Abstract
The ability of bone morphogenetic proteins (BMPs) to induce bone formation has led to a multitude of investigations into their use as bone graft substitutes in spinal surgery. The purpose of this multi-center clinical pilot study was to evaluate the safety and efficacy of BMP-7 (osteogenic protein 1, OP-1), in the form of a putty, combined with autograft for intertransverse process fusion of the lumbar spine in patients with symptomatic spinal stenosis and degenerative spondylolisthesis following spinal decompression. Twelve patients with spinal stenosis and degenerative lumbar spondylolisthesis underwent a laminectomy and partial or complete medial facetectomy as required for decompression of the neural elements, followed by an intertransverse process fusion by placing iliac crest autograft and OP-1 putty between the decorticated transverse processes. No instrumentation was used. Patients were followed clinically using the Oswestry scale and SF-36 outcome forms, and radiographically using static and dynamic radiographs to assess their fusion status over a 2-year period. Independent and blinded radiologists assessed the films for the presence of bridging bone between the transverse processes and measured translation and angulation on dynamic films using digital calipers. Radiographic outcome was compared to a historical control (autograft alone fusion without instrumentation for the treatment of degenerative spondylolisthesis). All adverse events were recorded prospectively. The results showed eight of the nine evaluable patients (89%) obtained at least a 20% improvement in their preoperative Oswestry score, while five of ten patients (50%) with radiographic follow-up achieved a solid fusion by the criteria used in this study. Bridging bone on the anteroposterior film was observed in seven of the ten patients (70%). No systemic toxicity, ectopic bone formation, recurrent stenosis or other adverse events related to the OP-1 putty implant were observed. A successful fusion was observed in slightly over half the patients in this study, using stringent criteria without adjunctive spinal instrumentation. This study did not demonstrate the statistical superiority of OP-1 combined with autograft over an autograft alone historical control, in which the fusion rate was 45%. There were no adverse events related to the OP-1 putty implant in this study, which supports findings in other studies suggesting the safety of bone morphogenetic proteins in spinal surgery.
Keywords: Lumbar spine, Fusion, BMP, Spondylolisthesis, OP-1 putty
Introduction
Degenerative lumbar spondylolisthesis with associated spinal stenosis may result in symptomatic low back and lower extremity pain in afflicted patients [40]. The most commonly performed surgical procedure to treat this condition in patients who do not respond to nonoperative therapy is a lumbar decompression and spinal arthrodesis. Although the majority of patients following this procedure report improvement in their symptoms of neurogenic claudication, a solid arthrodesis rate without instrumentation is only seen in approximately 45–90% of patients [2, 4, 6, 9, 22–24, 26, 32, 49, 52–54, 56]. While there are many factors that have been linked to fusion failure, including mechanical instability, multilevel procedures, infection, poor health, smoking, and certain medications, there is no way to accurately predict which patients will eventually go on to fusion non-healing [1, 5, 7, 8, 10, 11–13, 16, 27, 31, 33, 46, 47, 50]. Interestingly, failure to obtain a solid arthrodesis is not always associated with a suboptimal clinical outcome, as long as an adequate decompression is performed and spinal stability is obtained. Although some studies have demonstrated that spinal instrumentation may decrease the rate of fusion failure, the effects of instrumentation on the overall clinical improvement of the patient is frequently debated [24]. As a result, it becomes imperative to improve on methods of of providing spinal stability while potentially avoiding the complications, both short and long term, associated with the application of spinal instrumentation. Bone graft substitutes, and particularly bone morphogenetic proteins (BMPs), are one of the alternative methods being evaluated as a means of improving radiographic fusion success as well as functional improvement.
The discovery of osteoinductive protein factors by Marshal Urist in 1965 has prompted significant efforts to isolate and characterize these BMPs. These proteins have demonstrated the ability to stimulate bone formation and therefore offer the potential to enhance, augment or replace autogenous bone graft when attempting a spinal arthrodesis. BMPs stimulate pluripotent mesenchymal cells to differentiate into osteoblasts and produce matrix elements characteristic of a mature cell line. A member of the TGF-β superfamily, osteogenic protein 1 (OP-1), also known as BMP-7, strongly induces the formation of bone when implanted in soft tissues and is able to assist with fracture healing and bone fusions [17, 26, 51, 55].
Recombinant human OP-1 (rhOP-1), used as an adjunct or replacement for autologous bone graft has demonstrated successful outcomes without device related adverse events in a variety of animals models for both fracture healing and spinal fusion [14, 15, 20, 21, 29, 37, 38, 45]. Ongoing clinical studies are presently investigating the use of rhOP-1 in human spinal surgery. A 1-year follow-up study found that rhOP-1 (BMP-7) was safe to use as an adjunct to autologous iliac crest bone graft in the surgical management of symptomatic degenerative spondylolisthesis and spinal stenosis [55]. The purpose of this present study is to evaluate the 2-year safety and efficacy results of rhBMP-7 (OP-1 putty), used as an adjunct with autologous iliac crest bone graft, in the surgical treatment of lumbar degenerative spondylolisthesis
Materials and methods
After obtaining Human Investigations Committee approval at each of four participating centers, 12 patients with single-level, grade I or II degenerative spondylolisthesis (L3-L4 or L4-L5) and symptoms of neurogenic claudication were prospectively enrolled in this study. To meet the inclusion criteria for this study, patients had to be skeletally mature adults less than 81 years of age. All patients complained of disabling leg pain with or without back pain and had failed to improve with non-operative treatment for at least 6 months preoperatively, and had no previous fusion attempts at the affected level. Additionally, patients who showed signs of active spinal or systemic infections, smokers, morbidly obese patients, those with a known sensitivity to collagen, women of childbearing potential and anyone who was known at the time of enrollment to require additional surgery in the next 6 months were excluded. All patients completed a detailed demographic questionnaire including an Oswestry pain scale and SF-36 form. Preoperative anteroposterior, lateral, flexion and extension plain radiographs were obtained to evaluate the degree of translation and angulation at the level of the spondylolisthesis. Patients who had a degenerative spondylolisthesis grade III or IV, or who showed spinal instability measured on flexion/extension radiographs of greater than or equal to 20° of angular motion were excluded from this study. Advanced imaging studies were obtained (magnetic resonance imaging or myelogram/computed tomography) to confirm the presence of spinal stenosis at the level of the spondylolisthetic segment.
The study subjects (Table 1) included nine women and three men, with an average age of 68 and an age range of 45–79. Nine patients underwent surgery at the L4-L5 level, while three patients underwent surgery at the L3-L4 level.
Table 1.
Demographic and clinical details of the 12 study subjects
| Mean± SD | Range | |
|---|---|---|
| Age (years) | 68±8.5 | 45–79 |
| Height (cm) | 163.8±19.8 | 149.9–188 |
| Weight (kg) | 91.6±2.6 | 55.3–111.1 |
| Pre-op Oswestry score | 41±15.6 | 30–72.0 |
| n | % | |
|---|---|---|
| Gender | ||
| Female | 9 | 75 |
| Male | 3 | 25 |
| Level fused | ||
| L3-L4 | 3 | 25 |
| L4-L5 | 9 | 75 |
The OP-1 putty implant contained 3.5 mg of lyophilized rhOP-1 mixed with 230 mg carboxymethylcellulose (CMC) and 1 g type I bone collagen. One implant was used on each side of the spine. The powdered mixture was reconstituted at the time of surgery by the addition of saline to form putty.
Autograft bone was harvested from the posterior iliac crest. Amount of autograft taken was based on an intraoperative surgeon decision based on the amount of graft material needed to span the space between the transverse processes. This graft was then mixed with one unit of OP-1 putty per side.
Follow-up evaluation
The patients were evaluated at 6 weeks, 3 months, 6 months, 9 months, 12 months, and 24 months with radiographs and a physical exam 2. The Oswestry questionnaire was repeated at the 6-, 12- and 24- month time points. The SF-36 form was also repeated at the 24-month follow-up. Clinical success was defined as at least 20% improvement in the pre-operative Oswestry score as well as functional improvement as demonstrated by the SF-36 outcome measurement.
Table 2.
Radiographic and clinical outcome in the study subjects
| 24 month results | Success | Failure | Percentage of success |
|---|---|---|---|
| Clinical | 8 | 1 | 89 |
| Radiographic | 5 | 5 | 50 |
Two independent neuroradioiogists evaluated the radiographs using digital calipers to obtain measurements. The criteria for successful radiographic fusion were defined as the presence of bridging bone between the transverse processes at the spondylolisthetic segment (Fig. 1), ≤5° of angulation and ≤2 mm of translation on flexion/extension radiographs (Fig. 2). These criteria for demonstration of successful fusion were slightly more rigid than those established by the US FDA as part of the required follow-up for medical devices being evaluated under FDA approved IDE studies. All radiographic criteria had to be met to be classified as a radiographic success. In the event that there was disagreement between the radiologists regarding the fusion status, a third, independent, neuroradiologist was utilized as the tiebreaker. The radiographic outcomes were compared to those reported in a historical randomized, controlled, prospective study that included a fusion arm using autograft alone without instrumentation in the treatment of symptomatic degenerative spondylolisthesis [3]. All clinical adverse events, related or not to the use of OP-1, were recorded prospectively.
Fig. 1.
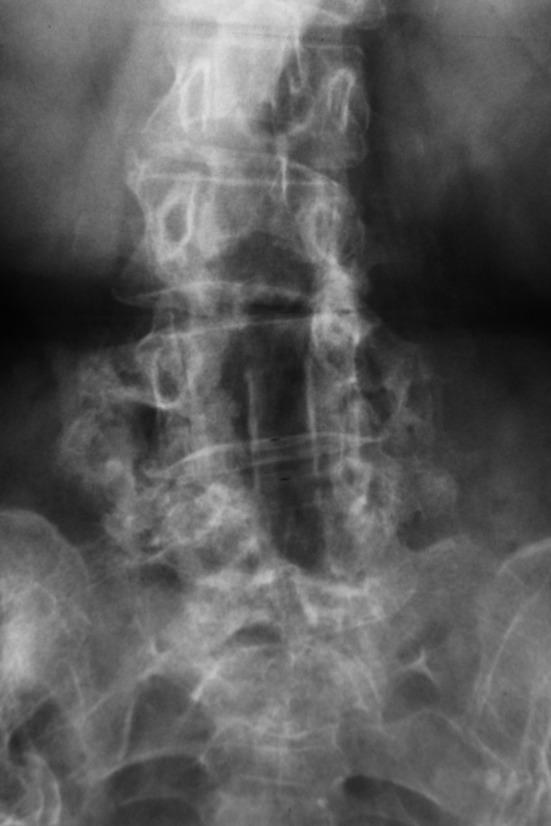
Twenty-four month follow-up antero-posterior radiograph of OP-1/autograft patient showing solid bridging bone between the transverse processes
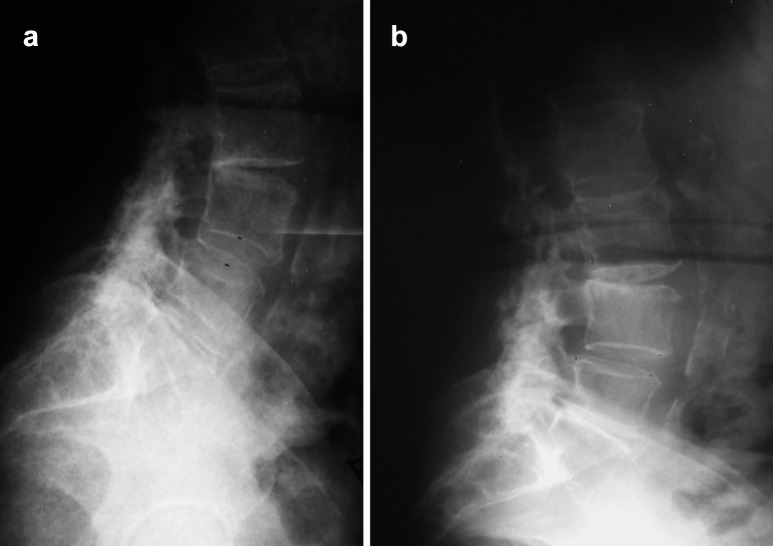
Fig. 2.
Lateral radiographs of an OP-1/autograft patient determined to be a radiographic success, in a flexion, and b extension
Results
A total of nine of the original 12 patients were available for clinical examination at the 24 month follow-up, and a total of ten patients had complete 24 month radiographic follow-up (this includes data for one patient without 24 month data but with 36 month data that was included in the analysis). Tables 3 and 4 show the outcome of the fusion operations. Clinical success at 24 months (20% improvement in the Oswestry score and functional improvement as demonstrated by the SF-36 outcome analysis) was achieved in eight of the nine patients available at follow-up (89%). Radiologic fusion at 24 months was achieved in five of ten patients at follow-up (50%) (Figs 1, 2). Two patients were lost to follow-up at approximately 6 months following their index procedure. One of these patients was diagnosed with a symptomatic pseudarthrosis at another institution and underwent a revision fusion procedure with pedicular fixation. One patient who was examined at 24 month follow-up underwent imaging evaluation but failed to complete his Oswestry and SF-36 outcome questionnaires leaving the total number of patients available for clinical follow-up at nine. If all patients that were lost to follow-up were considered to be failures, then the success rates for both the clinical and radiographic endpoints would have been lowered. If all lost patients were considered failures, the clinical success rate would have been 8/12 (67%) and the radiographic success rate would have been 5/12 (42%). Seven of ten patients (70%) were noted to have bridging bone between the transverse processes on their anteroposterior radiographs. No patient demonstrated progression of their spondylolisthesis and one patient underwent a revision posterior lumbar fusion for a clinical diagnosis of a pseudarthrosis.
Table 3.
Translation/angulation results of the reviewers (Rev.1, Rev. 2) for fusions classified as successful and those classified as failed. Seven of ten patients (70%) had bridging bone at 24 months
| Translation (mm) | Angulation (°) | Bridging bone on A/P films: Yes/No | |
|---|---|---|---|
| Rev. 1/Rev. 2 | Rev. 1/Rev. 2 | Rev. 1/Rev. 2 | |
| Failed fusions | |||
| 1 | 4.9/5.8 | 15.9/16.5 | y/y |
| 2 | 5.8/7.0 | 8.8/8.5 | n/y |
| 3 | 2.7/3.0 | 6.3/7.1 | n/y |
| 4 | 0.6/1.2 | 8.1/14.2 | n/y |
| 5a | 0.0/1.3 | 6.4/5.2 | y/y |
| Successful fusions | |||
| 1 | 0.6/1.0 | 1.6/0.0 | y/y |
| 2 | 0.6/0.0 | 1.9/0.0 | y/y |
| 3 | 1.49/0.3 | 1.5/1.2 | y/y |
| 4 | 0.6/1.2 | 2.7/1.3 | y/y |
| 5 | 0.6/1.5 | 2.5/1.7 | y/y |
a36-month data used for patient 5 in failed fusions
Table 4.
Summary table. NA not applicable
| Clinical | Radiographic | Bridging | |
|---|---|---|---|
| 1 | Success | Success | y |
| 2 | Not reported | Success | y |
| 3a | Lost | Lost | NA |
| 4 | Lost | Lost | NA |
| 5 | Success | Failure | y |
| 6 | Success | Failure | No |
| 7 | Success | Failure | No |
| 8 | Success | Success | y |
| 9 | Success | Success | y |
| 10 | Success | Success | y |
| 11 | Failure | Failure | No |
| 12b | Success | Failure | y |
aPatient 3 had revision surgery with pedicle screw instrumentation 9 months following the index procedure, following a diagnosis of pseudarthrosis
bUsing 36 month data for patient 12 because 24 month data was incomplete
Adverse events identified among the study group included one case of symptomatic pseudoarthrosis consisting of worsening back pain which eventually required a revision instrumented surgical fusion. Twenty-five percent of patients reported moderate donor site pain and no patients reported severe pain at 24 month follow-up 5. No patient in this study had any side effects attributable to the rhOP-1 product. There were no cases of systemic toxicity, ectopic bone formation or recurrence of spinal stenosis.
Table 5.
Donor site pain
| Visit window | None n (%) |
Mild n (%) |
Moderate n (%) |
Severe n (%) |
|---|---|---|---|---|
| 6 weeks | 50 | 30 | 20 | 0 |
| 3 months | 42 | 42 | 16 | 0 |
| 6 months | 46 | 27 | 18 | 9 |
| 9 months | 64 | 27 | 9 | 0 |
| 12 months | 63 | 0 | 37 | 0 |
| 24 monthsa | 75 | 0 | 25 | 0 |
a36 Month data used for patient 12
Discussion
Bone graft substitutes may be used clinically to replace the need for autograft bone or to act as an adjunct to either enhance the qualitative or quantitative nature of autologous bone during graft maturation. Though this is a limited human pilot study where data collected is preliminary in nature, the purpose of this investigation was 2-fold, to assess the safety of rhBMP-7 as a bone graft adjunct in spinal fusion, and to assess its efficacy as a bone graft adjunct compared to autograph alone in the setting of a lumbar degenerative spondylolisthesis.
This study, as well as other clinical investigations assessing the safety profile of BMPs in spinal fusion, suggest that these growth factors are safe for the indications evaluated. Animal studies utilizing a variety of BMPs have failed to demonstrate systemic toxicity or tumor formation in response to these substances [15, 17–21, 28, 38, 43–45]. In a clinical tibial non-union study by Friedlaender et al. [26], low levels of anti-OP-1 antibodies developed in approximately 10% of patients treated with OP-1. All of the anti-OP-1 antibody responses were transient and all titers were low. No adverse events related to sensitization were identified, and all patients who were found to have an anti-OP-1 antibody response were healed clinically and radiographically at the 24-month follow-up. Antibody titers were not available in our study population, as more advanced methods for testing for antibodies to OP-1 are currently in development.
At 2 year follow-up, this study demonstrated that OP-1 had an acceptable safety profile when used as an adjunct to iliac crest in posterolateral fusions in the setting of a degenerative spondylolisthesis. No patient demonstrated systemic toxicity, ectopic bone formation or implant migration into the laminectomy site.
Radiographically, the fusion success rate using the stringent criteria defined in this paper appeared to change very little between the 1 year and 2 year follow-up interval (55% versus 50% respectively). Clinically, results of the Oswestry scale and SF-36 similarly demonstrated no significant change in results over the same time period (75% versus 89% respectively). Fischgrund et al. [24] demonstrated the difficulty in obtaining a solid arthrodesis in the setting of a degenerative spondylolisthesis observing that in the absence of internal fixation, only 45% of patients went onto a solid arthrodesis. To improve the rate of fusion, some authors have recommended the use of supplemental instrumentation [9, 24, 42, 48], while others have argued that instrumentation may not lead to improved clinical outcomes [25, 35, 36, 39]. Our study found that the use of OP-1 as an adjunct to autologous iliac crest bone graft did not statistically improve the rate of fusion compared to historical controls. These findings may be a reflection of the prospective nature of this study and the application of very strict rules for judging fusion success.
The methods for judging fusion success varies widely between studies comparing the results of posterolateral fusion for degenerative spondylolisthesis, while the typical practice evaluation is based on clinical and radiographic (bridging bone) assessment. Although no method, short of surgical exploration, is considered completely accurate to determine the success of an arthrodesis, the radiographic criteria used in this study are commonly used as a non-invasive method to determine fusion success [9, 24, 32, 34, 35, 39, 48]. The use of digital calipers and independent neuroradiologists as employed in this study should increase the accuracy and objectivity compared with most other studies where less stringent fusion criteria are utilized. In addition, because instrumentation was not utilized in the current study, the ability of the radiographs to quantify ossification between the transverse processes and any residual motion is enhanced. It is known that some motion in the sagittal plane occurs in the setting of a solid posterolateral arthrodesis [3, 30, 41]; however, it is not clear how much motion should be evident to qualify as a true pseudoarthrosis.
Conclusion
The attainment of a solid posterolateral fusion in patients with degenerative spondylolisthesis in the absence of internal fixation continues to be a challenging fusion model. The present study evaluated the safety and efficacy of OP-1 putty in this patient population by combining OP-1 putty with autogenous bone for intertransverse process fusion. Although a successful fusion rate of only 50% was observed using stringent radiographic criteria, 70% of the patients demonstrated bridging bone, and clinical success was achieved in 89% of patients. No patient exhibited signs of systemic toxicity, ectopic bone formation or migration of the implant into the laminectomy site, and there were no complications related to the OP-1 putty product itself. The results presented in this small pilot study support the safety of OP-1 putty when used as an adjunct to autogenous iliac crest bone graft in uninstrumented posterolateral fusions. This study did not demonstrate an improved efficacy of OP-1 combined with autologous bone as compared to autologous bone graft alone in attempting to obtain an arthrodesis in the setting of a degenerative spondylolisthesis.
References
- 1.Albert TJ, Pinto M, Denis F. Management of symptomatic lumbar pseudarthrosis with anteroposterior fusion (discussion 130). A functional and radiographic outcome study. Spine. 2000;25:123–129. doi: 10.1097/00007632-200001010-00021. [DOI] [PubMed] [Google Scholar]
- 2.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Barrick WT, Schofferman JA, Reynolds JB, et al. Anterior lumbar fusion improves discogenic pain at levels of prior posterolateral fusion. Spine. 2000;25:853–857. doi: 10.1097/00007632-200004010-00014. [DOI] [PubMed] [Google Scholar]
- 4.Beirne JC, Barry HJ, Brady FA, Morris VB. Donor site morbidity of the anterior iliac crest following cancellous bone harvest. Int J Oral Maxillofac Surg. 1996;25:268–271. doi: 10.1016/s0901-5027(06)80053-6. [DOI] [PubMed] [Google Scholar]
- 5.Boden SD. The biology of posterolateral lumbar spinal fusion. Orthop Clin N Am. 1998;29:603–619. doi: 10.1016/s0030-5898(05)70034-1. [DOI] [PubMed] [Google Scholar]
- 6.Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Eng. 2000;6:383–399. doi: 10.1089/107632700418092. [DOI] [PubMed] [Google Scholar]
- 7.Boden SD, Sumner DR. Biologic factors affecting spinal fusion and degeneration. Spine. 1995;20:102S–112S. [PubMed] [Google Scholar]
- 8.Brantigan JW. Pseudarthrosis rate after allograft posterior lumbar interbody fusion with pedicle screw and plate fixation (discussion 1280) Spine. 1994;19:1271–1279. doi: 10.1097/00007632-199405310-00014. [DOI] [PubMed] [Google Scholar]
- 9.Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative spondylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–472. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brown CW, Orme TJ, Richardson HD. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine. 1986;11:942–943. doi: 10.1097/00007632-198611000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Buttermann GR, Glazer PA, Hu SS, Bradford DS. Revision of failed lumbar fusions. A comparison of anterior autograft and allograft. Spine. 1997;22:2748–2755. doi: 10.1097/00007632-199712010-00009. [DOI] [PubMed] [Google Scholar]
- 12.Cameron HU, Bridges A. Pseudoarthrosis in lumbar spine fusion. Prog Clin Biol Res. 1985;187:479–484. [PubMed] [Google Scholar]
- 13.Carpenter CT, Dietz JW, Leung KY, Hanscom DA, Wagner TA. Repair of a pseudarthrosis of the lumbar spine. A functional outcome study. J Bone Joint Surg [Am] 1996;78:712–720. doi: 10.2106/00004623-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Chen, et al. Osteogenic protein 1 induced bone formation in an infected segmental defect in the rat femur. J Orthop Res. 2002;20(1):142–150. doi: 10.1016/S0736-0266(01)00060-2. [DOI] [PubMed] [Google Scholar]
- 15.Chirossel Stryker Spine PEEK and titanium. 2000;cages:interbody. [Google Scholar]
- 16.Cohen DB, Chotivichit A, Fujita T, et al. Pseudarthrosis repair. Autogenous iliac crest versus femoral ring allograft. Clin Orthop. 2000;371:46–55. [PubMed] [Google Scholar]
- 17.Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669–671. [PubMed] [Google Scholar]
- 18.Cook SD, Baffes GC, Wolfe MW, Sampath TK, Rueger DC (1993) Recombinant human osteogenic protein-1 (rhOP01) heals segmental long bone defects in non-human primates. Presented at the 60th Annual meeting of the American Academy of Orthopaedic Surgeons, San Francisco
- 19.Cook SD, Dalton JE, Tan EH, Whitecloud TS, Rueger DC. In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusion. Spine. 1994;19:1655–1663. doi: 10.1097/00007632-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham BW, Kanayama M, Parker LM, et al. Osteogenic protein versus autologous interbody arthrodesis in the sheep thoracic spine. Spine. 1999;24:509–518. doi: 10.1097/00007632-199903150-00002. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham BW, Shimanoto N, Sefter JC, et al. Posterolateral spinal arthrodesis using osteogenic protein-1: an in-vivo time-course study using a canine model. New Orleans: NASS; 2000. [Google Scholar]
- 22.Torre JI, Tenenhaus M, Gallagher PM, Sachs SA. Harvesting iliac bone graft: decreasing the morbidity. Cleft Palate Craniofac J. 1999;36:388–390. doi: 10.1597/1545-1569_1999_036_0388_hibgdt_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 23.Palma AF, Rothman RH. The nature of pseudoarthrosis. Clin Orthop. 1968;59:113–118. [PubMed] [Google Scholar]
- 24.Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award Winner in Clinical Studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 25.France JC, Yaszemski MJ, Lauerman WC, et al. A randomized prospective study of posterolateral lumbar fusion. Outcomes with and without pedicle screw instrumentation. Spine. 1999;24:553–560. doi: 10.1097/00007632-199903150-00010. [DOI] [PubMed] [Google Scholar]
- 26.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg [Am] 2001;83:S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 27.Gertzbein SD, Hollopeter MR, Hall S. Pseudarthrosis of the lumbar spine. Outcome after circumferential fusion (discussion 2356–2357) Spine. 1998;23:2352–2356. doi: 10.1097/00007632-199811010-00021. [DOI] [PubMed] [Google Scholar]
- 28.Grauer et al. (2003) Evaluation of OP-1 in a novel model of Pseudarthrosis Repair. Presented at the Annual North American Spine Society (NASS) Meeting , San Diego
- 29.Grauer JN, Patel TC, Erulkar JS, Troiano MW, Panjabi MM, Friedlaender GE. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2000;26:127–133. doi: 10.1097/00007632-200101150-00004. [DOI] [PubMed] [Google Scholar]
- 30.Greenough CG, Peterson MD, Hadlow S, Fraser RD. Instrumented posterolateral lumbar fusion. Results and comparison with anterior interbody fusion. Spine. 1998;23:479–486. doi: 10.1097/00007632-199802150-00015. [DOI] [PubMed] [Google Scholar]
- 31.Heggeness MH, Esses SI. Classification of pseudarthroses of the lumbar spine. Spine. 1991;16:S449–S454. [PubMed] [Google Scholar]
- 32.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am] 1991;73:802–808. [PubMed] [Google Scholar]
- 33.Jacobson JA, Starok M, Pathria MN, Garfin SR. Pseudarthrosis: US evaluation after posterolateral spinal fusion: work in progress. Radiology. 1997;204:853–858. doi: 10.1148/radiology.204.3.9280271. [DOI] [PubMed] [Google Scholar]
- 34.Kanayama M, Cunningham BW, Weis JC, Parker LM, Kaneda K, McAfee PC. The effects of rigid spinal instrumentation and solid bony fusion on spinal kinematics. A posterolateral spinal arthrodesis model. Spine. 1998;23:767–773. doi: 10.1097/00007632-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 35.Kimura I, Shingu H, Murata M, Hashiguchi H. Lumbar posterolateral fusion alone or with transpedicular instrumentation in L4–L5 degenerative spondylolisthesis. J Spinal Disord. 2001;14:301–310. doi: 10.1097/00002517-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kuntz KM, Snider RK, Weinstein JN, Pope MH, Katz JN. Cost-effectiveness of fusion with and without instrumentation for patients with degenerative spondylolisthesis and spinal stenosis. Spine. 2000;25:1132–1139. doi: 10.1097/00007632-200005010-00015. [DOI] [PubMed] [Google Scholar]
- 37.Magin M, Delling G. Improved lumbar vertebral interbody fusion using rhOP-1. Spine. 2001;26:469–478. doi: 10.1097/00007632-200103010-00009. [DOI] [PubMed] [Google Scholar]
- 38.Magin MN. Enhancement of lumbar vertebral interbody fusion by human recombinant osteogenic protein-1 (BMP-7), in a sheep model. Spine. 2001;26:469–478. doi: 10.1097/00007632-200103010-00009. [DOI] [PubMed] [Google Scholar]
- 39.McCulloch JA. Microdecompression and uninstrumented single-level fusion for spinal canal stenosis with degenerative spondylolisthesis. Spine. 1998;23:2243–2252. doi: 10.1097/00007632-199810150-00020. [DOI] [PubMed] [Google Scholar]
- 40.Moller, et al. Symptoms, signs, and functional disability in adult spondylolisthesis. Spine. 2000;25(6):683–689. doi: 10.1097/00007632-200003150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Nachemson A, Zdeblick TA, O’Brien JP. Lumbar disc disease with discogenic pain. What surgical treatment is most effective? Spine. 1996;21:1835–1838. doi: 10.1097/00007632-199608010-00023. [DOI] [PubMed] [Google Scholar]
- 42.Nork SE, Hu SS, Workman KL, Glazer PA, Bradford DS. Patient outcomes after decompression and instrumented posterior spinal fusion for degenerative spondylolisthesis. Spine. 1999;24:561–569. doi: 10.1097/00007632-199903150-00012. [DOI] [PubMed] [Google Scholar]
- 43.Paramore CG, Lauryssen C, Rauzzino J, et al. The safety of OP-1 for lumbar fusion with decompression—a canine study. Neurosurgery. 1999;44:1151–1156. doi: 10.1097/00006123-199905000-00134. [DOI] [PubMed] [Google Scholar]
- 44.Patel, et al. Osteogenic protein 1 overcomes the inhibitory effect of nicotine on posterolateral fusion. Spine. 2001;26(15):1656–1661. doi: 10.1097/00007632-200108010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Patel TC, Erulkar JS, Grauer JS. OP-1 overcomes the inhibitory effects of nicotine on lumbar fusion. New Orleans: NASS; 2000. [DOI] [PubMed] [Google Scholar]
- 46.Quagliano PV, Hayes CW, Palmer WE. Vertebral pseudoarthrosis associated with diffuse idiopathic skeletal hyperostosis. Skeletal Radiol. 1993;23:353–355. doi: 10.1007/BF02416992. [DOI] [PubMed] [Google Scholar]
- 47.Raiszadeh R, Heggeness M, Esses SI. Thoracolumbar pseudarthrosis. Am J Orthop. 2000;29:513–520. [PubMed] [Google Scholar]
- 48.Rechtine GR, Sutterlin CE, Wood GW, Boyd RJ, Mansfield FL. The efficacy of pedicle screw/plate fixation on lumbar/lumbosacral autogenous bone graft fusion in adult patients with degenerative spondylolisthesis. J Spinal Disord. 1996;9:382–391. [PubMed] [Google Scholar]
- 49.Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine. 2001;26:1473–1476. doi: 10.1097/00007632-200107010-00018. [DOI] [PubMed] [Google Scholar]
- 50.Rothman RH, Booth R. Failures of spinal fusion. Orthop Clin N Am. 1975;6:299–304. [PubMed] [Google Scholar]
- 51.Salkeld SL, Patron LP, Barrack RL, Cook SD. The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg [Am] 2001;83:803–816. doi: 10.2106/00004623-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Schnee CL, Freese A, Weil RJ, Marcotte PJ. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine. 1997;22:2222–2227. doi: 10.1097/00007632-199710010-00005. [DOI] [PubMed] [Google Scholar]
- 53.Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop. 1992;284:80–90. [PubMed] [Google Scholar]
- 54.Summers BM, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg [Br] 1989;71:677–680. doi: 10.1302/0301-620X.71B4.2768321. [DOI] [PubMed] [Google Scholar]
- 55.Vaccaro, et al. A pilot safety and efficacy study of OP-1 putty (rhBMP-7) as an ajunct to iliac crest autograft in posterolateral lumbar fusions. Eur Spine J. 2003;12(5):495–500. doi: 10.1007/s00586-003-0561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]