW. Lubiprostone targets prostanoid EP receptors in ovine airways. Br J Pharmacol. 2011;162:508–520. doi: 10.1111/j.1476-5381.2010.01058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furukawa T, Horikawa S, Terai T, Ogura T, Katayama Y, Hiraoka M. Molecular cloning and characterization of a novel truncated from (ClC-2 beta) of ClC-2 alpha (ClC-2G) in rabbit heart. FEBS Lett. 1995;375:56–62. doi: 10.1016/0014-5793(95)01178-h. [DOI] [PubMed] [Google Scholar]
- 16.Jordt SE, Jentsch TJ. Molecular dissection of gating in the ClC-2 chloride channel. EMBO J. 1997;16:1582–1592. doi: 10.1093/emboj/16.7.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai Z, Scott-Ward TS, Sheppard DN. Voltage-dependent Gating of the Cystic Fibrosis Transmembrane Conductance Regulator Cl– Channel. The Journal of General Physiology. 2003;122:605–620. doi: 10.1085/jgp.200308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niemeyer MaI, Cid LP, Zúñiga L, Catalán M, Sepúlveda FV. A conserved pore-lining glutamate as a voltage- and chloride-dependent gate in the ClC-2 chloride channel. The Journal of Physiology. 2003;553:873–879. doi: 10.1113/jphysiol.2003.055988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham SA, Worrell RT, Benos DJ, Frizzell RA. cAMP-stimulated ion currents in Xenopus oocytes expressing CFTR cRNA. American Journal of Physiology - Cell Physiology. 1992;262:C783–C788. doi: 10.1152/ajpcell.1992.262.3.C783. [DOI] [PubMed] [Google Scholar]
- 20.Cuppoletti J, Tewari KP, Sherry AM, Kupert EY, Malinowska DH. ClC-2 Clchannels in human lung epithelia: activation by arachidonic acid, amidation, and acid-activated omeprazole. Am J Physiol Cell Physiol. 2001;281:C46–C54. doi: 10.1152/ajpcell.2001.281.1.C46. [DOI] [PubMed] [Google Scholar]
- 21.Tewari KP, Malinowska DH, Sherry AM, Cuppoletti J. PKA and arachidonic acid activation of human recombinant ClC-2 chloride channels. Am J Physiol Cell Physiol. 2000;279:C40–C50. doi: 10.1152/ajpcell.2000.279.1.C40. [DOI] [PubMed] [Google Scholar]
- 22.Park K, Begenisich T, Melvin JE. Protein kinase A activation phosphorylates the rat ClC-2 Cl- channel but does not change activity. J Membr Biol. 2001;182:31–37. doi: 10.1007/s00232-001-0026-0. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa T, Ogura T, Katayama Y, Hiraoka M. Characteristics of rabbit ClC-2 current expressed in Xenopus oocytes and its contribution to volume regulation. American Journal of Physiology - Cell Physiology. 1998;274:C500–C512. doi: 10.1152/ajpcell.1998.274.2.C500. [DOI] [PubMed] [Google Scholar]
- 24.Jentsch TJ. To the editor. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol. 2009;44:64. doi: 10.1080/10409230902814887. [DOI] [PubMed] [Google Scholar]
- 25.Joo NS, Wine JJ, Cuthbert AW. Lubiprostone stimulates secretion from tracheal submucosal glands of sheep, pigs, and humans. Am J Physiol Lung Cell Mol Physiol. 2009;296:L811–L824. doi: 10.1152/ajplung.90636.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacVinish LJ, Cope G, Ropenga A, Cuthbert AW. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol. 2007;150:1055–1065. doi: 10.1038/sj.bjp.0707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDonald KD, McKenzie KR, Henderson MJ, Hawkins CE, Vij N, Zeitlin PL. Lubiprostone activates non-CFTR-dependent respiratory epithelial chloride secretion in cystic fibrosis mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L933–L940. doi: 10.1152/ajplung.90221.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Lisle RC, Mueller R, Roach E. Lubiprostone ameliorates the cystic fibrosis mouse intestinal phenotype. BMC Gastroenterol. 2010;10:107. doi: 10.1186/1471-230X-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien CE, Anderson PJ, Stowe CD. Use of the chloride channel activator lubiprostone for constipation in adults with cystic fibrosis: a case series. Ann Pharmacother. 2010;44:577–581. doi: 10.1345/aph.1M642. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien CE, Anderson PJ, Stowe CD. Lubiprostone for constipation in adults with cystic fibrosis: a pilot study. Ann Pharmacother. 2011;45:1061–1066. doi: 10.1345/aph.1Q219. [DOI] [PubMed] [Google Scholar]