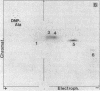
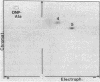
Abstract
Ribosome releasing factor (RR factor) releases ribosomes from mRNA at the termination codon in Escherichia coli. In the absence of this factor, polypeptides with molecular weights very close to the molecular weight of bacteriophage R17 coat protein were synthesized in vitro under the direction of a mutant R17 phage RNA having an amber mutation at codon 7 of the coat cistron. The major coat-protein-like product shared all the R17 coat protein sequence except that the seven NH2-terminal amino acids were missing. The minor product had the complete coat protein sequence starting from formylmethionine except for a probable amino acid substitution at codon 7 (UAG). Addition of RR factor inhibited the synthesis of the major protein. These results indicate that, in the absence of RR factor, the ribosome that has released the NH2-terminal hexapeptide at the amber codon stays on the mRNA and subsequently reinitiates translation "in phase" immediately after the amber codon without formylmethionine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M. On the release of the formyl group from nascent protein. J Mol Biol. 1968 May 14;33(3):571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- Berberich M. A., Kovach J. S., Goldberger R. F. Chain initiation in a polycistronic message: sequential versus simultaneous derepression of the enzymes for histidine biosynthesis in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1857–1864. doi: 10.1073/pnas.57.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brot N., Caldwell P., Weissbach H. Regulation of synthesis of Escherichia coli ribosomal proteins L1 and L11. Arch Biochem Biophys. 1981 Jan;206(1):51–53. doi: 10.1016/0003-9861(81)90064-3. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Bergquist P. L. Peptide mapping study of R17 coat protein synthesized in vitro. Biochemistry. 1970 Mar 17;9(6):1310–1317. doi: 10.1021/bi00808a003. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Cell-free protein synthesis programmed with R17 RNA: identification of two phage proteins. J Mol Biol. 1966 Oct 28;21(1):173–193. doi: 10.1016/0022-2836(66)90086-6. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Klein H. A. Characterization of three proteins involved in polypeptide chain termination. Cold Spring Harb Symp Quant Biol. 1969;34:469–477. doi: 10.1101/sqb.1969.034.01.053. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Platt T. Gene structure in the tryptophan operon of Escherichia coli. Nucleotide sequence of trpC and the flanking intercistronic regions. J Mol Biol. 1980 Oct 5;142(4):519–530. doi: 10.1016/0022-2836(80)90261-2. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Deutch C. E., Scarpulla R. C., Soffer R. L. Posttranslational NH2-terminal aminoacylation. Curr Top Cell Regul. 1978;13:1–28. doi: 10.1016/b978-0-12-152813-3.50005-7. [DOI] [PubMed] [Google Scholar]
- Files J. G., Weber K., Miller J. H. Translational reinitiation: reinitiation of lac repressor fragments at three internal sites early in the lac i gene of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Mar;71(3):667–670. doi: 10.1073/pnas.71.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoza M. C. The Ayerst Award Lecture 1976. Novel factors in protein biosynthesis. Can J Biochem. 1977 Apr;55(4):267–281. doi: 10.1139/o77-038. [DOI] [PubMed] [Google Scholar]
- Ganoza M. C., Tomkins J. K. Polypeptide chain termination in vitro: competition for nonsense codons between a purified release factor and suppressor tRNA. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1455–1463. doi: 10.1016/0006-291x(70)90031-8. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Nakada D. Non-continuous translation of coat protein and ribonucleic acid polymerase cistrons in MS2 bacteriophage ribonucleic acid. J Bacteriol. 1974 Jan;117(1):227–231. doi: 10.1128/jb.117.1.227-231.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Kaji A. Role of elongation factor G and a protein factor on the release of ribosomes from messenger ribonucleic acid. J Biol Chem. 1973 Nov 10;248(21):7580–7587. [PubMed] [Google Scholar]
- KAJI A., KAJI H., NOVELLI G. D. SOLUBLE AMINO ACID-INCORPORATING SYSTEM. I. PREPARATION OF THE SYSTEM AND NATURE OF THE REACTION. J Biol Chem. 1965 Mar;240:1185–1191. [PubMed] [Google Scholar]
- KAJI A., KAJI H., NOVELLI G. D. SOLUBLE AMINO ACID-INCORPORATING SYSTEM. II. SOLUBLE NATURE OF THE SYSTEM AND THE CHARACTERIZATION OF THE RADIOACTIVE PRODUCT. J Biol Chem. 1965 Mar;240:1192–1197. [PubMed] [Google Scholar]
- Kozak M. Evaluation of the "scanning model" for initiation of protein synthesis in eucaryotes. Cell. 1980 Nov;22(1 Pt 1):7–8. doi: 10.1016/0092-8674(80)90148-8. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Leder P. Deformylation and protein biosynthesis. Biochemistry. 1969 Jan;8(1):435–443. doi: 10.1021/bi00829a059. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Robertson H. D. Regulation of in vitro translation of bacteriophage f2 RNA. Cold Spring Harb Symp Quant Biol. 1969;34:655–673. doi: 10.1101/sqb.1969.034.01.076. [DOI] [PubMed] [Google Scholar]
- Malamy M. H. Frameshift mutations in the lactose operon of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:189–201. doi: 10.1101/sqb.1966.031.01.027. [DOI] [PubMed] [Google Scholar]
- Manley J. L. Synthesis of internal re-initiation fragments of beta-galactosidase in vitro and in vivo. J Mol Biol. 1978 Nov 15;125(4):449–466. doi: 10.1016/0022-2836(78)90310-8. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Silbert D. F., Smith W. E., Whitfield H. J., Jr Polarity in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):357–369. doi: 10.1016/0022-2836(66)90104-5. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Zipser D. Mapping of polypeptide reinitiation sites within the beta-galactosidase structural gene. J Mol Biol. 1969 May 14;41(3):341–347. doi: 10.1016/0022-2836(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Momose K., Kaji A. Soluble amino acid-incorporating system. 3. Further studies on the product and its relation to the ribosomal system for incorporation. J Biol Chem. 1966 Jul 25;241(14):3294–3307. [PubMed] [Google Scholar]
- Newton A. Re-initiation of polypeptide synthesis and polarity in the lac operon of Escherichia coli. J Mol Biol. 1969 May 14;41(3):329–339. doi: 10.1016/0022-2836(69)90279-4. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Kaji A. Requirement for ribosome-releasing factor for the release of ribosomes at the termination codon. Eur J Biochem. 1975 Oct 15;58(2):411–419. doi: 10.1111/j.1432-1033.1975.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Kaji A. Ribosome run through of the termination codon in the absence of the ribosome releasing factor. Biochim Biophys Acta. 1975 Sep 1;402(3):288–296. doi: 10.1016/0005-2787(75)90266-x. [DOI] [PubMed] [Google Scholar]
- Platt T., Yanofsky C. An intercistronic region and ribosome-binding site in bacterial messenger RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2399–2403. doi: 10.1073/pnas.72.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmus J., Deyl Z. Chromatographic methods in the analysis of protein structure. The methods for identification of N-terminal amino acids in peptides and proteins. Part B. J Chromatogr. 1972 Aug 23;70(2):221–339. doi: 10.1016/s0021-9673(00)92700-6. [DOI] [PubMed] [Google Scholar]
- Ryoji M., Karpen J. W., Kaji A. Further characterization of ribosome releasing factor and evidence that it prevents ribosomes from reading through a termination codon. J Biol Chem. 1981 Jun 10;256(11):5798–5801. [PubMed] [Google Scholar]
- Sarabhai A., Brenner S. A mutant which reinitiates the polypeptide chain after chain termination. J Mol Biol. 1967 Jul 14;27(1):145–162. doi: 10.1016/0022-2836(67)90357-9. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Tanemura S., Bauerle R. Internal reinitiation of translation in polar mutants of the trpB gene of Salmonella typhimurium. Mol Gen Genet. 1977 Jun 8;153(2):135–143. doi: 10.1007/BF00264728. [DOI] [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Tooze J., Weber K. Isolation and characterization of amber mutants of bacteriophage R17. J Mol Biol. 1967 Sep 14;28(2):311–330. doi: 10.1016/s0022-2836(67)80012-3. [DOI] [PubMed] [Google Scholar]
- Weber K. Amino acid sequence studies on the tryptic peptides of the coat protein of the bacteriophage R17. Biochemistry. 1967 Oct;6(10):3144–3154. doi: 10.1021/bi00862a023. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Zinder N. D. Fate of the message-ribosome complex upon translation of termination signals. J Mol Biol. 1969 Jun 28;42(3):425–439. doi: 10.1016/0022-2836(69)90234-4. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C., Squires C. L. Regulated in vitro synthesis of Escherichia coli tryptophan operon messenger ribonucleic acid and enzymes. J Biol Chem. 1974 Jan 25;249(2):465–475. [PubMed] [Google Scholar]